?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Thermodynamics of sodium butoxide-catalyzed polymerization of α-angelicalactone (a five-member unsaturated lactone) is studied by calorimetric methods. A polymer with Mw = 2,500 g/mol and the ratio of C–O to C–C inter-monomeric bonds 87–13% is obtained; this polymerization is characterized by the values of = −33.41 kJ/mol,
= −42.69 J/(mol∙K) and
= −20.68 kJ/mol. This and similar polymers and copolymers are biodegradable and can be produced from commercially available green bio-platform feedstock: renewable carbohydrates and levulinic acid.
Public Interest Statement
This work relates to polymers obtained from renewable plant matter. Specifically, the polymer of α-angelicalactone (a lactone obtained by acid-catalyzed carbohydrate conversion) that can become a biodegradable alternative to commonly used polyester materials; the presence of double bonds in the polymer allows for additional functionalization. In the present study, the thermodynamic parameters of α-angelicalactone polymerization are experimentally measured; these parameters are necessary to know under which conditions this polymerization may occur. It is shown that in this regard, α-angelicalactone behaves differently from other five-member lactones like γ-butyro- or γ-valerolactone: polymerization of the latter is thermodynamically restricted and only becomes feasible under stern conditions like exceedingly high pressure. On the other hand, α-angelicalactone polymerizes under mild conditions (80°C), and the obtained data indicate that in theory (with the right catalyst) it could polymerize under normal conditions.
Competing interests
The authors declare no competing interest.
1. Introduction
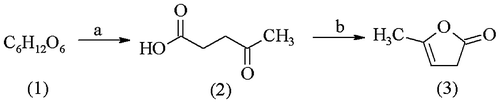
α-Angelicalactone (5-methyl-2(3H)-furanone, αAL) (3) is a product of levulinic acid dehydration (2). The latter can be obtained from hexose carbohydrates (1)—a renewable plant resource—as shown in Scheme .
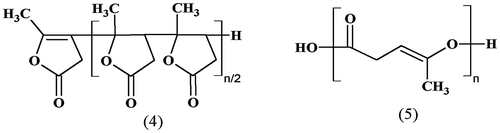
There are two polymerizable groups in a molecule of αAL: the lactone ring and the C–C double bond. Therefore, there are two possible polymerization pathways: the double bond opening resulting in the polyfuranone chain (4) and the formation of the unsaturated polyester—polyangelicalactone (PAL) (5) via ring opening (Figure ).
The double bond polymerization of αAL is caused by strong Lewis acids (Citation1,2), ionizing radiation, intensive mechanoactivation (Citation2) or basic catalysts (Citation3). A polyfuranone (Mn = 810, Mw = 862) (4) obtained from αAL in the presence of BF3 was found to be non-degradable by Saccharomyces cerevisae, Streptomyces chrysomallus and Streptomyces lividans (Citation4). Also, opening of the double bond occurs during copolymerization of αAL with methyladamantylmethacrylate, maleic anhydride, norbornene or polyhydroxystyrene (Citation5,6). In such copolymerization processes, the fraction of αAL included in the products did not exceed 30%.
The ring-opening polymerization (ROP) of αAL is more interesting. The product (5) of this reaction is supposedly biodegradable (Citation7–10). However, five-member lactones (at least, the saturated ones) are not predisposed to polymerization. For example, γ-butyrolactone does not polymerize, and the Gibbs free energy of this process is positive (Citation8). Similarly, there appears to be a lack of experimental reports about γ-valerolactone (γVL) polymerization, and this lactone can only undergo co-polymerization with other lactones (Citation11)—this suggests that the polymerization Gibbs free energy of pure γVL is also positive.
Nonetheless, it was recently found that αAL polymerizes under the action of sodium hydroxide, sodium butoxide (Citation4,12,13) and stannous octoate (Citation14), resulting in products with molecular weight ranging from 1,000 to 30,000 g/mol, and polydispersity index (PDI) = 1,6–2,2.
PALs obtained from αAL in the presence of 5 wt.% of sodium butoxide (ButONa) at room temperature are slightly yellow resins or solids with M = 1,120–1,800 g/mol and PDI = 1,8. 1H NMR data showed that 68–80% of the inter-monomeric bonds in the resulting polymers are esteric, and 20–32% of the bonds are formed due to the double bond opening (Citation4). Unlike polyfuranones (4), these PALs are fully degraded by S. cerevisae (over 1–1.5 weeks), S. chrysomallus (2–3 weeks) and S. lividans (3–4.5 weeks) (Citation4,12).
Solubilized in aprotic solvents, αAL can be easily polymerized when treated by sodium and aluminium alkoxides at 333–338 K for 40–60 min, which produces polyesters with M = 15,000–19,000 g/mol, and PDI = 1,6–2,2 (Citation12,15).
Copolymerization of the unsaturated PAL and styrene (Sty) results in further molecular weight increase, and improvement of the viscoelastic properties (Citation12). Fusion of the PAL and styrene (1–5 mol%) with boron trifluoride as the catalyst forms cross-linked polymers with M = 200,000–500,000 g/mol. These polymers are mostly or fully degradable after 20 weeks in grey forest soil. The obtained copolymers have the following characteristics: density 1.15–1.16 × 103 kg/m3, yield strength 40–60 MPa, ultimate strength 70–80 MPa and ultimate strain of 10–20% (Citation15).
There appears to be a lack of published data on the thermodynamic properties of PAL, and on the thermodynamics of its formation process. The hereinabove described facts about successful syntheses of angelicalactone polymers suggest that the thermodynamics of such polymerization does not follow the pattern of the corresponding parameters for other five-member lactones. Thus, there is incentive towards experimental measurement of the αAL polymerization thermodynamic parameters. In this work, we present the experimental study of the thermodynamic properties of PAL and αAL as dependences vs. temperature and phase composition of the reactants. The heat capacity of partially crystalline (67%) PAL was measured by DSC in the temperature interval 7–430 K. Finally, the standard thermodynamic functions for PAL and αAL formation were obtained from combustion calorimetric data.
2. Experimental section
2.1. Materials
α-Angelicalactone (Alfa Aesar, 98%) was purified by melt fractional crystallization. The total impurities content was 0.10 ± 0.02 mol%, according to 1H-NMR data. Spontaneous polymerization of the αAL during 9 months storage, and during experiments did not occur.
Sodium butoxide was synthesized according to the standard procedure (Citation16).
2.2. Polymerization of α-angelicalactone by sodium butoxide
10 g (~0.102 mol) of α-angelicalactone and 0.30 g (~3.06 × 10−3 mol) of sodium butoxide were sealed in a nitrogen-filled glass ampule, placed in a thermostat, and left there for 14 h at 350 ± 1 K. After that, the viscous yellow product was washed by decantation with several portions of cold diethyl ether, and then cleared of volatile matter at 80°C at 2 Torr for 1.5 h. There remained 8.2 g of a slightly yellow tacky solid with Mw 2,500 g/mol, PDI 2.3, the fraction of the ester inter-monomeric bonds was 0.87.
2.3. Equipment and analysis techniques
The structure of the polymer was elucidated by 1H-NMR spectroscopy (spectrometer Bruker DPX-200 W, Krasnoyarsk Regional Research Equipment Center of SB RAS) in CDCl3 solution (12 mg/ml)).
The relative molecular weight of the polymer was determined by phase-reversal chromatography relative to polystyrene standards. The column was Nova-Pak CIS (Waters Corporation, USA) packed with octadecyl-functionalized silica; evaporative light scattering detector device was used (model 500, Alltech Corporation, USA); the eluent was an acetonitrile–tetrahydrofuran mixture (MeCN/THF = 58/42), flow rate 1 ml/min.
The internal energy of combustion ΔUc was determined using a static isothermal combustion calorimeter V-08MA, according to the standard technique (Citation17). The initial temperature was 25.0 ± 0.2°C and the initial pressure was 2,940 kPa. The samples to be burnt were placed into weighted polyethylene ampules and weighed to the accuracy of 0.01 mg. The ampoule was placed into the bomb crucible in the proximity of the fuse used for ignition; if necessary, an auxiliary cotton fuse was used. The bomb was flushed with dried oxygen for three minutes, then sealed and filled with oxygen to the initial pressure mentioned above. The calorimeter constant was determined by calibration against benzoic acid combustion (the certified combustion energy 26,434.6 J/g). The calorimeter constant was calculated from at least ten combustion runs, resulting in the value of W = 15,126.6 ± 3.8 J/K.
After combustion, the gas mixture was evacuated from the bomb. The combustion gas products were analysed with the Gazochrom-3101 gas chromatograph. For CO2, the data error was 0.4%, and for CO—0.06%. The walls and fittings of the bomb were washed with 200 mL of double-distilled water. Three 50 mL samples of the solution were titrated with 0.1 N NaOH solution. The mean value from the three titrations was used for the calculation of the nitric acid amount resulting from the combustion.
The experimental values of combustion energy were corrected to the standard state (T = 298.15 K and P = 101.3 kPa) according to the Washburn equation (Citation18), recommended for the case of compounds comprising carbon, hydrogen and oxygen. The relative error in the determination of the combustion heat values was 0.022%.
The standard combustion enthalpies of the samples were calculated from the corresponding values of internal energy of combustion ΔUc.
DSC analyses were performed on a scanning calorimeter of the triple heat bridge-type assembled in-house according to the published specification (Citation19–23). Temperature and heat flow were calibrated using the standard materials (Citation23). The temperature data error was 0.05 K. The heat capacity determination error within different temperature intervals is: 7–160 K – ±0.4–0.6%, 160–200 K – ± 0.1–0.2%, 200–430 К – ± 1.5–2.5%. The error of phase transition enthalpy data was ±0.4%. The heat capacity of the PAL was measured within the range 7–400 K at the heating rate 0.02 K/s. The sample mass was 5.3759 g. The heat capacity of the PAL sample was 58% of the total heat capacity of the calorimeter in the entire temperature range. The heat capacity data error was 0.9 J/(mole K).
The glass-transition range and the heat capacity increase therein were determined from heat capacity vs. temperature curves. The glass-transition temperature was determined according to the Alford-Dole’s procedure (Citation24).
The melting point was determined by graphical evaluation from the Tf (v) vs. 1/v2 dependences, where Tf—temperature corresponding to the maximum magnitude of apparent heat capacity in the melting range, v—heating rate.
The enthalpy of melting was calculated by integration of
—the difference between the heat capacity in the melting interval and
= f(T) extrapolated from the beginning to the end of melting (Citation19). The entropy of melting
was determined from the dependency
.
The configurational entropy Sconf of the polymer was estimated according to the method described by Adam and Gibbs (Citation25). The zero entropy Sg(0) was found according to Lebedev and Rabinovich (Citation26).
Calculations of (H0(T)–H0(0)) and S0(T) were done by integration of the functional dependence Cp = f(T) and Cp = f(ln T), respectively, according to McCullough and Scott (Citation27).
The standard formation enthalpy of PAL at T = 298.15 К was calculated from its combustion enthalpy, (CO2(g)) and
(H2O(l)) (Citation28). The standard entropy of formation
(PAL) was calculated from the absolute entropy of PAL and reference data on carbon (graphite), hydrogen (gas) and oxygen (gas). Standard Gibbs free energy of formation was calculated from
and
. The enthalpy of polymerization ΔHpol of αAL in bulk at T = 298.15 К was calculated from
(αAL) and
(PAL). The Kirchhoff equation was used to calculate ΔHpol at different temperatures. The polymerization entropy
was found from the absolute entropies of αAL and PAL at the corresponding temperatures.
was calculated from the Gibbs–Helmholtz equation.
The limiting temperature of polymerization was estimated by the Dainton method (Citation29).
Ring strain energies for γ-butyrolactone, γ-valerolactone (γVL) and αAL were computed by molecular modelling using the program suite GAMESS (Citation30), (version 11 November 2017, R3 for GNU/Linux x86-compatible 32-bit). Evaluations of the strain energies were performed according to Dudev and Lim (Citation31). Geometry optimizations and frequency calculations were performed using the B3LYP density functional method (Citation32,33) in conjunction with the 6-31G(d) basis set (Citation34).
3. Results and discussion
3.1. Heat capacity
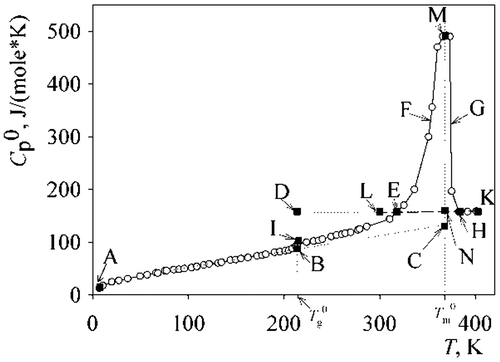
Heat capacity of the PAL smoothly increases with temperature (Figure ), and equals 135.83 J/(mol∙K) at 298 K. There are two sharp increases: (a) 210–225 K—devitrification (section BI on Figure ) and (b) 310–380 K—melting (section EM). The ~ 24 K wide section (EL) corresponds to a metastable state—the supercooled liquid—which occurred when cooling the liquid PAL. Upon further cooling, the sample spontaneous rapidly crystallized with a heating of the calorimeter.
3.2. Phase transition
Parameters of the glassy state and devitrification of the PAL together with the Lebedev’s (Citation8) data on poly-γ-butyrolactone and poly-δ-valerolactone are shown in Table . From the calorimetric data, the PAL sample crystallinity αc is 67%.
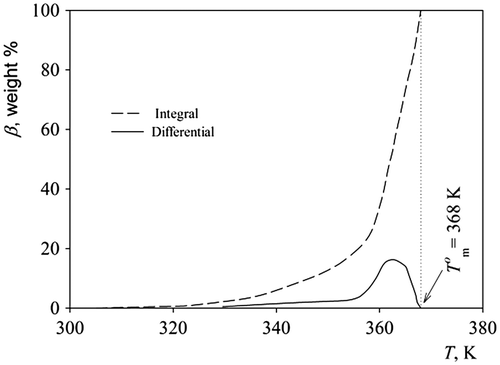
The PAL melting spans a 30 K wide temperature range, and the melting parameters are presented in Table together with the reference data (Citation8). Results of the calorimetric study of the PAL melting process are shown in Figure . The PAL is melting over a wide temperature range, and the largest portion of the crystals melt at T = 362 К, which is 6 K lower than . The values obtained for the phase transitions of PAL are lower comparing to literature data on the other polylactones (Citation8).
3.3. Combustion heats
The combustion experimental data are given with respect to the following reaction, the same for αAL and PAL:
C5H6O2 + 6.5 O2 = 5 CO2 + 3 H2O (Δn = −0.5)
These data were recalculated into the thermodynamic functions of αAL and the PAL. Table shows the combustion and standard formation enthalpies of liquid αAL, and the PAL in high-elastic state (67% crystallinity) at T = 298.15 K.
Table 3. Standard enthalpies of combustion (
 ) and formation (
) and formation (
 ) of αAL and the PAL (T = 298.15 K, po = 0.1 MPa)
) of αAL and the PAL (T = 298.15 K, po = 0.1 MPa)
3.4. Thermodynamic characteristics of the α-angelicalactone polymerization
Table shows the thermodynamic characteristics of the polymerization reaction of α-angelicalactone.
Table 4 Thermodynamic parameters of lactones polymerization (p = 101.325 kPa)
The results show that the monomer-polymer equilibrium is shifted towards the formation of PAL throughout the studied temperature range. The values increase with rising temperature. The enthalpy and entropy of polymerization are negative. Hence, there exists a ceiling temperature of polymerization. Calculated by the Dainton`s method, the ceiling temperature is T = 425.5 K.
The polymerization enthalpy of αAL into PAL = −33.41 ± 1.2 kJ/mol at 298 K. This value is in between the corresponding data for β-propiolactone (βPL) and δ-valerolactone (δVL) polymerization (
= −75 and −10.5 kJ/mol at 298 K, correspondingly), and is considerably lower than the datum for γ-butyrolactone (γBL) polymerization (
= + 5 kJ/mol at 298 K). Similar relation is observed for the Gibbs free energies of the lactones polymerization (see (Citation8) and Table ). The enthalpy obtained is lower than the earlier estimated enthalpy for the αAL oligomerization to the trimer (ΔHp = −26 kJ/mol (Citation14)).
The difference between the Gibbs energies of polymerization reactions of two five-member lactones—γBL and αAL—can be explained by the difference of their respective ring strain values. The strain energy of α-angelicalactone is greater than that of γ-butirolactone (Table ). Another saturated five-member lactone, γ-valerolactone, exhibits the strain energy similar to that of γBL; and as was described in Introduction, γVL is not prone to polymerization just like γBL. Therefore, we can conclude that the presence of the double bond in the αAL ring causes the strain in the five-member lactone structure that makes this compound sufficiently endergonic to thermodynamically enable the ring-opening polymerization.
Table 5. Strain energies (in kJ/mol) for δVL, γVL, αAL and γBL
4. Conclusions
Most five-member lactones are inactive in ring-opening polymerization, but α-angelicalactone can undergo such reaction. Explanation of this unusual behaviour is required, therefore we studied thermodynamic properties of α-angelicalactone and a polyangelicalactone. The polyangelicalactone (Mw = 2,500 g/mol) with the fractions of the C–O and C–C inter-monomeric bonds being 87 and 13%, respectively was studied by calorimetric methods. Thermodynamic functions of the PAL formation were determined. Heat capacity and thermodynamic parameters of phase transitions of the polymer were calculated.
Finally, the standard thermodynamic functions of the polymerization process were determined: = −33.41 kJ/mole,
= −42.69 J/(mole∙K) and
= −20.68 kJ/mole. Based on these, the ceiling temperature of αAL polymerization Tc = 425 K is estimated. The obtained standard thermodynamic functions of the α-angelicalactone polymerization are in a good agreement with the data on other lactones. The obtained results indicate that the double bond in the structure of αAL strains the ring and makes it active in the ring-opening polymerization.
Funding
The authors received no direct funding for this research.
Erratum
This article was originally published with errors. This version has been corrected. Please see Erratum (https://doi.org/10.1080/23312009.2018.1457128).
Additional information
Notes on contributors
Konstantin Leonidovich Kaygorodov
Konstantin Leonidovich Kaygorodov is a researcher at the Institute of Chemistry and Chemical Technology of Siberian Branch of Russian Academy of Sciences, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Krasnoyarsk, Russia (ICCT SB RAS). The author specializes in synthesis, structure, properties and reactivity of organic compounds, polymers and natural substances; analytical chemistry and molecular spectroscopy; catalysis; chemical thermodynamics; and kinetics.
Valery Evgenievich Tarabanko
Valery Evgenievich Tarabanko is head of Laboratory for Biomass Conversion at the ICCT SB RAS. His research areas are: wood chemistry, biodegradable polymers, production of fine chemicals from wood, reaction mechanisms of lignins and carbohydrates conversion.
Nikolay Tarabanko
Nikolay Tarabanko, PhD, is a researcher at the ICCT SB RAS. The author specializes in inorganic heterogeneous catalysis (by inclination), and in chemistry of renewable resources (by call of duty). This combination makes him a bit of an odd-man-out in the team, usually serving as the hitman who is called upon to deal with various research complications that require the attention of a flexible and meticulous mind.
References
- Marvel, C.S.; Levesque, C.L. The Structure of Vinyl Polymers. III. 1. The Polymer from α-Angelica Lactone. J. Am. Chem. Soc. 1939, 1, 1682–1684.10.1021/ja01876a013
- Lukeš, R.; Syhora, K. O dimerisaci laktonu α-angelokoveho [Dimerization of α-Angelicalactone]. Chemické listy 1954, 4, 560–568.
- Wolff, H.; Moyer, W.W. Canadian Patent No 516,357; Canadian Intellectual Property Office, Gatineau, 1952.
- Tarabanko, V.E.; Kaygorodov, K.L.; Sokolenko, V.A.; Chernyak, M. Issledovaniye polimerizatsii a-angelikalaktona [The Study of α-Angelicalactone Polymerization]. Khimiia rastitel’nogo syr’ia 2006, 2, 37–41.
- Yoon, K.S.; Jung, D.W.; Lee, S.H.; Kim, H.W.; Lee, S.; Woo, S.G.; Choi, S.J.. US Patent No 2,002,042,016; U.S. Patent and Trademark Office: Washington, DC, 2002.
- Brainard, R.L.. US Patent No 6,492,087; Washington, DC: U.S. Patent and Trademark Office, 2002.
- Stein, R.S. Polymer Recycling: Opportunities and Limitations. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 835–838.10.1073/pnas.89.3.835
- Lebedev, B.V. Thermodynamics of Polylactones. Russ. Chem. Rev. 1996, 65, 1063–1082.10.1070/RC1996v065n12ABEH000265
- Fomin, V.A.; Guzeev, V.V. Biorazlagayemyye polimery, sostoyaniye i perspektivy ispol’zovaniya [Biodegradable Polymers, Condition and Prospects of Use]. Plastichiskie massy 2001, 2, 42–48.
- Loshadkin, D.V. Biodegradiruyemyye plastiki: tipy materialov, osnovnyye svoystva i perspektivy ispol’zovaniya v promyshlennosti [Biodegradable Plastics: Types of Materials, Basic Properties and Prospects for Use in Industry]. Plastichiskie massy 2002, 7, 41–44.
- Lin, W.-J. Comparison of Thermal Characteristics and Degradation Properties of ε-Caprolactone Copolymers. J. Biomed. Mater. Res. 1999, 47, 420–423.10.1002/(ISSN)1097-4636
- Tarabanko, V.E.; Kaygorodov, K.L.; Chernyak, M. Polyesterification of alpha-Angelicalactone. J. Siberian Federal Univ. Chem. 2008, 1, 118–123.
- Tarabanko, V.E.; Kaygorodov, K.L. New Biodegradable Polymers Based on α-Angelicalactone. Chem Sustainable Develop. 2010, 3, 395–403.
- Chen, T.; Qin, Z.; Qi, Y.; Deng, T.; Ge, X.; Wang, J.; Hou, X. Degradable Polymers from Ring-Opening Polymerization of α-Angelica Lactone, a Five-Membered Unsaturated Lactone. Polym. Chem. 2011, 2, 1190–1194.10.1039/c1py00067e
- Tarabanko, V.E.; Kaygorodov, K.L. New Environmentally Benign Polymers Produced by Copolymerization with α-Angelicalactone. Macromolecular Symposia 2015, 354, 367–373.10.1002/masy.201400108
- Dyhanov, N.N.; Skripkina, V.T. Butilat natriya [Sodium Butoxide]. Metody polucheniya khimicheskikh reaktivov i preparatov 1964, 9, 28–30.
- Federal Agency on Technical Regulating and Metrology, Solid Mineral Fuel. Determination of Gross Calorific Value and Calculation of Net Calorific Value. Russian State Standart, GOST 147-2013, 2015.
- Cox, J.D.; Pilcher, V. Thermochemistry of Organic and Organometallic Compounds; Academic Press, London, 1970.
- Yagfarov, M. K voprosu opredeleniya kristallichnosti polimerov na osnove izmereniya teplovykh velichin [About determining the Crystallinity of Polymers Based on the Measurement of Thermal Quantities]. Vysokomolekulyarnye Soedineniya: Seriya A. 1979, 10, 2379–2384.
- Gusev, E.A.; Dalidovich, S.V.; Vecher, A.A. Equipment Design for Investigation of Substances Decomposed Under the Pressure of Self-Generated Atmosphere by Means of Thermal Analysis. Thermochimica Acta 1985, 92, 379–382.10.1016/0040-6031(85)85895-0
- Bernstein, V.A.; Egorov, V.M. Differencialnaya scaniruyuschaya calorimetriya v fizikokhimii polimerov [Differential Scanning Calorimetry in the Physicochemistry of Polymers]; Khimiya, USSR, Leningrad, 1990.
- Kabo, A.G.; Diky, V.V. Details of Calibration of a Scanning Calorimeter of the Triple Heat Bridge Type. Thermochim. Acta 2000, 347, 79–84.10.1016/S0040-6031(99)00419-0
- Federal Agency on Technical Regulating and Metrology, Plastics. Differential Scanning Calorimetry (DSC). Russian State Standart, GOST R 55134-2012, 2014.
- Alford, S.; Dole, M. Specific Heat of Synthetic High Polymers. VI. A Study of the Glass Transition in Polyvinyl Chloride. J Am. Chem. Soc. 1955, 77, 4774–4777.10.1021/ja01623a024
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139–146.10.1063/1.1696442
- Lebedev, B.V.; Rabinovich, I.B. Opredeleniye nulevoy entropii ryada stekloobraznykh polimerov po kalorimetricheskim dannym [The Determination of Zero Entropy for the Range of Glassy Polymers on Calorimetric Data]. Doklady Akademii Nauk SSSR 1977, 3, 641–644.
- McCullough, J.P.; Scott, D.W. Calorimetry of Non-reacting Systems; Butterworth, London, 1968.
- Lebedev, B.V. Termodinamika polimerov [Thermodynamics of Polymers]. USSR, Gorkij: Izdatelstvo gorkovskogo gosudarstvennogo universiteta, 1989.
- Dainton, F.S.; Ivin, K.J. Some Thermodynamic and Kinetic Aspects of Addition Polymerisation. Q. Rev. Chem. Soc. 1958, 12, 61–92.10.1039/qr9581200061
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Windus, T.L.; Su, S.; Dupuis, M.; Montgomery, J.A. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363.10.1002/(ISSN)1096-987X
- Dudev, T.; Lim, C. Ring Strain Energies from ab Initio Calculations. J. Am. Chem. Soc. 1998, 120 (18), 4450–4458.10.1021/ja973895x
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98 (7), 5648–5652.10.1063/1.464913
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron density. Phys. Rev. B 1988, 37 (2), 785–789.10.1103/PhysRevB.37.785
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta. 1973, 28 (3), 213–222.10.1007/BF00533485
- Brown, J.M.; Conn, A.D.; Pilcher, G.; Leitão, M.L.P.; Meng-Yan, Y. On the Strain Energy of 5-Ring and 6-Ring Lactones. J. Chem. Soc., Chem. Commun. 1989, 23, 1817–1819.10.1039/C39890001817