Abstract
Caralluma lasiantha is used as a traditional medicine in India to heal body heat and inflammations. In order to find out a scientific validation for the Indian traditional knowledge, antibacterial activity of C. lasiantha extracts was studied against inflammation causing bacteria (viz., Staphylococcus aureus, Escherichia coli, Streptococcus Sp., Bacillus subtilis, Enterobacter aerogenes, Klebsiella pneumoniae) along with other Gram-positive and Gram-negative bacteria. Solvents with different polarity were used for extraction from dry roots and stems. Minimum inhibitory concentrations (MIC) were also studied. Differential antibacterial activity was exhibited by extracts and higher inhibition potential against Gram-positive bacteria was explained. The observed antibacterial activities were correlated with the chemical structures of phytochemicals present in C. lasiantha. Anti-inflammation activities are related to C. lasiantha extracts through their antibacterial activities.
Public Interest Statement
Now a days, plant-based products are gaining much importance due to the reason that micro-organisms are showing resistance day by day toward several synthetic antibiotic drugs. In addition to this, lot of side effects are there due to use of synthetic drugs. Caralluma is one of the genus of Asclepiadaceae family possessing good pharmacological importance. One of the species of Caralluma genus namely Caralluma lasiantha was selected to carry out its anti bacterial activities to prove the scientific sanctity of its usage in Indian traditional medicine.
1. Introduction
Nowadays, an increase in awareness on traditional medicine is observed due to development of resistance by micro-organisms toward many synthetic antibiotics. The healing potential of plants is very well known from primeval times. The secondary metabolites produced in plants are responsible for their pharmacological activity (Citation1). As plants provide a number of drugs, herbal products have been used in the healing of an array of ailments (Citation2). The need of hour is the screening of local medicinal plants in view of their richness in antimicrobial agents (Citation3).
Caralluma is one of the prominent genus out of 200 genera and 2500 species of Asclepiadaceae family (Citation4, 5). It grows well in dry regions like India, Africa and the Middle East (Citation6). In folkloric medicine, as well as in Unani and Ayurvedic systems of medicine, the plants of Caralluma are being used for the treatment of diabetic patients and rheumatism (Citation7). Tribals consider some of them as food during famines (Citation8, 9) and also as a part of traditional medicinal system (Citation10). A spectrum of biological activities of Caralluma species can be expected due to the existence of pregnane glycosides (Citation11), stigmasterol and other phytochemicals (Citation12) in them.
Caralluma lasiantha (syn. Boucerosia lasiantha) belongs to the family Asclepiadaceae and its local name is Sirumankeerai (in Tamil) / Kundeti Kommulu (in Telugu) (Citation13). C. lasiantha is succulent in habit and is used as an indoor ornamental plant (Citation14). It grows wild in Anantapur, Chittoor, and surrounding places of Andhra Pradesh, India. This plant is medicinally important as it is rich in pregnane glycosides, which may possess different biological activities (Citation15) including anti-hyperglycemic effect (Citation16). In India, 10 grams of fresh root less plant is taken as such twice a day for a period of 3 days to reduce the body heat and inflamations (Citation17).
However to the knowledge of authors, no scientific validation was conducted for this Indian traditional knowledge of using C. lasiantha for the above-discussed medicinal usage. Hence, inflammation causing bacteria (viz., Staphylococcus aureus, Escherichia coli, Streptococcus Sp., Bacillus subtilis, Enterobacter aerogenes, Klebsiella pneumoniae) as well as other bacteria are selected in the present investigation in order to determine their susceptibility toward extracts obtained using different solvents having varying polarities. Steroidal glycosides (Citation15, 18), C21 pregnane steroid (Citation19) and C27 steroid (Citation20) were isolated using alcohol, chloroform, and n-hexane, respectively, as extracting solvents. The observed antibacterial activity of C. lasiantha extracts against those micro-organisms is very well substantiated by relating with different phytochemicals present in it, and also, relation between C. lasiantha extracts and anti-inflammation activity was discussed.
2. Materials and methods
Analytical Reagent grade chemicals of Merck India Co. Ltd. were used in the present study. Wherever it is necessary, they were purified according to the standard procedures (Citation21). Geological location (Citation22), season (Citation23), and plant collection time (Citation24) influence the chemical composition or active constituents of the plants which play a key role in exhibiting the biological activity by plant extracts. Fresh whole plants of C. lasiantha (Asclepiadaceae) were collected from Gooty, Anantapur District, Andhra Pradesh, India in February 2012. Voucher specimen of the plant was deposited in Herbarium, Department of Botany, Sri Krishna Devaraya University, Anantapur. Libermann–Burchard test (Citation25), Molisch test and Shinoda test (Citation26) were used, respectively, to test the presence of steroids, steroidal glycosides, and flavanoids in the extracts of C. lasiantha. Bacteria used of the present study were provided by Institute of Microbial Technology (IMTECH), Chandigarh. Antibacterial activity was carried out on four each Gram-positive and Gram-negative bacteria. Gram-positive bacteria are S. aureus (MTCC 3103), B. subtilis (MTCC 1305), Streptococcus Sp., (MTCC 9724), and Bacillus megaterium (MTCC 9554). Gram-negative bacteria are E. aerogenes (MTCC 8358), K. pneumoniae (MTCC 10309), E. coli (MTCC 9537), and Pseudomonas aeruginosa (MTCC 10636).
2.1. Preparation of extract
Roots and stems were dried separately under shade, powdered, sieved (sieve No.14), and stored in air tight containers. The weighed quantity (200 g) of dried powder was subjected to successive solvent extraction method in Soxhlet extractor using solvents with varying polarity (n-hexane, chloroform, and methanol). All the extracts were concentrated and last trace of solvent was removed by applying vacuum (Citation27, 28). The crude extracts were purified by recrystallization.
2.2. In vitro screening of antibacterial activity
The in vitro antibacterial activity was evaluated for the root and stem extracts of the C. lasiantha by agar well diffusion method (Citation29, 30). The medium (prepared by mixing peptone-5.0 g, sodium chloride-5.0 g, beef extract-1.5 g, agar-15.0 g in 1 l of distilled water) (Citation31) was poured in to petridishes under aseptic conditions in a laminar flow chamber. When the medium in the plates was solidified, 0.1 mL of 24 h old culture of test organism (Gram-positive bacteria such as Streptococcus Sp., B. subtilis, S. aureus, B. megaterium and Gram-negative bacteria such as E. aerogenes, K. pneumoniae, E. coli, P. aeruginosa) was inoculated. After inoculation, cups were scooped out with 6-mm sterile cork borer and the lids of the dishes were replaced. Different concentrations of extracts (1000, 750, 500, 250 μg/mL) were used for study. Dimethylsulfoxide (DMSO) is maintained as negative control and inhibitions shown by it are negligible. Chloramphenicol and Ampicillin were used as positive controls. The plates were kept in an incubator at 37 °C for 24 h. Inhibition zones were measured to nearest millimeter. MIC was also determined for each bacteria tested (Citation32). The antibacterial activities were carried out in triplicate and average values were compiled.
3. Results and discussion
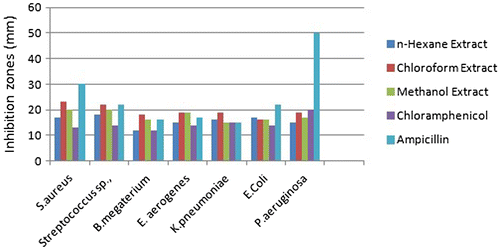
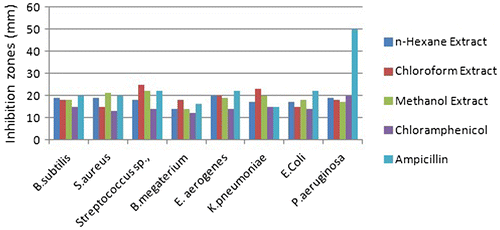
Antimicrobial activity of all the C. lasiantha extracts was studied against Gram-positive (B. subtilis, S. aureus, Streptococcus Sp., B. megaterium) and Gram-negative (E. aerogenes, K. pneumoniae, E. coli, P. aeruginosa) bacteria (Tables and ; Figures and ). The salient features of antibacterial analysis are summarized and given below.
| A. | Highlights of antibacterial activity of stem / root extracts against Gram-positive bacteria
| ||||||||||||||||||||||
| B. | Highlights of antibacterial activity of stem / root extracts against Gram-negative bacteria
| ||||||||||||||||||||||
| C. | Effect of polarity of solvent used extraction on antibacterial activity Against B. subtilis, Streptococcus Sp., E. aerogenes and E. coli, the order of biological activity is: n-hexane extract > chloroform extract > methanol extract. Against S. aureus, K. pneumoniae and P. aeruginosa, the order of biological activity is: n-hexane extract < chloroform extract < methanol extract. | ||||||||||||||||||||||
| D. | Minimum inhibitory concentration (MIC): These studies were carried out at different concentrations in order to find out MIC (Minimum inhibitory concentration) (Tables and ) of extracts for antibacterial activity. MIC values for stem extracts are as follows: 250 μg/mL for B. subtilis / S. aureus / Streptococcus Sp., (n-hexane, chloroform, and methanol extracts); 500 μg/mL for P. aeruginosa (n-hexane, chloroform, and methanol extracts); 750 μg/mL for B. megaterium (n-hexane extract), E. aerogenes/K. pneumoniae (n-hexane, chloroform, and methanol extracts); 1000 μg/mL for B. megaterium (chloroform and methanol extracts). MIC values for root extracts are as follows: 250 μg/mL for B. subtilis / S. aureus/ E. coli (n-hexane, chloroform, and methanol extracts), Streptococcus Sp. (n-hexane, chloroform extracts), P. aeruginosa (chloroform extract), 500 μg/mL for P. aeruginosa (n-hexane and methanol extracts), E. aerogenes (chloroform extract), K. pneumoniae (chloroform extract); 750 μg/mL for B. megaterium (n-hexane extract), E. aerogenes (n-hexane and methanol extracts), K. pneumoniae (n-hexane and methanol extracts); 1000 μg/mL for B. megaterium (chloroform and methanol extracts). | ||||||||||||||||||||||
| E. | Inhibition exhibited by plant extracts against Gram-positive bacteria is higher compared to that of Gram-negative bacteria. | ||||||||||||||||||||||
| F. | Statistical analysis of Figures and was carried out (excel sheets are attached). From the ANOVA test the following conclusions were drawn. On a particular bacterium, variation in the effect of type of extract is negligible as Fcal < Fcrit at 5% level of significance and hence, null hypothesis (H0) can be accepted. However, variation in the effect of type of extract on a particular bacteria is not negligible as Fcal > Fcrit at 5% level of significance and hence, null hypothesis (H0) can be rejected. | ||||||||||||||||||||||
Table 1. Antibacterial activity of crude extracts of Caralluma lasiantha Stems
Table 2. Antibacterial activity of crude extracts of Caralluma lasiantha Roots
Table 3. MIC of antibacterial activity of crude extracts of Caralluma lasiantha Stems
Table 4. MIC of antibacterial activity of crude extracts of Caralluma lasiantha Roots
3.1. Pharmacological activities and phytochemicals of Caralluma lasiantha
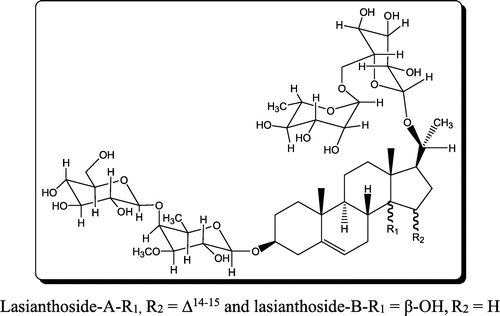
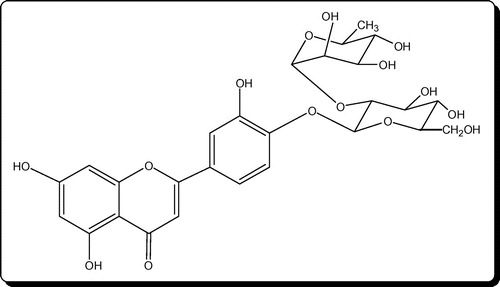
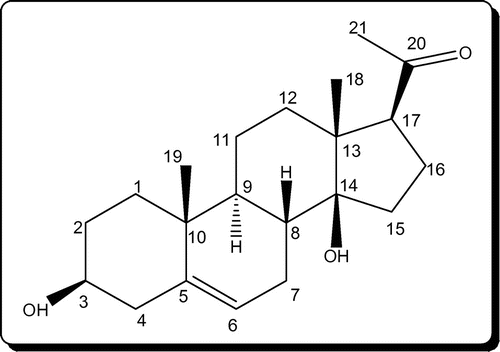
C. lasiantha is a part of Indian traditional medicinal usage to reduce body heat which is an indication of the antibiotic nature (Citation17). So, it can be expected that extracts of C. lasiantha may exhibit activity against pathogenic bacteria. In the present study, the crude extracts of stem and roots of C. lasiantha were tested against infectious bacteria. The rationality in exhibiting medicinal properties by the extracts of C. lasiantha (Tables and ) can be ascribed to active constituents existing in the extracts. The active constituents isolated from C. lasiantha by earlier researchers are steroidal glycosides (lasianthoside-A (Figure ), lasianthoside-B (Figure )) and flavone glycoside (Luteoline-4-O-neohesperiodoside Figure ) (Citation15, 18) using polar solvents like alcohols. In the present study, existence of steroids in the C. lasiantha extracts is verified from appearance of to observe red color in Libermann–Burchard test (Citation25). Similarly, existence of steroidal glycosides and flavanoids is confirmed from positive Molisch test and Shinoda test (Citation26) to observe violet color and no reaction, respectively. Based on the polarity, different solvents are capable of extracting different phytochemicals (Citation33). Hence in the present study, different solvents (non-polar to polar) were used.
Nowadays, Caralluma is gaining a great importance from scientific community due to exhibition of immunostimulating activities as active constituents like saponins and flavonoids are present in it (Citation34). The major bioactive constituents of Ayurvedic medicine are saponins, flavonoids, and polyphenols (Citation35). Previous investigations on essential medicinal plants disclosed that saponins exhibit good anti-inflammatory activity (Citation36) and antimicrobial activity (Citation37). Literature survey reveals that other species of Caralluma show antimicrobial activity due to the existence of tannins, flavonoids, and sterols in them (Citation38). For example, aqueous extracts of Caralluma adscendens were efficient against S. typhi, E. coli and P. aeruginosa (Citation39), and petroleum ether extract was effective against S. aureus and E. coli (Citation40). Antimicrobial activity can be explained based on interactions of saponins present in C. lasiantha with the membrane of cells which causes variation in the permeability of cells (Citation37). In addition, the presence of sugar moiety in saponins indicates a higher hemolytic activity (Citation41). Hence, the observed antimicrobial activity of extracts in the present study can be explained based on the existence of steroidal glycosides (lasianthoside-A (Figure ), lasianthoside-B (Figure ) in C. lasiantha (Citation15).
3.2. Antibacterial activity due to steroids
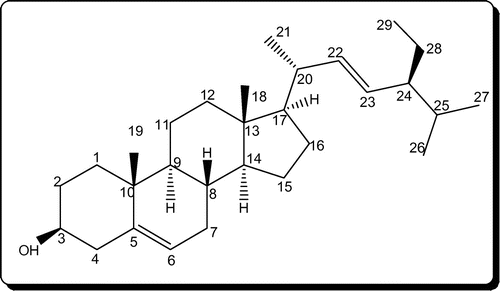
In our previous publications, we have reported the isolation and characterization of one C21 pregnane steroid, 3β,14β-dihydroxy-14β-pregn-5-en-20-one (Figure ) from chloroform extracts (Citation19) and stigmasterol (Figure ) from n-hexane extracts (Citation20) of C. lasiantha. In addition, Qiu et al. (Citation15) and Ramesh et al. (Citation18) reported the isolation of steroidal glycosides from alcohol extracts. Hence from the above observations mentioned under “effect of polarity of solvent used extraction on antibacterial activity”, higher activity observed against B. subtilis, Streptococcus Sp., E. aerogenes and E. coli by n-hexane extract can be attributed to stigmasterol. Similarly, steroidal glycosides of methanol extract might be active against S. aureus, K. pneumoniae and P. aeruginosa.
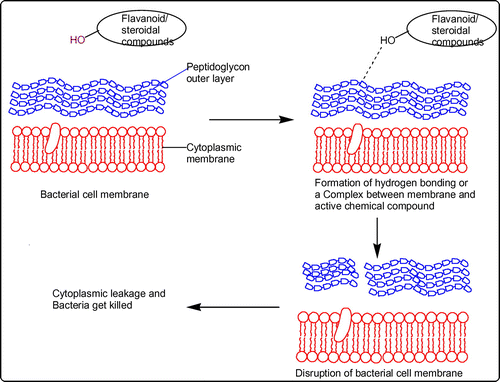
The difference in reactivities of epimers of sterols can be established based on type of conformation of the hydroxyl group located at position-3. Equatorial 3-hydroxyl groups experience low shielding effect compared to axial groups. Hence, esters of equatorial hydroxyl groups hydrolyze easily and hence high reactivity is noticed. Epimers containing equatorial hydroxyl groups form stable complexes and are willingly sorbed on various carriers (Citation42, 43) due to formation of hydrogen bonding. As hydroxyl group is present at position-3 in both C21 pregnane steroid (3β,14β-dihydroxy-14β-pregn-5-en-20-one) and stigmasterol, their contribution toward antibacterial activity can be understood as sterols are integrated into biological membrane by forming complexes with primary phospholipids of the membrane (Citation44). The plausible mechanism is depicted in Figure .
3.3. Antibacterial activity due to flavonoids and flavone glycosides
It is well known that many traditional medicinal plants synthesize bioactive aromatic compounds like saponins, flavonoids, and polyphenols which form the basis for Ayurvedic medicine (Citation35). Flavonoids, flavones, and flavonols demonstrate antimicrobial activity against a range of micro-organisms as these phytochemicals are synthesized in plants due to microbial infection (Citation45). Moreover, antibacterial activity of saponins can be explained by release of strong antibiotic compounds after the hydrolysis of saponins (Citation38, 46). As flavonoids are phenolic compounds, they form irreversible complexes with extracellular/soluble proteins (Citation33) with involvement of hydroxyl group (Citation47) which restrain protein synthesis in bacteria (Citation48) and hence antibacterial activity is exhibited by them (Citation2, 49). Other postulates on antibacterial activity of flavonoids are (1) disruption of microbial membranes by lipophilic flavonoids (Citation50) (2) release of hydrogen peroxide by some flavonoids in aqueous media at physiological pH (Citation51), (3) inhibition of enzymes and precipitation of proteins in micro-organisms due to interference of glycosylated flavonoids (Citation52, 53), and (4) inhibition of phosphor kinases and ATP by flavonoids (Citation54). Hence, antibacterial activity of C. lasiantha can be explained based on the presence of flavone glycoside (Luteoline-4-O-neohesperiodoside) (Citation15, 18).
3.4. Higher activity of plant extracts toward Gram-positive bacteria
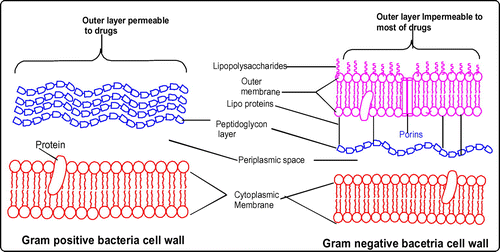
In the present study, it is observed that inhibition displayed by all stem/root extracts against all the selected Gram-positive bacteria is higher than Gram-negative bacteria (Tables and ). It is similar to the previous reports which suggested a higher susceptibility of food-borne pathogenic Gram-positive bacteria to tea flavonoids in comparison with Gram-negative bacteria (Citation55, 56). Higher activity against Gram-positive bacteria can be explained in terms of specificity of saponins toward them. Literature survey shows that saponins present in Medicago species exhibited high efficacy against Gram-positive bacteria (like B. subtilis, S. aureus, and Bacillus cereus) whereas unsuccessful against Gram-negative bacteria (Citation41). The differential activities of plant extracts against these two types of bacteria can be elucidated taking into account of their cell outer layers. In the case of Gram-positive bacteria, cell outer barrier is made up of peptidoglycan layer which is ineffective and permeable. Hence, drug constituents are permeable through the cell wall of Gram-positive bacteria. However, in Gram-negative bacteria, cell wall is made up of multilayered peptidoglycan and phospholipidic membrane which is impermeable to drug constituents (Citation57). In addition, glycosylated flavonoids selectively inhibit topoisomerases in Gram-positive bacteria which shows a negative effect on replication and transcription mechanics (Citation58). Hence, C. lasiantha extracts have preferable activity against Gram-positive bacteria (Figure ).
The observed differential antibacterial activity of C. lasiantha extracts against Gram-positive and Gram-negative bacteria can also be explained based on the existence of flavone glycoside (Luteoline-4-O-neohesperiodoside) in it (Citation18). It is a well-known fact that presence of flavonoids is one of the reasons for antibacterial activity of plant extracts (Citation2, 49) due to formation of a complex between extracellular protein on bacteria and carbonyl group on flavonoids (Citation33). For example, (+)-catechin, a monomeric flavan sub-unit is capable of linking with the lipopolysaccharide present on the bacterial cell surface (Citation59). Hence, all those polyphenols of plant origin can display various biological activities including anti bacterial activities (Citation60).
3.5. Relation between anti-inflammation activity and Caralluma lasiantha extracts
In India, C. lasiantha powder is used to reduce body heat, a characteristic of inflammation (Citation17) and methanolic extracts of C. lasiantha exhibit anti-inflammatory activity (Citation61). Moreover, anti inflammatory action is exhibited by polyphenols of plant origin (Citation60). Hence in the present study, bacteria were chosen which cause inflammation. For example, local inflammation is created by S. aureus as it replicates in metaphyseal capillary loops (Citation62). About 70 to 80% of urinary tract infections (UTI) are due to E. coli infection (Citation63) and Streptococcus Sp., (Citation64). B. subtilis causes Endocarditis (inflammation of the endocardium) (Citation65). E. aerogenes causes respiratory tract nosocomial infections (Citation66). K. pneumoniae is responsible for pneumoniae (the destructive lung inflammation disease) (Citation67). Exceptional antimicrobial activity against E. coli by lower concentrated C. lasiantha extracts helps to visualize their potential to cure inflammation of urinary bladder. This is supported by the fact that Ramesh et al., (Citation7) reported the anti-inflammatory activity by flavone glycosides which are also present in C. lasiantha (Citation15). As C. lasiantha extracts exhibit exceptional growth inhibition against these bacteria, the anti-inflammation activity of C. lasiantha can be substantiated.
4. Conclusion
From the present in vitro studies, it can be concluded that the crude plant extracts of C. lasiantha exhibit good antimicrobial activity against the selected bacteria due to the presence of steroids and flavones in plant extracts. Anti-inflammation activities are related to C. lasiantha extracts through their antibacterial activities. Hence, further in vivo studies have to be carried out using pure phytochemicals to review their usage in the treatment of infectious diseases caused by these micro-organisms and to establish the exact mechanism of action.
Funding
The authors received no direct funding for this research.
Acknowledgments
The authors acknowledge the support of Mr Kullayappa, SK University, India for identification and collection of plant sample.
Additional information
Notes on contributors
Sireesha Malladi
Sireesha Malladi has completed MSc (2005) and MPhil (2008) in Chemistry from Acharya Nagarjuna University, Guntur and Periyar University, Salem, respectively. She is an assistant professor in Chemistry since 2005 in the Department of S&H, Vignan’s University, India. Currently, she is on academic leave and pursuing PhD from JNTU, Anantapur in the research area of Natural Products and Organic Synthesis.
References
- Hartmann, T. The Lost Origin of Chemical Ecology in the Late 19th Century. Proc. Natl. Acad. Sci. 2008, 105 (12), 4541–4546. doi: 10.1073/pnas.0709231105.
- Bruneton, J. Pharmacognosy, Phytochemistry: Medicinal plants, 2nd ed.; Lavoisier: France, 1999.
- Patel, N.B.; Patel, K.C. Molecular Docking Study on Anisomeles Indica Inn. Wild Ethnomedicinal Plant for Therapeutic Purpose. Life Sci. Leaflets 2012, 11, 33–39.
- Rajendra; Ramaswamy; Kamala. USP Filed – 4. 6376657, 2004.
- Qiu, S.X.; Lin, L.Z.; Cordell, G.A.; Ramesh, M.; Kumar, B.R.; Radhakrishna, M.; Mohan, G.M.; Reddy, B.M.; Rao, Y.N.; Thomas, N.S.; Rao, A.V.N.R. Acylated C-21 Steroidal Bisdesmosidic Glycosides from Caralluma umbellata. Phytochemistry 1997, 46 (2), 333–340. doi: 10.1016/S0031-9422(97)00237-9.
- Zakaria, M.N.M.; Islam, M.W.; Radhakrishnan, R.; Chen, H.B.; Kamil, M.; AlGifri, A.N.; Al-Attas, A. Anti-Nociceptive and Anti-Inflammatory Properties of Caralluma arabica. J. Ethnopharmacol. 2001, 76 (2), 155–158. doi: 10.1016/S0378-8741(01)00208-2.
- Ramesh, M.; Rao, Y.N.; Rao, A.A.; Prabhakar, M.C.; Rao, C.S.; Muralidhar, N.; Reddy, B.M. Antinociceptive and Anti-Inflammatory Activity of a Flavonoid Isolated from Caralluma attenuata. J. Ethnopharmacol. 1998, 62 (1), 63–66. doi: 10.1016/S0378-8741(98)00048-8.
- Gandhi, R. Carallumas of the Indian subcontinent, Indian society of Cactii and Succulents; Ram Gandhi: New Delhi, 1999.
- Ahmad, V.U.; Usmanghani, K.; Rizwani, G.H. New Pregnane Glycosides from Caralluma tuberculata. J. Nat. Prod. 1988, 51 (6), 1092–1097.10.1021/np50060a007
- Abdul-Aziz Al-Yahya, M.; Abdel-Sattar, E.; Guittet, E. Pregnane Glycosides from Caralluma russeliana. J. Nat. Prod. 2000, 63 (10), 1451–1453. doi: 10.1021/np990530c.
- Abdel-Sattar, E.; Ahmed, A.A.; Hegazy, M.E.F.; Farag, M.A.; Al-Yahya, M.A.A. Acylated Pregnane Glycosides from Caralluma russeliana. Phytochemistry 2007, 68 (10), 1459–1463. doi: 10.1016/j.phytochem.2007.03.009.
- Bader, A.; Braca, A.; De Tommasi, N.; Morelli, I. Further Constituents from Caralluma negevensis. Phytochemistry 2003, 62 (8), 1277–1281. doi: 10.1016/S0031-9422(02)00678-7.
- Arinathan, V.; Mohan, V.R.; Britto, A.; Murugan, C. Wild Edibles used by Palliyars of the Western Ghats, Tamil Nadu. Indian J. Tradit. Know. 2007, 6 (1), 163–168 http://hdl.handle.net/123456789/857.
- Reddy, S.R.; Reddy, A.M.; Yasodamma, N. Exploration of Wild Ornamental Flora of YSR District, Andhra Pradesh, India. Indian J. Fundam. Appl. Life Sci. 2012, 2 (1), 192–199.
- Qiu, S.X.; Cordell, G.A.; Kumar, B.R.; Rao, Y.N.; Ramesh, M.; Kokate, C.; Rao, A.V.N.A. Bisdesmosidic Pregnane Glycosides from Caralluma lasiantha. Phytochemistry 1999, 50 (3), 485–491. doi: 10.1016/S0031-9422(98)00569-X.
- Kumar, V.H. Anti-Hyperglycemic Effect of Caralluma lasiantha Extract on Hyperglycemia Induced by Cafeteria-Diet in Experimental Model. Int. J. Pharm. Sci. Res. 2016, 7 (6), 2525–2530. doi: 10.13040/IJPSR.0975-8232.7(6).2525-30.
- Vikneshwaran, D.; Viji, M.; Rajalakshmi, K. Ethnomedicinal Plants Survey and Documentation Related to Paliyar Community. Ethnobot Leaflets 2008, 12, 1108–1115.
- Ramesh, M.; Rao, Y.N.; Kumar, M.R.; Mohan, G.K.; Kumar, B.R.; Rao, A.A.; Radha Krishn, A.M.; Reddy, B.M. Flavone glycoside from Three Caralluma Species. Biochem. Syst. Ecol. 1999, 27 (1), 85–86.10.1016/S0305-1978(98)00069-6
- Sireesha, M.; Nadh, R.V.; Suresh Babu, K.; Pullaiah, T. C21 Pregnane Steroid in Caralluma lasiantha. Orient. J. Chem. 2017a, 33 (1), 963–967.
- Sireesha, M.; Nadh, R.V.; Suresh Babu, K.; Pullaiah, T. Phytochemical Investigation of Caralluma lasiantha: Isolation of Stigmasterol, an Active Immunomodulatory Agent. Int. J. Chem. Sci. 2017b, 15 (1), 399–405.
- Vogel, A.I. Text-Book of Practical Organic Chemistry, 3rd ed.; ELBS: London, 1971.
- Adoum, O.A.; Akinniyi, J.A.; Omar, T. The Effect of Geographical Location on the Antimicrobial Activities and Trace Element Concentration in the Root of Calotropis procera (Ait.) R. Br. Ann. Borno 1997, 13 (14), 199–207.
- World Health Organization. Traditional Medicine. Fact sheet Number 134, 2008.
- Odugbemi, T. A Textbook of Medicinal Plants from Nigeria; University of Lagos Press, Nigeria, 2008; pp 20–323.
- Harborne, J.B. Phytochemical Methods: A guide to modern techniques of plant analysis; Champman and Hall: London, 1998.
- Krishnaswamy, N.R. Chemistry of Natural Products: A Laboratory Handbook, 1st ed.; Universities Press: India, 2003.
- Evans, W.C. Trease and Evans Pharmacognosy, 15th ed.; Elsevier Health Sciences: New York, NY, 2002; pp 21–24.
- Gupta, A.K. Introduction to Pharmaceutics-1; CBS publication, New Delhi, 2004; p 145.
- Nolte, F.S.; Metchock, B. Mycobacterium. In Manual of clinical microbiology, 6th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press, Washington, DC, 1995; pp 400–437.
- Olurinola, P.F. A laboratory manual of pharmaceutical microbiology: Nigeria: Idu. Abuja 1996, 69, 1–105.
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC press, 2010, ISBN: 1439804087, 9781439804087.10.1201/EBK1439804063
- Wayne, P.A. National Committee for Clinical Laboratory Standards: Performance Standards for Antimicrobial Disc Susceptibility Testing. 2002, 12.
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582.
- Kamil, M.; Jayaraj, A.F.; Ahmad, F.; Gunasekhar, C.; Samuel, S.; Chan, K.; Habibullah, M. Identification and Quantification of Flavonoids from Caralluma arabica and its Quality Control Studies. J. Pharm. Pharmacol. 1999, 51, 225–229.
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The Antioxidant Properties of Alcoholic Extracts from Sambucus nigra L. (Antioxidant Properties of Extracts). LWT-Food Sci. Technol. 2006, 39 (3), 308–315 doi: 10.1016/j.lwt.2005.01.005.
- Tanaka, O.; Tamura, Y.; Masuda, H.; Mizutani, K. Application of Saponins in Foods and Cosmetics: Saponins of Mohave yucca and Sapindus mukurossi. In Saponins used in Food and Agriculture; Waller G.R., Yamasaki K. Eds.; Advances in Experimental Medicine and Biology, Vol 405; Springer: US, 1996; pp 1–11.
- Yildirim, A.; Mavi, A.; Kara, A.A. Antioxidant and Antimicrobial Activities of Polygonum cognatum Meissn Extracts. J. Sci. Food Agric. 2003, 83 (1), 64–69. doi: 10.1002/jsfa.1288.
- Osbourn, A. Saponins in Cereals. Phytochemisty 2003, 62, 1–4.
- Naik, J.B.; Jadge, D.R. Anti-Bacterial and Anti-Fungal Activity of Actiniopteris radiata and Caralluma adscendens. Int. J. PharmTech Res. 2010, 2 (3), 1751–1753.
- Kulkarni, A.; Mute Vaishali, S.M.; Dhamane Suchita, P.; Gadekar, A.S. Evaluation of Antibacterial Activity of Caralluma adscendens Roxb Stem. Int. Res. J. Pharm. 2012, 3 (9), 269–270.
- Tava, A.; Avato, P. Chemical and Biological Activity of Triterpene Saponins from Medicago Species. Nat. Prod. Commun. 2006, 1 (12), 1159–1180.
- Fieser, L.F.; Fieser, M. Reagents for Organic Synthesis (Translated into Russian); Izd. Mir.: Moscow, 1971; Vol. 5, p 107.
- Gibbons, G.F.; Mitropoulos, K.A.; Myant, N.B. Biochemistry of Cholesterol; Elsevier: New York, NY, 1982; p 63.
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45 (4), 493–496. PMID: 5325707.
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Enzymology and Molecular Biology. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 55 (1), 69.
- Osbourn, A. Saponins and Plant Defence—A Soap Story. Trends in Plant Sci. 1996, 1 (1), 4–9.10.1016/S1360-1385(96)80016-1
- Chaurasia, S.C.; Vyas, K.K. In vitro Effect of Some Volatile Oil Against Phytophthora parasitica var. piperina. J. Res. Indian Med. Yoga Homeopathy 1977, 1977, 24–26.
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30 (12), 3875–3883.10.1016/0031-9422(91)83426-L
- Tharan, N.T.; Vadivu, R.; Palanisamy, M.; Justin, V. Antibacterial Activity of Evolvulus alsinoides. Indian Drugs 2003, 40 (10), 585–586.
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative Study on the Antibacterial Activity of Phytochemical Flavanones Against Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50 (1), 27–34. doi: 10.1016/0378-8741(96)85514-0.
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active Oxygen Generation by Flavonoids. Biol. Pharm. Bull. 1998, 21 (2), 93–96. doi: 10.1248/bpb.21.93.
- Naz, S.; Siddiqi, R.; Ahmad, S.; Rasool, S.A.; Sayeed, S.A. Antibacterial Activity Directed Isolation of Compounds from Punica granatum. J. Food Sci. 2007, 72 (9), 341–345. doi: 10.1111/j.1750-3841.2007.00533.x.
- Fan, W.; Chi, Y.; Zhang, S. The Use of a Tea Polyphenol Dips to Extend the Shelf Life of Silver Carp (Hypophthalmicthys molitrix) During Storage in Ice. Food Chem 2008, 108 (1), 148–153. doi: 10.1016/j.foodchem.2007.10.057.
- Newman, D.J. Natural Products as Leads to Potential Drugs: An Old Process or the New Hope for Drug Discovery. J. Med. Chem. 2008, 51 (9), 2589–2599. doi: 10.1021/jm0704090.
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal Catechins Damage the Lipid Bilayer. Biochim Biophys Acta (BBA)-Biomembr. 1993, 1147 (1), 132–136. doi: 10.1016/0005-2736(93)90323-R.
- Toda, M.; Okubo, S.; Hiyoshi, R.; Shimamura, T. The Bactericidal Activity of Tea and Coffee. Lett. Appl. Microbiol. 1989, 8 (4), 123–125. doi: 10.1111/j.1472-765X.1989.tb00255.x.
- Ravikumar, S.; Syed Ali, M.; Ramu, A.; Ferosekhan, M. Antibacterial Activity of Chosen Mangrove Plants Against Bacterial Specified Pathogens. World Appl. Sci. J. 2011, 14 (8), 1198–1202.
- Winter, J.E.A.N.E.T.T.E.; Moore, L.H.; Dowell, V.R.; Bokkenheuser, V.D. C-Ring Cleavage of Flavonoids by Human Intestinal Bacteria. Appl. Environ. Microbiol. 1989, 55 (5), 1203–1208.
- Delehanty, J.B.; Johnson, B.J.; Hickey, T.E.; Pons, T.; Ligler, F.S. Plant Proanthocyanidins Bind to and Neutralize Bacterial Lipopolysaccharides. NRL Rev 2008, 101–107. Url : ADA517872.
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. K.α-Glucosidase Inhibitory Profile of Catechins and Theaflavins. J. Agric. Food Chem. 2007, 55 (1), 99–105. doi: 10.1021/jf0627672.
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J. Pharm. Pharmacol. 2014, 66 (10), 1351–1368. doi: 10.1111/jphp.12265.
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. The Lancet 2004, 364 (9431), 369–379. doi: 10.1016/S0140-6736(04)16727-5.
- El-Mosalamy, H.; Salman, T.M.; Ashmawey, A.M.; Osama, N. Role of Chronic E. coli Infection in the Process of Bladder Cancer-an Experimental Study. Infect. Agents Cancer 2012, 7 (1), 19. doi: 10.1186/1750-9378-7-19.
- Cheesbrough, M. District Laboratory Practice in Tropical Countries, 2nd ed.; Cambridge university press. ISBN-13 978-0-511-34842-6, 2006.10.1017/CBO9780511543470
- Reller, L.B. Endocarditis Caused by Bacillus subtilis. Am. J. Clin. Pathol. 1973, 60 (5), 714–718.10.1093/ajcp/60.5.714
- Jarvis, W.R.; Martone, W.J. Predominant Pathogens in Hospital Infections. J. Antimicrob. Chemother. 1992, 29 (Suppl A), 19–24. doi: 10.1093/jac/29.suppl_A.19.
- de Souza Lopes, A.C.; Rodrigues, J.F.; de Morais Júnior, M.A. Molecular Typing of Klebsiella pneumoniae Isolates from Public Hospitals in Recife, Brazil. Microbiol. Res. 2005, 160 (1), 37–46. doi: 10.1016/j.micres.2004.09.007.