Abstract
Biofortified open pollinated maize varieties (OPV) could be used to address the problem of micronutrient deficiencies in developing countries. This study aimed at investigating the effects of maturity 20, 27, and 34 days after pollination (DAP) and processing (boiling with and without husks) on the bioactive components (carotenoids, phytic acid, tannins, and vitamin C) on fresh orange OPV maize. The fresh and processed samples were analysed for bioactive components using standard methods of analysis. Carotenoids, phytate, and vitamin C showed a general significant (P ≥ 0.5) increase in concentrations across the studied harvest maturity stages. The optimum retention for most bioactive compounds was found at 27 DAP for cobs of orange OPV maize boiled with and without husks. Boiled maize with husks showed higher retention of most bioactive compounds than boiled maize without husks where the mean concentrations of the bioactive compounds increased across the harvesting stages except for tannin and vitamin C that showed a decrease at 34 DAP. Varieties 1 and 5 showed a higher provitamin A value than the grand mean of 6.04 μg/g at 27 DAP but variety 5 had the highest concentration of 10.2 μg/g. Variety 1 showed a higher concentration of provitamin A value than the respective grand mean at the three harvest maturity stages for OPV maize boiled with husk intact. The retention of more bioactive compounds during boiling with or without husks is found to be genotype dependent. The information from this study could guide the food scientists, nutritionists, and consumers on the best boiling methods to process OPV orange maize for optimum retention of bioactive components.
GRAPHICAL ABSTRACT
PUBLIC INTEREST STATEMENT
Human beings require at least 49 nutrients (those require in large quantity (macro) and small quantity (micro)) to meet their body needs and one of these micronutrients is Vitamin A. The use of the orange maize varieties with enhanced pro-vitamin A carotenoids (bioactive) levels will be of value in reducing incidences of micronutrient deficiency of particularly the low-income communities of the developing countries. Orange maize is preferred as fresh maize and consumed boiled or roasted on the cob to bridge the hunger gap after a long dry season. The best time to have very high bioactive compounds was found at 27 days after pollination for cobs of orange OPV maize boiled with and without husks. Boiled maize with husks showed higher retention of most bioactive compounds than boiled maize without husks. The retention of more bioactive compounds during boiling with or without husks is found to be variety dependent.
Competing Interests
The authors declare no competing interests.
1. Introduction
Under-nutrition is characterized not only by an energy deficit owing to a reduction in all macronutrients but also by a deficit in many micronutrients. Orange maize is reported to have principal micronutrient antioxidants such as carotene, xanthophylls, polyphenols, and vitamins C, E, and D (Adom & Liu, Citation2002; Alamu, Menkir, Maziya-Dixon, & Olaofe, Citation2014a; Kurilich & Juvik, Citation1999; Menkir, Weiping, Wendy, Maziya–Dixon, & Rocheford, Citation2008; Weber, Citation1987). Within the maize embryo, elevated levels of tocopherols are present while carotenoids are commonly associated with the kernel endosperm (Weber, Citation1987). In recent years, considerable efforts have been made to develop open pollinated varieties (OPV) of maize with high levels of provitamin A through plant breeding (biofortification) and genetic modification; such OPV may be used by farmers and consumers as green maize when food shortages are severe. Through these efforts, some orange maize lines (OPV and hybrids) have been found to have very high levels of bioactive compounds, especially carotenoids (Alamu et al., Citation2014a; Egesel, Wong, Lambert, & Rocheford, Citation2004; Menkir & Maziya–Dixon, Citation2004; Menkir et al., Citation2008; Muzhingi et al., Citation2008). There are many improved varieties of orange OP maize both in the pipelines and available for the consumers; identifying the content of these lines would help maize breeders, agronomists, and human nutritionists to determine which lines with high levels of bioactive compounds are best suited to particular climates, soil types, and cultures for different parts of the world. Reproduction of OPV is in one of two ways: by cross-pollination between two plants (via wind or insects) or from separate flowers on the same plant. Lower levels of lignin makes silage more digestible, but also create problems with lower standability. The development of some structural and material components of maize kernels leads to the attainment of maturity. The endosperm is the most implicated because it constitutes the main store of the dry matter accumulated during plant growth that contains most of the nutrients found in maize.
Polyphenols and carotenoids are referred to as antioxidant micronutrients and could play an important part in preventive nutrition, but they are susceptible to high variation among cultivars and growth conditions. Rice-Evans, Miller, Bolwell, Bramley, and Pridham (Citation1995) reported that flavonoids and polyphenols have greater antioxidant activity than either vitamins C or E. Plant phenolics are increasingly gaining importance in relation to human health and well-being, as they exhibit anticarcinogenic, antioxidant, antiviral, antimicrobial, anti-inflammatory, and hypertensive properties (Cowan, Citation1999). Adom and Liu (Citation2002) reported that corn had the highest total phenolic content (15.55) of the grains tested, followed by wheat (7.9) and rice (5.56) measured in micromole of gallic acid equivalent per gram of grain. Rice and oat flours contain approximately the same quantity of phenolic acids as wheat flour but the content in maize flour is reported to be three times as high (Shahidi & Naczk, Citation1995). The consumption of cereal products contributes to the phenolic acid intake only when whole grains are used for their manufacture or processing (Adom & Liu, Citation2002). The phytic acids in unprocessed products mainly appear as inositol hexaphosphate (IP6). Phytic acid is one of the bioactive compounds that are being intensively studied to evaluate their effects on health; it has been shown to have potential as an anticancer agent that affects only malignant cells and not normal cells and tissues (Vucenik & Shamsuddin, Citation2003). A variety of benefits of phytic acid on human health have also been reported including its potential as an anticancer property in soft tissues, colon, prostate, metastatic, and mammary cancers. It may also act as an inhibitor for renal stone development (Dost & Tokul, Citation2006). Khan, Zaman, and Elahi (Citation1991) studied the effect of heat treatments on the phytic acid content of maize products and reported that the processing of maize (Zea mays L. fresh and dry) for the production of various traditional products results in the loss of phytic acid. Horvatic and Balint (Citation1996) studied the relationship among phytic acid and protein contents during maize grain maturation. Phytic acid increased significantly (P = 0.05) until the late stage of dough grain maturity. Afterwards, until full grain maturity, no significant changes of phytic acid content have been obtained. Vitamin C is used as an index of the health-related quality of fruits, because, compared to other beneficial compounds, it is more sensitive to degradation from processing and storage (Odriozola-Serrano, Hernandez–Jover, & Martin–Belloso, Citation2007). Maize is quite a good source of vitamin C. Although the amount present is lower than that in the guava or citrus fruits, it exceeds those in apples and pears (Asami, Hong, Diane, & Alyson, Citation2003). Systematic studies were not conducted to determine the effect of processing on the bioactive composition of green maize harvested fresh from orange OPV maize and also to determine the extent of loss in bioactive content when the cobs are harvested green and consumed, boiled on the cob, with or without husks. This study is therefore designed to evaluate the effects of location, boiling methods, and maturity on the bioactive characteristics of eight OPV of orange maize.
2. Materials and experimental methods
2.1. Source materials and study design
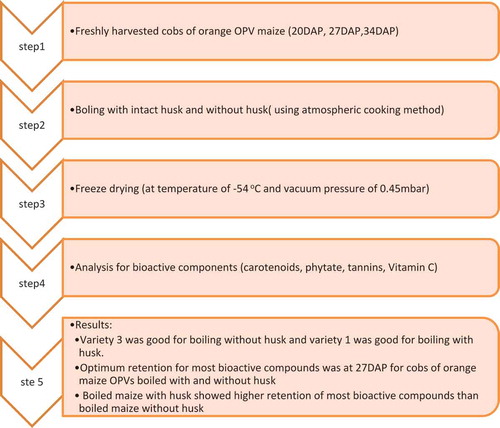
The freshly harvested cobs from eight biofortified OPV of orange maize were used for this research work and obtained from the research farms of International Institute of Tropical Agriculture (IITA), Ibadan. The eight OPV were planted in 2010 in two separate trials at Ibadan (7°22 N, 3°58ʹE, altitude 150 masl) and Ikenne (10°40ʹN, 8°77ʹE, altitude 730 masl). The varieties were arranged in a randomized complete block design (RCBD) with replications. Self-pollination was done to minimize contamination from other pollen sources. The harvest maturity stages were 20, 27, and 34 days after pollination (DAP) (50% anthesis/pollen shed or 50% silk emergence which was 57 days after planting). All chemicals used were of analytical grade.
2.2. Sampling and sample preparation
Samples were obtained at 20, 27, and 34 DAP for each OPV. They were harvested at 08.00 h on the relevant dates. A total of 20 selected cobs of each OPV were harvested from each plot and pooled to give 60 cobs per OPV per harvest. They were packed in mailing sacks and taken to the laboratory as soon as possible (Alamu et al., Citation2014a). The cobs for each variety were divided into 6 sets of 10 cobs each and subjected to chemical assays, as well as boiling with/without husks. All the harvested cobs were processed within 24 h after harvesting.
2.3. Processing of freshly harvested orange maize
The 15 selected cobs of each orange OPV were boiled with intact husks and another 15 selected cobs were dehusked and boiled at 100°C in stainless pots on domestic gas cookers in 2 L of water according to the local practice (Alamu et al., Citation2014a; Osanyintola, Marek, & Akingbala, Citation1992). The cooking time varied with harvest times for both forms of boiling. Dehusked cobs from harvesting at 20, 27, and 34 DAP were cooked for 25, 35, and 45 min, respectively. Intact cobs from harvesting at 20, 27, and 34 DAP were cooked for 35, 45, and 55 min, respectively. The samples of fresh and processed orange maize were carefully shelled, then freeze-dried using Labconco Freezone 4.5 L (at temperature of −54°C and vacuum pressure of 0.45 mbar), milled (sieve size, 0.5 mm), packed in the polythene whirl-pack, and stored at 4°C. The samples targeted for carotenoid analysis were stored at ‒80°C. All laboratory analyses were done in duplicate.
3. Determination of other bioactive compounds
3.1. Carotenoid analysis method
The method of Howe and Tanumihardjo (Citation2006) was employed as modified by Alamu et al. (Citation2014a). The extraction of carotenoids from dried maize (0.6 g) was done by adding ethanol (6 mL) containing 0.1% butylated hydroxyl toluene (BHT), mixing by vortex, and ethanol precipitation in a water bath at 85°C for 5 min. Potassium hydroxide (500 µL, 80% w/v) was added to the mixture to saponify the interfering oil. Samples were vortexed again and returned to the water bath for an additional 5 min. Upon removal, they were immediately placed in an ice bath where 3 mL of cold deionized water was added. Carotenoids were separated three times with addition of 3 mL of hexane, vortexed, and centrifuged (1200 g) for 5 min. The combined hexane fractions were washed with de-ionized water three times, vortexed, and centrifuged for 5 min at 1200 g. The hexane fractions were dried down in a concentrator TurboVap LIV under nitrogen gas. Samples were reconstituted in methanol/dichloromethane (1 mL, 50:50 v/v), and 50 µL was injected into the HPLC. Waters HPLC system (Water Corporation, Milford, MA) consisted of a guard-column, C30 YMC Carotenoid column (4.6 × 250 mm, 3 µL), Waters 626 binary HPLC pump, 717 auto-sampler, and a 2996 photodiode array detector (PDA) and was used for carotenoids quantification. The solvent A used consisted of methanol/water (92:8 v/v) with 10 mM ammonium. Solvent B used consisted of 100% methyl tertiary-butyl ether. Gradient elution was performed at 1 mL/min with the following conditions: 29 min linear gradient from 83% to 59% A, 6 min linear gradient from 59% to 30% A, 1 min hold at 30% A, 4 min linear gradient from 30% to 83% A and a 4 min hold at 83%. β-carotene eluted at ~25 min. Chromatograms were generated at 450 nm and identification of α-carotene, β-carotene (cis and trans isomers), and β-cryptoxanthin was determined using external standard methods based on the calibration curve from pure standards, verification of absorption spectrum, and co-elution with available authentic standards. Standards of α-carotene, β-carotene, and β-cryptoxanthin were purchased from CaroteNature, GmbH (Lupsingen, Switzerland). Solvents were HPLC grade.
3.2. Determination of ascorbic acid (vitamin C)
Ascorbic acid was determined using the dyestuff titration method as described by AOAC (Citation2005) and Adegunwa, Adelekan, Adebowale, Bakare, and Alamu (Citation2017). The sample (5 g) was digested with 0.4 g/100 g oxalic acid. The aliquot was titrated against dyestuff that was previously standardized by the standard ascorbic acid solution. The ascorbic acid content was calculated using the following expression.
Vitamin C (mg/100 g) = Titre value × 0.606 × 100/Weight of Sample
3.3. Phytic acid analysis
Phytic acid was determined by a combination of two methods. The extraction and precipitation of phytic acid were done according to the method of Wheeler and Ferrel (Citation1971) as described by Alamu, Maziya-Dixon, Okonkwo, and Asiedu (Citation2014b). A 4:6 Fe/P atomic ratio was used to calculate the phytic acid content.
3.4. Determination of Tannin (polyphenols)
Tannin content was determined by the Folins–Dennis colorimetric method described by Alamu et al. (Citation2014b) in which the 5 g sample was weighed and dispersed in 50 mL of distilled water and the mixture stood for 30 min at 28°C before it was filtered through Whatman No. 42-grade filter paper. A 2 mL of the extract was dispensed into a 50 mL volumetric flask; Folins reagent was added to the flask with 2.5 mL of saturated Na2CO3 solution and allowed to incubate for 90 min at 28°C. The absorbance was read in a spectrophotometer at 260 nm.
4. Statistical analysis
Data generated from all experiments were subjected to analysis of variance (ANOVA) and descriptive statistics using the statistical analysis system (SAS) software package. Least significant difference (LSD) test was used for mean comparison. The percentage true retention for β-carotene and PVA was calculated using the method recommended and described by Murphy, Criner, and Gray (Citation1975).
5. Results and discussion
5.1. Effects of harvest maturity stages on bioactive components of unprocessed fresh orange OP maize
Table provided the summary of descriptive statistics for bioactive components of unprocessed fresh cobs of orange OPV maize at different stages of harvest maturity. The carotenoids found were lutein, zeaxanthin, β-cryptoxanthin, all trans-β-carotene, 9-cisβ-carotene, and 13-cisβ-carotene which were the same as in the orange maize lines reported in the literature (Alamu et al., Citation2014a; Howe & Tanumihardjo, Citation2006; Kurilich & Juvik, Citation1999; Menkir et al., Citation2008; Muzhingi et al., Citation2008). The mean concentration of carotenoids (lutein, zeaxanthin, α-carotene, total β-carotene, and provitamin A) showed a decrease between 20 and 27 DAP followed by an increase at 34 DAP. However, other bioactive components showed a different pattern to that observed for carotenoids. The phytate and tannin showed a general decrease in mean concentrations across the three stages of harvest maturity while vitamin C showed an increase as the maize matured. The differences in the mean concentrations of phytate, tannin, and vitamin C were significant across the harvest maturity stages but differences in mean concentrations for zeaxanthin, β-cryptoxanthin, and α-carotene were significant at 20 and 27 DAP but not at 34 DAP. The carotenoids showed no significant differences between 20 and 27 DAP but differences were significant at 34 DAP. The results are slightly different from those reported by Horvatic and Balint (Citation1996) in which phytic acid increased significantly (P = 0.05) until the late stage of dough grain maturity; however, they did not state the actual stage of maturity as the starting stage used in their study was exactly 57 days after planting. However, they found out that fresh mature corn contains less phytic acid (1.71 g/kg) than dry corn (7.15–7.60 g/kg) in results that supported the findings of this present study that showed a lowest value for phytic acid at 34 DAP which is a later stage of maturity. The difference in lutein content at 20 and 27 DAP was not significant but significant at 34 DAP. The increase in contents with the increase in maturity days found for most of the bioactives could be due to the maximum increase in granule size. Boyer, Shannon, Garwood, and Creech (Citation1976) found that although granule size distributions vary among genotypes, maximum granule size increases markedly from about 18 to 36 DAP for maize varieties. It was also reported that there is at least 80% dry matter weight accumulation in kernel development between 15 and 30 DAP (Kurilich & Juvik, Citation1999). In addition, among all OPVs investigated, varieties 1, 3, 4, and 8 showed higher provitamin A concentrations above the grand mean value of 3.9 μg/g at 20 DAP. Varieties 1, 2, 3, 4, and 5 showed provitamin A contents above the grand mean value of 3.48 μg/g at 27 DAP. Varieties 2, 3, 4, 7, and 8 showed higher provitamin A above the grand mean value of 4.66 μg/g at 34 DAP. Therefore, varieties 3 and 4 showed higher provitamin A content at the three harvest maturity stages, well above the commonly grown OPV maize used as control (variety 8).
Table 1. Bioactive contents of unprocessed fresh orange OPV maize at different harvest maturity stages (N = .64 for each maturity stage)
5.2. Effect of boiling methods on bioactive contents of maturing fresh orange OPV maize
Tables and showed the summary of descriptive statistics of bioactive compounds in fresh orange OPV maize when boiled with or without husks. In OPV maize boiled without husks, the mean concentrations of the bioactive compounds increased across the harvesting stages except for tannin and vitamin C that showed a decrease at 34 DAP. The pattern obtained for the boiled OPV maize differed from that observed for hybrid maize reported by Alamu et al. (Citation2014a) and for unprocessed fresh orange OPV maize in this study. This observation suggested that boiling influences the bioactive contents of fresh orange OPV maize. The mean values of all bioactive compounds increased at 20 and 27 DAP followed by a decrease at 34 DAP. When OPV maize cobs were boiled with husks, the level of Vitamin C showed a decrease in concentration at 20 and 27 DAP followed by an increase in concentration at 34 DAP. Mean concentrations of the bioactive components in OPV maize boiled with husks were higher than those boiled without husks. This observation suggested that husks showed an effect on the retention of bioactive contents in boiled OPV maize. Husks have no effect on the carotenoid profile of boiled fresh orange OPV maize but showed an effect on the peak area of the isomers of β-carotene (9-cis and 13-cis) across the maturity stages. However, when OPV maize was boiled without husks, varieties 1, 2, 3, 4, 5, and 7 showed higher provitamin A than the grand mean concentration of 4.05 μg/g at 20 DAP but variety 3 had the highest concentration of provitamin A of 5.10 μg/g. Varieties 1, 2, 3, and 4 showed higher provitamin A than the grand mean concentration of 4.89 μg/g at 27 DAP but variety 3 had the highest provitamin A of 9.01 μg/g. Varieties 2, 3, 4, and 5 showed higher provitamin A than the grand mean concentration of 6.38 μg/g at 34 DAP but variety 3 had the highest concentration of 11.7 μg/g. Varieties 2, 3, and 4 showed higher provitamin A at all three harvest maturity stages but variety 3 was the best variety for boiling without husks at all maturity stages.
Table 2. Bioactive contents of boiled fresh orange OPV maize without husk at different harvest maturity stages (N = 64 for each maturity stage)
Table 3. Bioactive contents of boiled fresh orange OPV maize with husk at different harvest maturity stages (N = 64 for each maturity stage)
The mean concentrations of bioactive compounds of OPV boiled with husks at the three maturity stages showed that varieties 1, 2, 3, 4, and 8 showed higher provitamin A value than the grand mean value of 4.35 μg/g at 20 DAP but variety 1 had the highest concentration of 5.19 μg/g. Varieties 1 and 5 showed higher values for provitamin A than the grand mean of 6.04 μg/g at 27 DAP but variety 5 had the highest concentration of 10.2 μg/g. Varieties 1, 2, 3, 4, 5, and 6 showed higher provitamin A values than the grand mean value of 5.22 μg/g at 34 DAP but variety 4 had the highest concentration of 6.41 μg/g. Variety 1 showed a higher concentration of provitamin A value than the respective grand mean at the three harvest maturity stages for boiled OPVs maize with husks intact. This suggested that there are some genotypes that can retain more provitamin A during boiling with or without husks as observed for hybrid maize studied by Alamu et al. (Citation2014a).
5.3. Percentage true retention or change of bioactive contents of boiled fresh orange OPV maize across two locations
Tables and showed percentage true retention or change of bioactive contents of boiled fresh orange OPV maize across two locations and two seasons. When the cobs were boiled with/without husks, there was a gain in lutein content at each stage of harvest maturity. Percentage retention showed that lutein was not lost into the boiling water. It was found that boiling with husks prevented the loss of lutein. Zeaxanthin content for boiled OPV maize with/without husks showed a gain at all stages of harvest maturity except for OPV maize cobs boiled without husks that showed a loss of 5.73% at 20 DAP. Content of β-cryptoxanthin for boiled OPV maize with/without husks showed a gain at stages of all harvest maturity except for boiled OPV maize boiled without husks that showed a loss of 1.33% at 20 DAP. It could be observed that OPV maize boiled with husks had the highest retention of lutein and zeaxanthin at 27 DAP. Contents of α-carotene, total β-carotene, and provitamin A for OPV maize boiled with/without husks showed gains at all stages of harvest maturity. The optimum retention of α-carotene, total β-carotene, and provitamin A contents was found to be at 27 DAP where OPV maize boiled with husks showed a higher retention than OPV maize boiled without husks. The result obtained in the present study is in close agreement with those reported by Muzhingi et al. (Citation2008) who found that the boiling of matured orange dried corn (<45 DAP) at 100°C for 30 min increased the carotenoid concentration. This study also confirmed previous reported results showing that boiling did not alter the carotenoid profile in vegetables but the amounts of carotenoids quantified were higher when compared to those in unprocessed samples (Mosha, Pace, Adeyeye, Laswai, & Mtebe, Citation1997; Park, Citation1987). Khachik et al. (Citation1992) showed that conventional blanching and cooking resulted in a significant (P < 0.05) increase in the concentration of carotenoids in the leaves of cowpea, peanut, and pumpkin. This could be because of the easy extraction of carotenoids due to the breakdown of the food matrix (Khachik et al., Citation1992). It has been reported that cooking can increase the extractability and bioavailability of carotenoids (Dietz, Sri, & Erdman, Citation1988; Hart & Scott, Citation1995). The phytate content for OPV maize boiled without husks showed a loss of 47.8% at 20 DAP, a gain of 3.49% at 27 DAP, and of 41.5% at 34 DAP while OPV maize boiled with husks showed losses of 38.4% at 20 DAP and 27.5% at 27 DAP but a gain of 3.94% at 34 DAP. The result on phytate content in the present study was in close agreement with those of Khan et al. (Citation1991) and Nawab Khan and Manzoor (Citation2006) who reported losses in phytic acid during heat treatment. The tannin content of OPV maize boiled without husks showed a loss of 44.0% at 20 DAP, a gain of 13.5% at 27 DAP, and a loss of 27.4% at 34 DAP while OPV maize boiled with husks showed a loss of 13.6% at 20 DAP and gains of 17.7% at 27 DAP and 51.1% at 34 DAP. Phytate and tannin contents in boiled OPV maize had the greatest losses at 20 DAP. Shahidi and Naczk (Citation1995) reported that polyphenols are not evenly distributed in plant tissues and food fractionation during processing may result in a loss or an enrichment of some phenolic compounds, as observed in the present study. Vitamin C content for boiled OPV maize without husks showed a gain of 20.5% at 20 DAP, losses of 40.1% at 27 DAP, and 38.3% at 34 DAP while OPV maize boiled with husks showed losses of 1.13% at 20 DAP and 41.4% at 27 DAP, and a gain of 29.4% at 34 DAP. The losses could be due to degradation from processing as Vitamin C is highly sensitive to heat treatment (Odriozola-Serrano et al., Citation2007).
Table 4. Percentage true retention of bioactive content of boiled orange OPV maize without husk at two locations (N = 64 for each maturity stage)
Table 5. Percentage true retention of bioactive content of boiled orange OPV maize with husk at two locations (N = 64 for each maturity stage)
6. Conclusion
In conclusion, variety 3 was good for boiling without husks and variety 1 was good for boiling with husks. The optimum retention for most bioactive compounds was found at 27 DAP for cobs of orange maize OPVs boiled by both methods. Maize boiled with husks showed higher retention of most bioactive compounds than when boiled without husks. Thus, boiling maize with husks is recommended as the better method to process maize for optimum bioactive retention. Such information will not only increase the understanding of the level of retention of these antioxidant phytochemicals after processing that will be available for lowering incidence of ageing and chronic diseases but will also help breeders to adjust their germplasm development activities for high content of such phytochemicals. This information can also help researchers in choosing the proper cooking methods to be used to increase the retention of high levels of carotenoids in orange maize that can be delivered to consumers through nutrition education.
Acknowledgements
The authors acknowledge support from CRP Maize program under CGIAR, a global research partnership for a food-secure future.
Additional information
Funding
Notes on contributors

Busie Maziya-Dixon
Dr. ALAMU, Emmanuel Oladeji, a Nigerian, is an Associate Scientist (Food Science and Technology) working with the International Institute of Tropical of Agriculture(IITA), Zambia. He holds a doctorate degree in Food Chemistry with over 12 years of research experience and strong analytical skills in food science and nutrition, and experienced in carrying out nutrition-sensitive agricultural research using different tools and techniques. He has many publications in local and foreign journals to his credit. Specifically, his research lines primarily examined: the physical and bioactive characteristics of biofortified and non-biofortified crops such as soybean, maize, cowpea, cassava, yam; retention studies on the bioactive compounds in unprocessed and processed biofortified crops and foods; anti-oxidant activities/capacities of unprocessed and processed biofortified crops; bioavailability and bioefficacy of processed biofortified crops and associated products; sensory characteristics of products from biofortified crops.
References
- Adegunwa, M. O., Adelekan, E. O., Adebowale, A. A., Bakare, H. A., & Alamu, E. O. (2017). Evaluation of nutritional and functional properties of plantain (Musa paradisiaca L.) and tigernut (Cyperus esculentus L.) flour blends for food formulations. Cogent Chemistry, 3, 1383707. doi:10.1080/23312009.2017.1383707
- Adom, K. K., & Liu, R. H. (2002). Antioxidant activity of grains. Journal of Agricultural and Food Chemistry, 50((21), 6182‒6187. doi:10.1021/jf0205099
- Alamu, E. O., Maziya-Dixon, B., Okonkwo, C. C., & Asiedu, R. (2014b). Physicochemical and bioactive properties of selected white yam (Dioscorea rotundata) varieties adapted to riverine areas of Nigeria. African Journal of Food Science, 8(7), 402‒409. doi:10.5897/AJFS2014.1154
- Alamu, E. O., Menkir, A., Maziya-Dixon, B., & Olaofe, O. (2014a). Effect of husk and harvesting time on the carotenoids and acceptability of roasted orange maize hybrids. Food Science & Nutrition, 2(6), 811–820. doi:10.1002/fsn3.179
- AOAC. 2005. Official methods of analysis. Association of official analytical chemists, Vol. 252. Published by the Association of Official Analytical Chemists, Inc. Suite 400 2200 Wilson Boulevard: Arlington. Virginia 22201 USA 152‒154.
- Asami, D. K., Hong, Y., Diane, M., & Alyson, M. E. (2003). Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry and corn grown using conventional, organic and sustainable agricultural practices. Journal of Agricultural and Food Chemistry, 51, 1237‒1241. doi:10.1021/jf020635c
- Boyer, C. D., Shannon, J. C., Garwood, L., & Creech, R. G. (1976). Changes in starch granules size and amylose percentage during kernel development in several Zea mays l. genotypes. Cereal Chemistry, 53, 327‒337.
- Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Review, 12, 564‒582.
- Dietz, J. M., Sri, K. S., & Erdman, J. W., Jr. (1988). Reversed phased HPLC analysis of α carotene and β-carotene from selected raw and cooked vegetables. Plant Foods for Human Nutrition, 38, 333‒341.
- Dost, K., & Tokul, O. (2006). Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Analytical Biochemistry, 119, 413‒417.
- Egesel, C. O., Wong, J. C., Lambert, R. J., & Rocheford, T. R. (2004). Gene dosage effects on carotenoid concentration in maize grain. Maydica, 48, 183‒190.
- Hart, D. J., & Scott, K. J. (1995). Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chemistry, 54(1), 101–111. doi:10.1016/0308-8146(95)92669-B
- Horvatic, M., & Balint, L. (1996). Relationship among the phytic and protein content of maize grain. Journal of Agronomy and Crop Science, 176(2), 73–77. doi:10.1111/j.1439-037X.1996.tb00448.x
- Howe, J. A., & Tanumihardjo, S. A. (2006). Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp). Journal of Agricultural and Food Chemistry, 54, 7992–7997. doi:10.1021/jf062256f
- Khachik, F., Goli, M. B., Beecher, G. R., Holden, J., Lusby, W. R., Tenorio, M. D., & Barrera, M. R. (1992). Effects of food preparation on quantitative distribution of major carotenoid constituents of tomatoes and several vegetables. Journal of Agricultural and Food Chemistry, 40, 390–398. doi:10.1021/jf00015a006
- Khan, N., Zaman, R., & Elahi, M. (1991). Effect of heat treatments on phytic acid content of maize products. Journal of Science Food and Agriculture, 54, 153–156. doi:10.1002/jsfa.2740540117
- Kurilich, A. C., & Juvik, A. J. (1999). Quantification of carotenoid and tocopherol antioxidants. Zea Mays. Journal of Agricultural and Food Chemistry, 47, 1948–1955. doi:10.1021/jf981029d
- Menkir, A., & Maziya–Dixon, B. (2004). Influence of genotype and environment of β–Carotene content of tropical orange endosperm maize genotypes. Maydica, 49, 313–318.
- Menkir, A., Weiping, L., Wendy, S. W., Maziya–Dixon, B., & Rocheford, T. (2008). Carotenoids diversity in tropical–Adapted orange maize inbred lines. Food Chemistry, 109, 521–529. doi:10.1016/j.foodchem.2008.01.002
- Mosha, T. C., Pace, R. D., Adeyeye, S., Laswai, H. S., & Mtebe, K. (1997). Effect of traditional processing practices on the content of total carotenoids, β–Carotene, α–Carotene and vitamin A activity of selected Tanzanian vegetables. Journal of Plant Foods for Human Nutrition, 50(3), 189–201. doi:10.1007/BF02436056
- Murphy, E. W., Criner, P. E., & Gray, B. C. (1975). Comparison of methods for calculating retention nutrients in cooked foods. Journal of Agriculture and Food Chemistry, 23, 1153–1157. doi:10.1021/jf60202a021
- Muzhingi, T., Kyung-Jin, Y., Robert, M. R., Elizabeth, J. J., Jian, Q., & Guangwen, T. (2008). Determination of carotenoids in orange maize, the effects of saponification and food preparations. International Journal of Vitamin and Nutrition Reserves, 78(3), 112–120. doi:10.1024/0300-9831.78.3.112
- Nawab Khan, R. Z., & Manzoor, E. (2006). Effect of heat treatment on the phytic acid content of maize products. Journal of the Science of Food and Agriculture, 54(1), 153–156. doi:10.1002/jsfa.2740540117
- Odriozola-Serrano, I., Hernandez–Jover, T., & Martin–Belloso, O. (2007). Comparative evaluation of UV–HPLC methods and reducing agents to determine vitamin C in fruits. Food Chemistry, 105, 1151–1158. doi:10.1016/j.foodchem.2007.02.037
- Osanyintola, O. J., Marek, J. H., & Akingbala, J. O. (1992). Effect of time of harvest and variety on the quality of boiled green field maize (Zea mays Linn). Tropical Science, 32, 369–376.
- Park, Y. W. (1987). Effect of freezing, thawing, drying and cooking on carotene retention in carrots, broccoli and spinach. Journal of Food Sciences, 52(4), 1022–1025. doi:10.1111/jfds.1987.52.issue-4
- Rice-Evans, C. A., Miller, N. J., Bolwell, P. G., Bramley, P. M., & Pridham, J. B. (1995). The relative antioxidant activities of plant–Derived polyphenolic flavonoids. Free Radical Research, 22(4), 375‒383.
- Shahidi, F., & Naczk, M. (1995). Food phenolics: Sources, chemistry, effects and applications (pp. 340). Lancaster: Technomic Publishing Co. Inc.
- Vucenik, I., & Shamsuddin, A. M. (2003). Cancer inhibition by inositol hexaphosphate (ip6) and inositol: From laboratory to clinic. Journal of Nutrition, 133(11 Suppl 1), 3778S–3784S. doi:10.1093/jn/133.8.2622
- Weber, E. J. (1987). Carotenoids and tocols of corn grain determined by HPLC. Journal of the American Oil Chemists’ Society, 64, 1129–1134. doi:10.1007/BF02612988
- Wheeler, E. L., & Ferrel, R. E. (1971). A method for phytic acid determination in wheat fractions. Cereal Chemistry, 48, 312–316.
