Abstract
K2CO3/Al2O3 (KCA) is an efficient heterogeneous catalyst for the Michael addition reaction of 1,3-dicarbonyl compounds to nitro olefin under thermal solvent-free conditions. The results showed that the catalyst has high activity and the desired products were obtained in high yields. Furthermore, the products could be separated simply from the catalyst, and the catalyst could be recycled and reused with only slight reduction in its catalytic activity.
Public Interest Statement
Michael reaction of nitro-olefins represents a convenient access to nitroalkanes that are versatile intermediates in organic synthesis. The nitro functionality can be easily transformed into amine, nitrile oxide, ketone or carboxylic acid, hydrogen, etc., providing a wide range of synthetically interesting compounds. Therefore, in this paper, a simple and efficient method for the synthesis of these compounds by the conjugate addition reaction of 1,3-dicarbonyl compounds to nitro-olefins using K2CO3/Al2O3 as catalyst has been reported. The method was fast and the desired products were obtained within a few minutes in high yields under solvent-free conditions. Other advantages of this protocol include inexpensive and easily obtained catalyst, simple work-up, and the recyclability and reusability of the catalyst.
Competing interests
The author declare no competing interest.
1. Introduction
Among the C–C bond-forming reactions, the Michael addition of various nucleophiles to highly electron deficient nitro olefins has received great attention (Citation1). It provides a convenient access to valuable synthones and building blocks, which may lead to a variety of important biologically active compounds and pharmaceuticals, due to the ease of transformation of the nitro group (Citation2, 3).
The conjugate addition of enolates to activated olefins remains an active field of research. Indeed, the conjugate addition of carbon-based nucleophiles to electron deficient nitro olefins is one of the classical methods for C–C bond construction. Direct Michael addition and Michael-type conjugate reactions are among the most simple, efficient and atom-economical ways to achieve this transformation. These reactions are typically performed with stoichiometric amounts of inorganic bases such as alkali metal alkoxides (Citation4). Several undesirable side reactions can be caused by these strongly basic catalysts, including rearrangements, secondary condensations, isomerizations, polymerizations, bis additions, retrogressions, and trans-esterifications. In the Literature survey, some of improved procedures have been reported using LiClO4/Et3N (Citation5), bis(2,4-pentanedionato)nickel (II) (Citation4b), NaOH/BMImPF6 system (Citation6), transition metal complexes of BINOL-derived salens (Citation7), a quaternary ammonium salt (Citation8), L-proline (Citation9), cinchona alkaloids (Citation10), imidazolidine (Citation11), DBU (Citation12), TBD (Citation13a) and bifunctional thiourea (Citation14). On the other side, excellent enantioselective Michael reactions have been developed using catalysts, like bis(oxazoline)-Mg(OTf)2 (Citation1c), demethylquinine salts in water (Citation15), and Nickel(II)-diamine complexes (Citation16). However, some of these methods suffer from the drawback of not having use of green chemistry and have been associated with several short comings such as long reaction times, expensive reagents, low product yields and difficulty in recovery and reusability of the catalysts. Due to these problems, development of an efficient and versatile method using heterogeneous and reusable catalysts is still in demand.
In recent years, heterogeneous catalysts have gained great importance in different areas of organic synthesis due to their economic and environmental compatibility combined with the good yield and selectivity that can be achieved (Citation17). In addition, the activity and selectivity of a reagent dispersed on the surface of a support are improved as the effective surface area of the reagent is increased significantly, and hence they are expected to perform more effectively than the individual reagents (Citation18). Among the various supported catalysts, particularly, alumina and silica supported reagents have advantages of low cost, ease of preparation, and catalyst recycling (Citation19).
On the other hand, the current considerable interest has been devoted to finding new methodologies for the synthesis of organic compounds in solvent-free conditions (Citation19c, Citation20). The toxicity and volatile nature of many organic solvents have posed a serious threat to the environment. Thus, design of solvent-free catalytic reaction has received tremendous attention in recent times in the area of green synthesis (Citation21).
Considering the above facts and due to our interest in the solid catalysts and in solid-supported synthesis for organic reactions (Citation22), we report herein the investigation of K2CO3/Al2O3 (KCA) as an efficient, low cost and heterogeneous reusable catalyst for the reaction of electron-deficient nitro-olefins with 1,3-dicarbonyl compounds under solvent-free conditions.
2. Result and discussion
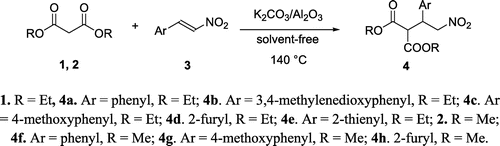
In the present work and as a reliable starting point, diethyl malonate 1 (1.1 mmol) was used as the Michael donor and nitro olefin 3a (1 mmol) as Michael acceptors. After that, the performance of K2CO3/Al2O3 (KCA) (Citation23) as a solid base supported catalyst along with its ability as a reusable catalyst was evaluated for this conjugate addition reaction, which chosen as model to afford the product 4a (Ar = phenyl, R = Et, Scheme ) under solvent-free conditions. A summary of obtained results is provided in Table .
Table 1. Synthesis of compound 4a using KCA as catalyst
Initially, to establish the optimal conditions, a set of experiments varying the amount of the catalyst and temperature were carried out. As can be seen from Table , the efficiency of the reaction is affected mainly by the amount of the catalyst (KCA). Low yield of the product 4a was produced in the absence of the catalyst at 140°C (entry 1) indicating that the catalyst KCA is necessary for a high yield. Increasing the amount of the catalyst increased the yield of the product 4a. The best result was obtained when the reaction was run at 140°C in the presence of 0.15 g as optimal amount of KCA under solvent-free conditions (entry 10, 98%). An increase in the reaction temperature and amount of the catalyst did not improve the yields significantly. Also the model reaction was tested in the presence of only K2CO3 at 140°C under solvent-free conditions; however, this reagent was not efficient separately (entry 12). Indicating that, it is necessary to support K2CO3 on the alumina (Al2O3).
Subsequently, we examined the recycling performance of KCA in the model reaction. Entry 10 described the yields of five consecutive additions leading to 4a. In these experiments, the product was isolated by filtration and washing the solid residues with ethanol. Thus, the remaining catalyst, which always works the same, begins reloading with fresh reagents for further runs. We found that the catalyst could be used at least five times with only a slight reduction in activity, demonstrating the efficiency of K2CO3/Al2O3 (KCA) as a catalyst in Michael addition reactions. Therefore, the solid KCA is a truly heterogeneous efficient catalyst for this conjugate addition.
The previously mentioned results indicated that K2CO3/Al2O3 reaction at 140°C under solvent-free condition furnished maximum yield of the conjugate addition reactions in the short time. Furthermore, KCA is reusable and environmentally benign, presenting fewer disposal problems, which can be a good alternative.
After achieving the optimized reaction conditions, we examined the generality of the catalytic efficiency of KCA using other nitro olefins having different substituents. In all cases the reactions were complete in few minutes affording products in high yields. The results are summarized in Table . Instead of diethyl malonate, dimethyl malonate has been used with similar success to provide the corresponding products (4f–h, Table ). The structures of all products were confirmed by 1H NMR and 13C NMR spectroscopy and compared with those reported in the literature (Citation13, 14).
Table 2. Scope of the malonate Michael addition with nitroalkenes 3a–e
3. Conclusion
In summary, we have established successful conjugate addition reaction of 1,3-dicarbonyl compounds to nitro olefin in the presence of a catalytic amount of K2CO3/Al2O3 as an efficient, reusable, and green heterogeneous catalyst under solvent-free conditions. Moreover, the method offers several advantages such as ease of workup, high yields, stability and recyclability and reusability of the catalyst. The catalyst can be used at least five times without substantial reduction in its catalytic activity. Hence, we believe that this method will find wide application in organic synthesis as well as industry.
4. Experimental
To a mixture of dialkyl malonate (1.1 mmol) and nitro olefin (1 mmol) in a round-bottom flask connected to a reflux condenser was added K2CO3/Al2O3 (0.15 g), and the resulting mixture was stirred in an oil-bath at 140 °C for an appropriate duration of time (8–13 min). The reaction was monitored by thin-layer chromatography (TLC). Upon completion, the reaction mixture was cooled to room temperature, hot ethanol (20 ml) was added and filtered to remove the catalyst. Again the catalyst was washed with 10 ml of absolute ethanol (hot). The remaining catalyst was reloaded with fresh reagents for further runs. The solvent of combined filtrate was evaporated by rotary evaporator, and the crude product was recrystallized from ethyl acetate/n-hexane (1:3) or ethanol.
4.1. Selected data
Compound 4a was obtained in 98% yield. MP 43–45 °C; νmax 1735 (b, 2 × CO), 1556, 1375 (NO2); 1H NMR (300 MHz, CDCl3): δ 1.03 (t, J = 7.0 Hz, 3H, OCH2CH3), 1.25 (t, J = 7.3 Hz, 3H, OCH2CH3), 3.82 (d, J = 9.4 Hz, 1H, (CO)2–CH), 3.99 (q, J = 7.3 Hz, 2H, OCH2CH3), 4.18–4.29 (m, 3H, [OCH2CH3, Ar–CH]) 4.81–4.98 (m, 2H, CH2–NO2), 7.21–7.35 (m, 5H, ArH); 13C NMR (75 MHz, CDCl3): δ 13.6, 13.8, 42.8, 54.9, 61.8, 62.0, 77.5, 127.9, 128.2, 128.8, 136.1, 166.7, 167.3; MS: m/z 263 [M − (NO2)]+, 189 (100%).
Compound 4f was obtained in 90% yield. Mp 42–44°C; νmax 1735, 1728 (2 × CO), 1556, 1373 (NO2); 1H NMR (300 MHz, CDCl3): δ 3.55 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.86 (d, J = 9.0 Hz, 1H, (CO)2–CH), 4.22–4.27 (m, 1H, Ar–CH), 4.81–4.96 (m, 2H, CH2–NO2), 7.21–7.35 (m, 5H, ArH); 13C NMR (75 MHz, CDCl3): δ 42.8, 52.7, 52.9, 54.6, 77.4, 127.8, 128.3, 128.9, 136.0, 167.2, 167.8; MS: m/z 235 [M – (NO2)]+, 175 (100%).
Funding
The author received no direct funding for this research.
Supporting information
Full experimental details and 1H and 13C spectra are available. This material can be found via the “Supplemental Content” section of this article’s Web page.
Supplementary material
Supplementary material for this article can be accessed here https://doi.org/10.1080/23312009.2018.1455346.
OACH_A_1455346_Supplementary_Material.doc
Download MS Word (4.3 MB)Acknowledgments
We are Grateful to Prof. R.S. Kusurkar for helpful guidance, B.S. Kalshetti for IR spectra, J.P. Choudhary for NMR spectra and D.S. Shishupal for GCMS. AMAS is thankful to MOHE and UOH for the support.
Additional information
Notes on contributors
Abdullah M.A. Shumaila
Abdullah M.A. Shumaila, born in 1977, January, Yemen; I studied chemistry at Sana’a University, Yemen, where I received BSc in 2001; my MSc degree in Organic Chemistry received in 2005 from Pune University, Pune, India; where I completed my PhD in Organic Chemistry in 2011 under the supervision of Prof. Radhika S. Kusurkar at the same University. Currently, I am working as a Assistant Professor at the Department of Industrial chemistry, Faculty of Applied Science, Hajjah University, Hajjah, Yemen. I have published five peer-reviewed articles. My current research interest is on heterocyclic chemistry, catalysis and new synthetic methodologies.
References
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric Michael Additions to Nitroalkenes. Eur. J. Org. Chem. 1877, 2002. doi:10.1002/1099-0690(200206)2002; (b) Ono, N. Nitro Group in Organic Synthesis; Wiley-VCH, New York, NY, 2001; (c) Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. doi:10.1021/ja036972z.
- (a) Winn, M.; von Geldern, T.W.; Opgenorth, T.J.; Jae, H.-S.; Tasker, A.S.; Boyd, S.A.; Kester, J.A.; Mantei, R.A.; Bal, R.; Sorensen, B.K.; et al. 2,4-Diarylpyrrolidine-3- Carboxylic AcidsPotent ETA Selective Endothelin Receptor Antagonists. 1. Discovery of a-127722. J. Med. Chem. 1996, 39, 1039–1048. doi:10.1021/jm9505369; (b) Wu- Wong, J.R.; Dixon, D.B.; Chiou, W.J.; Dayton, B.D.; Novosad, E.I.; Adler, A.L.; Wessale, J.L.; Calzadilla, S.V.; Hernandez, L.; Marsh, K.C.; et al. Pharmacology of a- 216546: A Highly Selective Antagonist for Endothelin ETA Receptor. Eur. J. Pharmacol. 1999, 366, 189–201; (c) Barnes, D.M.; Ji, J.; Fickes, M.G.; Fitzgerald, M.A.; King, S.A.; Morton, H.E.; Plagge, F.A.; Preskill, M.; Wagaw, S.H.; Wittenberger, S.J.; et al. Development of a Catalytic Enantioselective Conjugate Addition of 1,3-Dicarbonyl Compounds to Nitroalkenes for the Synthesis of Endothelin-a Antagonist ABT-546. Scope, Mechanism, and Further Application to the Synthesis of the Antidepressant Rolipram. J. Am. Chem. Soc. 2002, 124, 13097–13105. doi:10.1021/ja026788y.
- (a) Pinnick, H.W. The Nef Reaction. Org. React. 1990, 38, 655–792. doi:10.1002/0471264180.or038.03; (b) Tamura, R.; Kamimura, A.; Ono, N. Displacement of Aliphatic Nitro Groups by Carbon and Heteroatom Nucleophiles. Synthesis 1991, 423–434. doi:10.1055/s-1991-26484; (c) Mukayama, T.; Hoshino, T. The Reactions of Primary Nitro- Paraffins with Isocyanates. J. Am. Chem. Soc. 1960, 82, 5339–5342. doi:10.1021/ja01505a017.
- (a) Jung, M.E. In Comprehensive Organic Synthesis, Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, 1991, 4, pp 1–67; (b) Nelson, J.H.; Howells, P.N.; DeLullo, G.C.; Landen, G.L. Nickel-Catalyzed Michael Additions of β- Dicarbonyls. J. Org. Chem. 1980, 45, 1246–1249.
- Saidi, M.R.; Azizi, N.; Akbari, E.; Ebrahimi, F. LiClO4/Et3N: Highly Efficient and Active Catalyst for Selective Michael Addition Ofactive Methyleneb compounds under Solvent-Free Condition. J. Mol. Cat. a: Chem. 2008, 292, 44–48. doi:10.1016/j.molcata.2008.06.003.
- Yang, W.; Luo, S.; Yu, C.; Wang, B.; Yue, H.; Wang, L.; Xu, D. Base-Catalyzed Michael Reaction of Malonate with Α, β-Unsaturated Compounds in Ionic Liquids. Huaxue Yanjiu Yu Yingyong 2006, 18, 537–541.
- Annamalai, V.; DiMauro, E.F.; Carroll, P.J.; Kozlowski, M.C. Catalysis of the Michael Addition Reaction by Late Transition Metal Complexes of BINOL-Derived Salens. J. Org. Chem. 2003, 68, 1973–1981. doi:10.1021/jo025993t.
- Corey, E.J.; Zhang, F.-Y. Enantioselective Michael Addition of Nitromethane to Α, β- Enones Catalyzed by Chiral Quaternary Ammonium Salts. A Simple Synthesis of (R)- Baclofen. Org. Lett. 2000, 2, 4257–4259. doi:10.1021/ol0068344.
- Hanessian, S.; Pham, V. Catalytic Asymmetric Conjugate Addition of Nitroalkanes to Cycloalkenones. Org. Lett. 2000, 2, 2975–2978. doi:10.1021/ol000170g.
- Li, H.; Wang, Y.; Tang, L.; Deng, L. Highly Enantioselective Conjugate Addition of Malonate and β-Ketoester to Nitroalkenes: Asymmetric C-C Bond Formation with New Bifunctional Organic Catalysts Based on Cinchona Alkaloids. J. Am. Chem. Soc. 2004, 126, 9906–9907. doi:10.1021/ja047281l.
- Halland, N.; Aburel, P.S.; Jorgensen, K.A. Highly Enantioselective Organocatalytic Conjugate Addition of Malonates to Acyclic Alpha, Beta-Unsaturated Enones. Angew. Chem., Int. Ed. 2003, 42, 661–665. doi:10.1002/anie.200390182.
- Ballini, R.; Rinaldi, A. Michael Addition of Nitroalkanes to Dimethyl Maleate with DBU. a New Direct Method for the Synthesis of Polyfunctionalized Α, β–Unsaturated Esters. Tetrahedron Lett. 1994, 35, 9247–9250.10.1016/0040-4039(94)88479-X
- (a) Weiping, Y.; Junye, X.; Chin-Tong, T.; Choon-Hong, T. 1,5,7-Triazabicyclo[4.4.0] Dec-5-Ene (TBD) Catalyzed Michael Reactions. Tetrahedron Lett. 2005, 46, 6875–6878. doi:10.1016/j.tetlet.2005.08.010; (b) Tu, Z.; Jang, Y.; Lin, C.; Liu, J.T.; Hsu, J.; Sastry, M.N.V.; Yao, C.-F. The Study of Reaction Mechanism for the Transformation of Nitronate into Nitrile by Phosphorus Trichloride. Tetrahedron 2005, 61, 10541–10551. doi:10.1016/j.tet.2005.08.041.
- Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. Enantio- and Diastereo- Selective Michael Reaction of 1,3-Dicarbonyl Compounds to Nitroolefins Catalyzed by a Bifunctional Thiourea. J. Am. Chem. Soc. 2005, 127, 119–125. doi:10.1021/ja044370p.
- Chen, F.-X.; Shao, C.; Wang, Q.; Gong, P.; Zhang, D.-Y.; Zhanga, B.-Z.; Wanga, R. An Enantioselective Michael Addition of Malonate to Nitroalkenes Catalyzed by Low Loading Demethylquinine Salts in Water. Tetrahedron. Lett. 2007, 48, 8456–8459. doi:10.1016/j.tetlet.2007.09.168.
- Evans, D.A.; Mito, S.; Seidel, D. Scope and Mechanism of Enantioselective Michael Additions of 1,3-Dicarbonyl Compounds to Nitroalkenes Catalyzed by Nickel(II)- Diamine Complexes. J. Am. Chem. Soc. 2007, 129, 11583–11592. doi:10.1021/ja0735913.
- (a) Clark, J.H.; Rhodes, C.N. Clean Synthesis Using Porous Inorganic Solid Catalysts and Supported Reagents; Royal Society of Chemistry, Cambridge, 2000; (b) Gerard, V.S.; Notheisz, F. Heterogeneous Catalysis in Organic Chemistry; Elsevier, San Diego, 2000; (c) Rafiee, E.; Rashidzadeh, S.; Azada, A. Silica-Supported Heteropoly Acids: Highly Efficient Catalysts for Synthesis of Α-Aminonitriles, Using Trimethylsilyl Cyanide or Potassium Cyanide. J. Mol. Catal. A: Chem. 2007, 261, 49–52. doi:10.1016/j.molcata.2006.07.058; (d) Davoodnia, A.; Bakavoli, M.; Barakouhi, G.; Tavakoli-Hoseini, N. A New Route to the Synthesis of Thieno[2,3-D]Pyrimidin-4(3H)-One Derivatives Catalyzed by 12-Tungstophosphoric Acid (H3PW12O40). Chin. Chem. Lett. 2007, 18, 1483–1486. doi:10.1016/j.cclet.2007.10.013; (e) Tavakoli-Hoseini, N.; Davoodnia, A. Silica Gel-Supported Polyphosphoric Acid: A Mild, Efficient and Reusable Catalyst for the One-Pot Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles. Asian J. Chem. 2010, 22, 7197–7200; (f) Davoodnia, A.; Tavakoli-Nishaburi, A.; Tavakoli-Hoseini, N. Carbon- Based Solid Acid Catalyzed One-Pot Mannich Reaction: A Facile Synthesis of β-Amino Carbonyl Compounds. Bull. Korean Chem. Soc. 2011, 32, 635–638. doi:10.5012/bkcs.2011.32.2.63.
- (a) Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412; (b) Niknam, K.; Karimi, B.; Zolfigol, M.A. Silica Sulfuric Acid Promoted Aromatization of 1,2-Dihydroquinolines by Using NaNO2 Asoxidizing Agent under Mild and Heterogeneous Conditions. Catal. Commun. 2007, 8, 1427–1430; (c) Niknam, K.; Zolfigol, M.A.; Khorramabadi-Zad, A.; Zare, R.; Sheygan, M. Silica Sulfuric Acid as an Efficient and Recyclable Catalyst for the Methoxymethylation of Alcohols under Solvent-Free Conditions. Catal. Commun. 2006, 7, 494–498; (d) Kozhevinkov, I.V. In Catalysis by Poly Oxometalates; Wiley, Chichester, 2002; pp 2–22; (e) Hajipour, A.R.; Ruoho, A.E. Nitric Acid in the Presence of P2O5supported on Silica Gel – A Useful Reagent for Nitration of Aromatic Compounds under Solvent-Free Conditions. Tetrahedron Lett. 2005, 46, 8307–8310. doi:10.1016/j.tetlet.2005.09.178; (f) Kantevari, S.; Bantu, R.; Nagarapu, L. HClO4–SiO2 and PPA–SiO2 Catalyzed Efficient One-Pot Knoevenagel Condensation, Michael Addition and Cyclo-Dehydration of Dimedone and Aldehydes in Acetonitrile, Aqueous and Solvent Free Conditions: Scope and Limitations. J. Mol. Catal. a: Chem. 2007, 269, 53–57. doi:10.1016/j.molcata.2006.12.039; (g) Davoodnia, A.; Roshani, M.; Malaeke, S.H.; Bakavoli, M. A Rapid Synthesis of Isoxazolo[5,4-D]Pyrimidin-4(5H)-Ones under Microwave Irradiation with Solid Acid Catalysis in Solvent-Free Conditions. Chin. Chem. Lett. 2008, 19, 525–528. doi:10.1016/j.cclet.2008.01.037.
- (a) Khan, A.T.; Ghosh, S.; Choudhury, L.H. Perchloric Acid Impregnated on Silica Gel (HClO4/SiO2): a Versatile Catalyst for Michael Addition of Thiols to the Electron Deficient Alkenes. Eur. J. Org. Chem. 2006, 9, 2226–2231. doi:10.1002/ejoc.200600006; (b) Zeinali-Dastmalbaf, M.; Davoodnia, A.; Heravi, M.M.; Tavakoli-Hoseini, N.; Khojastehnezhad, A.; Zamani, H.A. Silica Gel-Supported Polyphosphoric Acid (PPA- SiO2) Catalyzed One-Pot Multi-Component Synthesis of 3,4-Dihydropyrimidin-2(1H)- Ones and -Thiones: An Efficient Method for the Biginelli Reaction. Bull. Korean Chem. Soc. 2011, 32, 656–658. doi:10.5012/bkcs.2011.32.2.656; (c) Khojastehnezhad, A.; Davoodnia, A.; Bakavoli, M.; Tavakoli- Hoseini, N.; Zeinali-Dastmalbaf, M. Silica Gel- Supported Polyphosphoric Acid (PPA/SiO2): an Efficient and Reusable Heterogeneous Catalyst for Facile Synthesis of 14-Aryl-14H-Dibenzo[a]Xanthenes under Solvent-Free Conditions. Chin. J. Chem. 2011, 29, 297–302. doi:10.1002/cjoc.201190081; (d) Li, H.; Yua, X.; Tua, S.T.; Yanb, J.; Wanga, Z. Catalytic Performance and Characterization of Al2O3-Supported Pt–Co Catalyst Coatings for Preferential CO Oxidation in a Micro- Reactor. Appl. Catal. A 2010, 387, 215–223. doi:10.1016/j.apcata.2010.08.030; (e) Emrani, A.; Davoodnia, A.; Tavakoli-Hoseini, N. Alumina Supported Ammonium Dihydrogenphosphate (NH4H2PO4/Al2O3): Preparation, Characterization and Its Application as Catalyst in the Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles. Bull. Korean Chem. Soc. 2011, 32, 2385–2390. doi:10.5012/bkcs.2011.32.7.2385.
- Davoodnia, A.; Khojastehnezhad, A.; Tavakoli-Hoseini, N. Carbon-Based Solid Acid as an Efficient and Reusable Catalyst for the Synthesis of 1,8-Dioxodecahydroacridines under Solvent-Free Conditions. Bull. Korean Chem. Soc. 2011, 32, 2243–2248. doi:10.5012/bkcs.2011.32.7.2243.
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. doi:10.1021/cr940089p.
- (a) Shumaila, A.M.A.; Kusurkar, R.S. Silica Gel, an Effective Catalyst for the Reaction of Electron-Deficient Nitro-Olefins with Nitrogen Heterocycles. Synth. Commun. 2010, 40, 2935–2940. doi:10.1080/00397910903340694; (b) Shumaila, A.M.A.; Puranik, V.G.; Kusurkar, R.S. Diastereoselective Synthesis of Tetrasubstituted-Octahydro-3,6- Diazacarbazoles and Tetrasubstituted-3,6-Diazacarbazoles via Double Pictet-Spengler Reaction. Tetrahedron Lett. 2011, 52, 2661–2663. doi:10.1016/j.tetlet.2011.03.060; (c) Shumaila, A.M.A.; Puranik, V.G.; Kusurkar, R.S. Synthesis of Tetrahydro-5-Azaindoles and 5-Azaindoles Using PicteteSpengler Reactiondappreciable Difference in Products Using Different Acid Catalysts. Tetrahedron 2011, 67, 936–942. doi:10.1016/j.tet.2010.12.003; (d) Dattatray, G.H.; Shumaila, A.M.A.; Kusurkar, R.S. Silica Gel-Supported Bismuth Nitrate Pentahydrate:A Highly Active Catalyst under Solvent Free Conditions towards the Synthesis of Dihydropyrimidin-2(1H)-Ones and Their Sulphur Analogues. Indian J. Chem., Sec B 2013, 52, 1161–1165.
- The catalyst (KCA) was prepared with the help of Procedure reported by: Yamaguchi, T.; Zhu, J.-H.; Wang, Y.; Komatsu, M.-A.; Ookawa, M. Supported K-Salts as a New Solid Base Catalyst. Chem. Lett. 1997, 989–990. doi:10.1246/cl.1997.989.