Abstract
Xylan polysaccharides previously obtained from argan (Argania spinosa) leaves by sequential alkaline extractions and purified by chromatography on Sepharose CL-4B were fragmented by acid hydrolysis in 2 M HCl. The resulting fragments were separated by gel filtration on Biogel P2. The oligosaccharide fractions obtained were then characterized by gas–liquid chromatography (GLC) and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). The results show the presence of acidic oligosaccharide motifs, some of which composed of xylose and 4-O-methylglucuronic acid and other ones comprising rhamnose and galacturonic acid.
Public Interest Statement
In the context of the valorisation of the Algerian southern trees, it seemed useful to us to be interested in parietal polysaccharides of the Argan leaves and more particularly hemicelluloses. These macromolecules constitute a source of chemicals that the agro-alimentary, cosmetic, and pharmaceutical industries exploit for their remarkable properties. In this study, xylan hemicellulosic extracts, subjected to a fractionation step by size exclusion chromatography on a Sepharose CL-4B gel column, have been hydrolyzed with HCl. This acid hydrolysis is a non-selective method which, thanks to its random breaking mode, makes it possible to obtain a wide range of different oligosaccharides. The oligosaccharide fractions thus obtained have been purified on a Biogel P2 column. Acid hydrolysis of the xylan fractions revealed the presence of some acidic oligosaccharide units, composed of xylose and 4-O-methylglucuronic acid and others ones, of pectic nature, comprising rhamnose and galacturonic acid.
Competing interests
The authors declare no competing interest.
1. Introduction
Structural analysis of a polysaccharide often relies on obtaining oligosaccharide units which are further characterized either in their native form by MALDI MS, 1H and 13C NMR or, after derivatization, by GC and GC-MS. Polysaccharide fragmentation can be carried out in various ways, acid or enzymatic hydrolysis, under variable operating conditions (with different acids, hydrolysis times, etc.), but the type of rupture and the extent of depolymerization do not solely depend on the experimental conditions but also on the structure of the polymer itself.
Acid hydrolysis is sometimes a complementary approach to enzymatic hydrolysis. By virtue of the random nature of the acid hydrolysis, some structural motifs of the polysaccharide can be identified in different oligosaccharides, which can make it possible to reconstruct the polymer from the oligosaccharide structures (Citation1).
We recently characterized the structure of xylan-type hemicelluloses from the leaves of the argan tree growing in Southwestern Algeria, this remnant of former tropical vegetation, is well adapted to extreme conditions of aridity and temperature and is actually the last defense against desertification (Citation2). Enzymatic treatment of these polysaccharides was conducted with an endo-(2-5)-β-d-xylanase. Applied to xylan-type polysaccharides, this enzymatic hydrolysis generated oligomers whose repeating unit was determined as Ara-Xyl(3–5)-4-O-MeGlcA(1–2). In the present study, our aim was to investigate the structure of xylan polysaccharide fractions from Algerian argan leaves obtained by hydrolysis in 2 M HCl.
2. Experimental
2.1. Biological material
Xylan polysaccharide fractions used in this study were isolated from the leaves of Argania spinosa (L.) Skeels collected in June 2010 from Tindouf province (southwestern Algeria). The plant material was botanically identified by Pr. Meriem Kaid-Harche (Department of Biotechnology, Oran University of Science and Technology-Mohamed Boudiaf-), and voucher (UMTSB 612010) specimen has been deposited at the Herbarium of the Department of Biology, Faculty of Sciences, Dr Moulay Tahar University of Saïda, Algeria.
2.2. Acid hydrolysis and purification
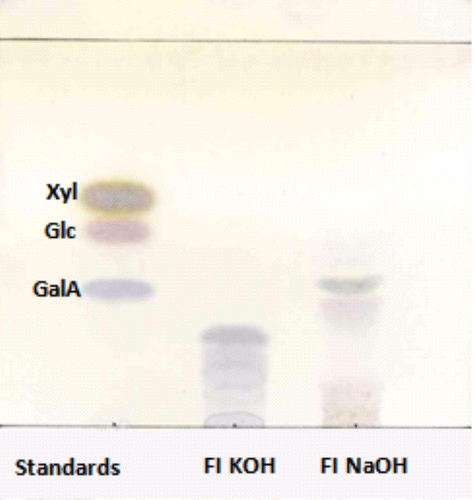
The xylan fractions (20 mg) were placed in the presence of 2 M HCl (10 mL) in a water bath at 100°C for one hour. After cooling, the hydrolyzate was neutralized with NaOH and then the non-hydrolyzed fraction was precipitated by adding 3 volumes of ethanol. After filtration, the hydrolyzate was concentrated by evaporation under vacuum. The acid hydrolysis profile was monitored by thin-layer chromatography (TLC) using butanol/acetic acid/water (2/1/1 v/v/v) as eluent. The revelation of spots was carried out by spraying orcinol-sulfuric acid reagent (0.001% of orcinol m/v in a 10% H2SO4 solution v/v) and heated at 100°C.
The oligosaccharide hydrolyzate was then purified by size exclusion chromatography on Biogel P2 column (Biorad; MWCO 100–1800 Da; column Ø: 2,5 cm × 70 cm), and eluted at 10 mL/h with water, at room temperature. The column effluent was monitored using a refractive index detector.
2.3. Chemical characterizations
2.3.1. Monosaccharide analysis
Monosaccharide determinations of the acid hydrolyzates were carried out after methanolysis (1 N MeOH/HCl, 24 h, 80°C) by gas–liquid chromatography of per-trimethylsilylated methylglycosides as previously described by Hachem et al. (Citation2).
2.3.2. Mass spectrometry
MALDI-TOF MS was performed on a Q-TOF Global mass spectrometer (Waters, UK) and a MALDI Autoflex III Smartbeam (Bruker Daltonics, Bremen, Germany). Mass spectra were recorded in the reflection mode using N,N-dimethylaniline (DMA)/2,5-dihydroxybenzoic acid (DHB) (100 mg·mL−1) as the matrix (Citation2).
3. Results and discussion
3.1. Chemical isolation of cell wall polysaccharides
Xylan-type hemicelluloses from the leaves of argan were obtained by a series of successive extractions and purified according to Hachem et al. (Citation2). Briefly, the first step consists in removing pigments, circulating sugars, tannins and mineral elements with an acetone/isopropanol solution (fraction S0) followed by extraction with 80% ethanol (fraction S1). Extraction of pectins is carried out in two steps: HM pectins (highly methyl-esterified) are extracted with hot water (fraction S2) and LM (low methyl-esterified) pectins are extracted in presence of ammonium oxalate (fraction S3), a calcium chelator which destabilizes the “egg box” structures. Hemicelluloses are then extracted in two steps (fractions S4 and S5) with alkaline solutions (KOH and NaOH, respectively). Each hemi-cellulosic extract was purified by size exclusion chromatography on a Sepharose CL-4B column with sodium acetate solution as eluent. We recovered the main fractions FI (the first elution fraction) and FIII (the third elution fraction) from the KOH-soluble hemicellulosic extracts, and FI and FIII for the NaOH-extracted hemicellulosic extracts.
3.2. Acid hydrolysis
We first monitored the products of acid hydrolysis in 2 M HCl in function of time (30 min, 1 h, 1h30 and 2 h) in order to find the optimum conditions for the release of intermediate-size oligosaccharides. On the basis of this kinetics, the xylan fractions (FI and FIII) were incubated with 2 M HCl and placed in a water bath at 100°C for one hour. After cooling, the hydrolyzate was neutralized by NaOH and then the non-hydrolyzed fractions precipitated by adding 3 volumes of ethanol. The oligosaccharide hydrolyzate was then purified by size exclusion chromatography on Biogel P2 column and the fractions collected were analyzed by TLC. The chromatographic profile (Figure ) of the hydrolysis products revealed the presence of acid oligosaccharides containing glucuronic acid.
3.3. Monosaccharide composition
The monosaccharide composition of the various oligosaccharide fractions is presented in Table corresponding to oligosaccharides resulting from acid hydrolysis of the main fractions FI and FIII of the hemicelluloses extracted with KOH (S4) and NaOH (S5). The fractions obtained do not display the same characteristics as the starting extracts S4 and S5. The acid nature of the oligosaccharides obtained is clearly apparent. The analysis shows significant percentages of galacturonic acid, between 22.8 and 62.5%, and rhamnose between, 17.1 and 31.6%, whereas these levels were relatively low in the fractions of the starting extracts (less than 7.2%) (Citation2). The presence of glucuronic acid appears to be an artifact, derived from the demethylation of 4-O-methylglucuronic acid during acid hydrolysis (Citation3). Indeed, the literature on the distribution of acids shows very variable contents (Citation4). They are often found in dicotyledons and can reach a higher ratio after acid hydrolysis as in birch wood (Citation3).
Table 1. Monosaccharide composition determined by GLC of oligosaccharides fractions derived from the hemicellulosic fractions by acid hydrolysis
Glucose is present in significant amounts since it can reach 38.7% in the oligosaccharides NaOH FI and FIII and this can be explained by the contamination of these oligosaccharides with xyloglucan (Citation5). Xylose is also present in amounts less than 4.6–11.2% although xylose and arabinose were shown to be the predominant monosaccharides of the main fractions (24.9–33.3% xylose and 31.9–39.4% arabinose) (Citation2). This low level of xylose and its absence in the FI KOH fraction together with some neutral oses such as arabinose in the various fractions of oligosaccharides can be explained by the hydrolysis conditions (reaction time and high temperature) that lead to their degradation (Citation6).
3.4. Mass spectrometry analysis
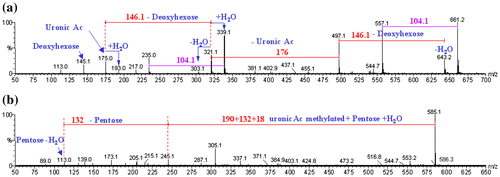
The molecular weights and the degree of polymerization (DP) of the oligosaccharides present in the acid hydrolyzates were determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). The samples were studied in the native form. The results are shown in Table .
Table 2. Positive-ion mode matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) of the oligosaccharides generated from the hemicellulosic fractions by acid hydrolysis
The chemical species in the different hydrolyzates identified with MALDI and electrospray ionization (ESI) corresponded mainly to a series of disaccharides: deoxyhexose, uronic acid, probably rhamnose, galactucuronic acid, and to a lesser extent, pentose residues bearing a methylated uronic acid, probably xylose, 4-O-methyl-glucuronic acid with a degree of polymerization (DP) equal to 4 (Figure ).
Figure 2. Mass spectrum from positive-mode MALDI-TOF MS (0.1 mg mL−1) generated by acid hydrolysis for (a) the FI KOH hemicellulosic fraction; and (b) the FI NaOH hemicellulosic fraction.
The present results showed some differences with those previously reported about xylan isolated from the leaves of Algerian argan (Citation2): in the latter case, the enzymatic hydrolysate showed only arabinoxylo-oligosaccharide substituted with a 4-O-methyl-glucuronic acid. The absence of arabinose in the xylo-oligosaccharide obtained by acid hydrolysis can be explained by its degradation as a result of the temperature rise (Citation6). However, implementation of enzymatic hydrolysis recommends mild temperature conditions and requires as many different enzymes as the types of bonds involved in the polysaccharides (Citation7).
Rhamnose and galacturonic acid appear to be integral parts of the xylan structure of argan leaves. Shimizu and Samuelson (Citation8) have shown that these units characterize the xylan reducing end. The presence of this motif can explain the resistance of xylans to alkaline peeling (Citation9). Indeed, complexes of xylans and pectic polysaccharides have also been isolated, such as a the glucuronoxylan-pectin complex obtained from beech (Citation10) and the type II xylan-arabinogalactan from suspension cultured cells of white campion (Citation11).
Some hemicelluloses containing uronic acids are more difficult to hydrolyze than neutral polysaccharides. This is due to the particular resistance of the ose-uronic acid bond to acid hydrolysis. Under these conditions, hydrolysis leads to the formation of a mixture of monosaccharides and aldobiuronic acids (dimers consisting of a neutral sugar and a uronic acid). The relative stability of these disaccharide in acid media is explained by the effects of the C-5 carboxyl group (Citation12). The doublet of oxygen, already engaged in a hydrogen bond with carboxyl at C-5, is less available to stabilize, by delocalization, the carbocation which would form during acid hydrolysis.
4. Conclusion
In a complementary way to selective hydrolysis using specific enzymes, acid hydrolysis is a non-selective method which, thanks to its random breaking mode, makes it possible to obtain a wide range of oligosaccharides. These, once compared, can reveal a common pattern that will give an image as close as possible to the structure of the xylans in the leaves of Algerian argan trees.
Acid hydrolysis with 2 M HCl of the hemicellulosic fractions of the Algerian Argan leaves revealed the presence of acidic oligosaccharide motifs. We showed that the xylan fraction consisted of xylose and 4-O-methylglucuronic acid and a pectic fraction comprised rhamnose and galacturonic acid. Further studies by 1H and 13C NMR spectroscopy are needed to fully identify the oligosaccharides and their relationships.
Funding
The authors received no direct funding for this research.
Acknowledgments
The authors acknowledge Vincent Sol, director of the Laboratory of Chemistry of Natural Substances (LCSN-EA 1069—France) for his warm and friendly welcome, David Ropartz for Mass Spectrometry analyses, and Dr Michel Guilloton for manuscript editing.
Additional information
Notes on contributors
Kadda Hachem
Our group is particularly interested in polysaccharides, of plant or microbial origin, due to their quantitative importance in terms of biomass and their very high structural richness; this large diversity is responsible for their various chemical properties and their specific biological activities. In addition to polysaccharides, we are currently developing the study of the chemical and biological properties of phenolic derivatives found in plant biomass, with a view to their valorization.
References
- Vuorinen, T.; Alén, R. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Sjöström, E. and Alén, R., Eds.; Springer-Verlag, Berlin, 1999.
- Hachem, K.; Faugeron, C.; Kaid-Harche, M.; Gloaguen, V. Structural Investigation of Cell Wall Xylan Polysaccharides from the Leaves of Algerian Argania spinosa. Molecules. 2016, 21, 1587.10.3390/molecules21111587
- Shimizu K. In Wood and Cellulosic Chemistry: Hon DN-S., Shiraishi N., Eds.; Marcel Dekker, New York; 1991.
- Wilkie, K.C.B. The hemicelluloses of grasses and cereals. Adv. Carbohydr. Chem. Biochem. 1979, 36, 215–264.10.1016/S0065-2318(08)60237-1
- Coimbra, M.A.; Rigby, N.M.; Selvendran, R.R.; Waldron, K.W. Investigation of the Occurrence of Xylan-xyloglucan Complexes in the Cell Walls of Olive Pulp (Olea europaea). Carbohydr. Polym. 1995, 27, 277–284.10.1016/0144-8617(95)00067-4
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299.10.1016/0008-6215(83)88244-5
- Dekker, R.F.H.; Richards, G.N. Hemicellulases: Their Occurrence, Purification, Properties, and Mode of Action. Adv. Carbohydr. Chem. Biochem. 1976, 32, 277–352.10.1016/S0065-2318(08)60339-X
- Shimizu, K.; Samuelson, O. Uronic Acids in Birch Hemicelluloses. Svensk. Papperstidning. 1973, 76, 150–155.
- Johansson, M.H.; Samuelson, O. Reducing End Groups in Birch Xylan and Their Alkaline Degradation. Wood Sci. Technol. 1977, 11, 251–263.10.1007/BF00356924
- Hromádková, Z.; Ebringerová, A.; Kačuráková, M.; Alföldi, J. Interactions of the Beechwood Xylan Component with Other Cell Wall Polymers. J. Wood Chem. Technol. 1996, 16, 221–234.10.1080/02773819608545805
- Kwan, J.S.; Morvan, H. Extracellular Branched Xylans and Acidic Arabinogalactans from Suspension Cultured Cells of White Campion (Silene alba (Miller) E.H.L. Krause). Food Hydrocolloids. 1991, 5, 163–166.10.1016/S0268-005X(09)80305-6
- Timell, T.E.; Enterman, W.; Spencer, F.; Soltes, E.J. The Acid Hydrolysis of Glycosides: II. Effect of Substituents at C-5. Can J Chem. 1965, 43, 2296–2305.10.1139/v65-310