?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lichens (a composite organism) are known for their secondary metabolites and have several properties as photoprotection, allelopathy, antioxidant, antimicrobial, and antiviral. In this study, based on alarming situation of prevalence and developing resistance in dermatophytes, the new biological source in the form of lichens was screened for their antidermatophytic potential. Three dermatophytes viz. Microsporum canis, Trichophyton mentagrophytes, and Trichophyton rubrum were procured from Microbial Type Cell Culture, Chandigarh, India and susceptibility of aforementioned pathogens were tested via Clinical Laboratory and Standard Institute recommended broth microdilution procedure for filamentous fungi. Five lichens viz. Bulbothrix setschwanensis, Myelochroa aurulenta, Parmotrema nilgherrense, Parmotrema reticulatum, and Ramalina conduplicans were tested for their antidermatophytic activity (fungistatic and fungicidal concentrations) in the form of MIC and MFC values. M. aurulenta exhibited most promising MIC and MFC values against all dermatophytes and provides new leads in the form of secalonic acid A and leucotylic acid for future investigations.
Public Interest Statement
The developing resistance against first line clinical drug in pathogens is an evolutionary process for survival. Due to developing resistance in pathogens, we have to search for new drugs or compounds or herbals for our backup. We humans always rely on nature for our needs and mostly nature provides us solution in the form of any biological source. In present study, one of the less explored biological sources i.e. lichens were investigated. Lichens are a composite organism (composed of a fungus and a photobiont (algae and cyanobacteria or both)), slow growing in nature and acts as a pollution indicator. Lichens are used since long back in human civilization for their several benefits viz. antimicrobial, perfume, edible and many more. The present study enhances the existing knowledge and provides new leads for the cure of prevalent disease dermatophytosis (fungal infection of skin).
Competing Interests
The authors declare no competing interest.
1. Introduction
Filamentous keratinophilic fungi causing cutaneous mycoses are called dermatophytes and are classified into three genera, Trichophyton, Microsporum, and Epidermophyton (Peres, Maranhão, Rossi, & Martinez-Rossi, Citation2010). So far, about 30 species of dermatophytes have been identified among human pathogens (White, Oliver, Graser, & Henn, Citation2008). Dermatophytes are cosmopolitan in distribution. A study involving 16 European countries showed that 35–40% of the analyzed individuals had infection of the foot (tinea pedis) caused by dermatophytes (Burzykowski et al., Citation2003). A study involving children’s in US revealed that between 22 and 55% were having hair scalp infection of dermatophytes (Abdel-Rahman, Simon, Wright, Ndjountche, & Gaedigk, Citation2006). Although, the prevalence of drug resistance in dermatophytes is rare but resistance cases have been reported for griseofulvin, terbinafine, and fluconazole (Orozco et al., Citation1998; Peres et al., Citation2010; Smith et al., Citation1986; Stephenson, Citation1997; Wingfield, Fernandez-Obregon, Wignall, & Greer, Citation2004). In present study, based on the alarming situation documented in aforementioned literatures, the less explored biological source in the form of lichens was screened for their antidermatophytic potential. Lichen (a composite organism) thallus is a consortium of mycobiont and photobiont in a mutualistic relationship (Hawksworth, Citation2000). Apart from photobiont and mycobiont the lichen thallus is comprised of several other lichenicolous fungi, endophytic fungi, and bacteria (Grube & Berg, Citation2009). Lichens are known for their secondary metabolites which are quite unique to them and have several properties as photoprotection, allelopathy, antioxidant, antimicrobial, and antiviral (Molnar & Farkas, Citation2010).
2. Results and discussion
2.1. Percent yield
Percent yield obtained for Bulbothrix setschwanensis was highest i.e. 5.5%; Parmotrema nilgherrense was 4.5%; Parmotrema reticulatum was 4.45%; Myelochroa aurulenta was 3.75%; and least for Ramalina conduplicans i.e. 3.5%.
2.2. Antidermatophytic activity of lichens
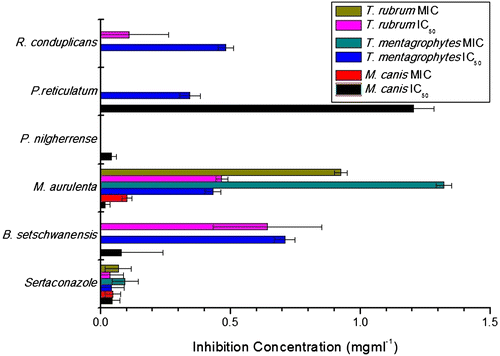
The antidermatophytic activity of various lichen extracts were tabulated in Table and graphically represented in Figure in the form of IC50 (concentration of drug/extract required to inhibit the pathogens growth up to 50 percent) and MIC (minimum concentration required to inhibit the growth of pathogens) values along with the standard drug used i.e. Sertaconazole. Sertaconazole nitrate exhibited very low MIC values in other studies against Trichophyton rubrum (1 μg ml−1) and Trichophyton mentagrophytes (8 μg ml−1) (Carrillo-Munoz, Tur-Tur, Cardenes, Estivill, & Giusiano, Citation2011). The difference in the MIC values is due to the size of inoculum. In the previous study, the initial inoculum size was 4.7 × 103 to 1.5 × 104 CFU/ml whereas in present study the inoculum size was ca 0.5 × 106 CFU/ml. Sertaconazole nitrate a highly active chemical drug having low fingistatic and fungicidal activities but it cause inflammation and itching to the patients (Liebel, Lyte, Garay, Babad, & Southall, Citation2006). Lichen extracts possess antidermatophytic activity but the most potent lichen extract was of M. aurulenta which had MIC values against all three pathogens present in this study. B. setschwanensis exhibited activity against all three pathogens but only 50% inhibition was achieved. R. conduplicans showed activity against Trichophyton spp. whereas; P. nilgherrense exhibited activity against Microsporum sp. and was least effective amongst all; P. reticulatum exhibited activity against Microsporum canis and T. mentagrophytes.
Table 1. Antidermatophytic activity of lichen extracts and reference standard i.e. sertaconazole
Fungicidal activities were reported in the form of minimum fungicidal concentration (MFC) in Sertaconazole and M. aurulenta. Sertaconazole exhibited fungicidal activity against M. canis and T. rubrum at 0.078 mg ml−1; 0.156 mg ml−1 for T. mentagrophytes; while M. aurulenta exhibited fungicidal concentration for M. canis at 0.156 mg ml−1 and 1.25 mg ml−1 for T. rubrum.
2.3. Statistical analysis
The significance level in the mean difference of control and treated columns in independent samples t-test are: Sertaconazole were 0.011 (M. canis), 0.047 (T. mentagrophytes), 0.025 (T. rubrum); B. setschwanensis were 0.011 (M. canis), 0.001 (T. mentagrophytes), 0.898 (T. rubrum); M. aurulenta were 0.029 (M. canis), 0.001 (T. mentagrophytes), 0.043 (T. rubrum); P. nilgherrense were 0.002 (M. canis); P. reticulatum were 0.277 (M. canis), 0.001 (T. mentagrophytes); R. conduplicans were 0.001 (T. mentagrophytes), 0.890 (T. rubrum).
Secondary metabolites reported from M. aurulenta are zeorin, atranorin, secalonic acid A, and leucotylic acid (Singh & Sinha, Citation2010). Among aforementioned compounds atranorin and zeorin exhibits weak antifungal properties. Atranorin doesn’t exhibit any activity against filamentous fungi but was found active against Candida albicans and Candida glabrata with MIC values of 500 μg for each (Turk, Yilmaz, Tay, Turk, & Kivanc, Citation2006; Yilmaz, Turk, Tay, & Kivanc, Citation2004). On the other hand, zeorin exhibited MIC values above 3 mg ml−1 against filamentous fungi (Marijana, Branislav, & Slobodan, Citation2010). Both the aforementioned compound either possess no or very weak antifungal activity. Thus, secalonic acid A and leucotylic acid need to be tested against dermatophytes or it might be possible that there was a synergy in between aforementioned compounds is the future leads of this present investigation.
3. Material and methods
3.1. Collection of lichens
Lichen thalli were collected from Chakrata district, Uttarakhand, India and identified with the help of relevant key (Awasthi, Citation2007). The voucher specimens were submitted in Central Regional Circle, Allahabad, Botanical Survey of India viz. B. setschwanensis (BSA-8763), M. aurulenta (BSA-8761), P. nilgherrense (BSA-8757), P. reticulatum (BSA-8762), and R. conduplicans (BSA-8759).
3.2. Preparation of lichen extracts
Two grams of lichen thalli were taken for cold extraction in 50% acetone (50 ml) (the solvent is a mixture of polar and non-polar solvent so that maximum amount of secondary as well as primary metabolites can diffuse into the solvent). Subsequently, the extracts were filtered by Whatman No. 1 filter paper and crude extract was obtained using rotary evaporator and weighed. The weight of crude extracts obtained were 0.11 g, 0.075 g, 0.09 g, 0.089 g, and 0.07 g for B. setschwanensis, M. aurulenta, P. nilgherrense, P. reticulatum, and R. conduplicans, respectively. Percent yield of crude extracts were calculated according to below mentioned formula:
Stock solutions (50 mg/ml) of crude extracts were prepared in dimethyl sulphoxide (DMSO) for antifungal susceptibility test.
3.3. Test pathogens and inocula preparation
Fungal cultures of M. canis (MTCC No. 3270), T. mentagrophytes (MTCC No. 7687), and T. rubrum (MTCC No. 296) were procured from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India, which were subcultured on SDA medium under laminar flow cabinet (Laminar flow ultra clean air unit, Micro-Filt, India). Inocula were prepared in saline media and then adjusted to a 0.5 McFarland standard, corresponding to ca 0.5 × 106 CFU/ml and transmittance of inoculum prepared was 70–72% at 520 nm for each culture (Santos, Barros, & Hamdan, Citation2006).
3.4. Antidermatophytic susceptibility test
Antidermatophytic susceptibility test was performed according to the Clinical Laboratory Standard Institute (CLSI) recommended broth microdilution method for filamentous fungi in RPMI-1640 medium HEPES modification (Sigma Aldrich) supplemented with MOPS buffer (3-morphollinopropane-1-sulfonic acid) (Qualigens Fine Chemicals) (Rex et al., Citation2008). Six 96-well plates were used separately i.e. five for lichen extracts and one for sertaconazole. Brief steps involved per plate were as follows: inocula prepared was diluted 1:50 times in testing media i.e. RPMI 1640; test was performed in 96-well flat-bottom microtitre plates; column 1 named as negative control consist of 100 μl RPMI-1640 broth media and 100 μl of inocula prepared and formaldehyde less than 1%; column 2 named as broth control consist of 200 μl of media; columns 3 and 4, 6 and 7, 9 and 10 were vertically diluted with extract having final concentration of 1.25, 0.625, 0.313, 0.156, 0.078, 0.039, 0.019, and 0.009 mg/ml and named as treated (The final concentration of DMSO is equal to and less than 2.5% which does not interfere the growth of tested pathogens); column 5, 8, and 11 were taken as positive control and contains only 100 μl inocula and 100 μl of RPMI-1640 broth media; column 12 named as extract control and contains vertically diluted extract/drug in the aforementioned concentrations. To encounter the color of extract, optical density (O.D.) of extract control was subtracted from treated columns corresponds to extract treated (Pathak et al., Citation2015). Percent inhibition was calculated using following equation:
Minimum Inhibition Concentrations (MICs) were calculated based on optical density recorded with a spectrophotometer (SpectraMax Plus384, Molecular Devices Corporation, USA) at 530 nm after 96 h incubated at 30 ± 2 ºC (Table ).
The antifungal activity of lichen acetone extracts was performed along with Sertaconazole Nitrate BP (Glenmark Pharmaceuticals, Nasik, India) as standard chemical. 50 mg ml−1 of stock solution of sertaconazole was prepared and tested at same concentrations as lichen extracts.
MFC was determined by classic method for microbial MBC determination method but with slight modification. 20 μl from treated columns from above MIC wells were transferred into 7 ml tubes of fresh RPMI 1640 medium. Tubes were incubated at 30 ± 2 ºC for 4 weeks and checked for the turbidity. Aforementioned procedure was performed with sertaconazole nitrate BP and the concentration at which no turbidity has been achieved was defined as MFC (Veinović et al., Citation2013).
3.5. Statistical analysis
Independent sample t-test was performed between the control and treated dermatophytes for the measure of Levene’s Test for Equality of Variances and t-test for equality of means via SPSS v20.
4. Conclusion
M. aurulenta exhibited antidermatophytic activity and provides new leads for the exploration of new compounds viz. secalonic acid A and leucotylic acid.
Acknowledgements
Thanks are due to Head, Department of Botany, University of Allahabad, Allahabad, India for providing library facilities; Dr D.K. Upreti, Scientist, National Botanical Research Institute-CSIR, Lucknow, India for determining the lichens; Dr G.P. Sinha, Head of Office, Central Regional Circle, Botanical Survey of India, Allahabad, India for providing accession numbers.
Additional information
Funding
Notes on contributors
Ashutosh Pathak
The main focus area of our research group is the bioprospection of new biological resources viz. plants, lichens and microbes, available in India and we are working in this particular field since last three decades. The other areas of interest of our group is microbiology of human pathogen and agricultural microbes; taxonomy of angiosperms and lichens. We are trying to develop new biological products for human welfare and have been succeeded in some which have been patented by us. Recently, the group is also working with synthesis, characterization, formulation of various metal, metal oxide and polymeric nanoparticles with their application in human as well as plant health.
References
- Abdel-Rahman, S. M., Simon, S., Wright, K. J., Ndjountche, L., & Gaedigk, A. (2006). Tracking Trichophyton tonsurans through a large urban child care center: Defining infection prevalence and transmission patterns by molecular strain typing. Pediatrics, 118, 2365–2373.10.1542/peds.2006-2065
- Awasthi, D. D. (2007). A compendium of the macrolichens from India, Nepal and Sri Lanka. Dehra Dun: Bishen Singh Mahendra Pal Singh. ISBN 978-81-211-0600-9.
- Burzykowski, T., Molenberghs, G., Abeck, D., Haneke, E., Hay, R., Katsambas, A., ... Marynissen, G. (2003). High prevalence of foot diseases in Europe: Results of the Achilles Project. Mycoses, 46, 496–505.10.1046/j.0933-7407.2003.00933.x
- Carrillo-Munoz, A. J., Tur-Tur, C., Cardenes, D. C., Estivill, D., & Giusiano, G. (2011). Sertaconazole nitrate shows fungicidal and fungistatic activities against Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum, causative agents of tinea pedis. Antimicrobial Agents and Chemotherapy, 55, 4420–4421.10.1128/AAC.00219-11
- Grube, M., & Berg, G. (2009). Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biology Reviews, 23, 72–85.10.1016/j.fbr.2009.10.001
- Hawksworth, D. L. (2000). Freshwater and marine lichen-forming. In K. D. Hyde, W. H. Ho, & S. B. Pointing (Eds.), Aquatic mycology across the millennium, (Vol. 5, pp. 1–7). Hong Kong: Fungal Diversity.
- Liebel, F., Lyte, P., Garay, M., Babad, J., & Southall, M. D. (2006). Anti-inflammatory and anti-itch activity of sertaconazole nitrate. Archives of Dermatological Research, 298, 191–199.10.1007/s00403-006-0679-8
- Marijana, K., Branislav, R., & Slobodan, S. (2010). Antimicrobial activity of the lichen Lecanora frustulosa and Parmeliopsis hyperopta and their divaricatic acid and zeorin constituents. African Journal of Microbiology Research, 4, 885–890.
- Molnar, K., & Farkas, E. (2010). Current results on biological activities of lichen secondary metabolites: A Review. Zeitschrift für Naturforschung C, 65, 157–173.
- Orozco, A., Higginbotham, L., Hitchcock, C., Parkinson, T., Falconer, D., Ibrahim, A., … Filler, S. G. (1998). Mechanism of fluconazole resistance in Candida krusei. Antimicrobial Agents and Chemotherapy, 42, 2645–2649.
- Pathak, A., Shukla, S. K., Pandey, A., Mishra, R. K., Kumar, R., & Dikshit, A. (2015). In vitro antibacterial activity of ethno medicinally used lichens against three wound infecting genera of enterobacteriaceae. Proceedings of National Academy of Sciences India Section B. doi:10.1007/s40011-015-0540-y
- Peres, N. T. A., Maranhão, F. C. A., Rossi, A., & Martinez-Rossi, N. M. (2010). Dermatophytes: Host-pathogen interaction and antifungal resistance. Anais Brasileiros de Dermatologia, 85, 657–667. doi:10.1590/S0365-05962010000500009
- Rex, J. H., Alexander, B. D., Andes, D., Arthington-Skaggs, B., Brown, S. D., Chaturveli, V., … Walsh, T. J. (2008). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi (Approved Standard-2nd ed., Vol. 28(16)). Clinical and Laboratory Standard Institute (CLSI), M38-A2.
- Santos, D. A., Barros, M. E. S., & Hamdan, J. S. (2006). Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. Journal of Clinical Microbiology, 44, 98–101.10.1128/JCM.44.1.98-101.2006
- Singh, K. P., & Sinha, G. P. (2010). Indian lichens: An annotated checklist. Kolkata: Botanical Survey of India. ISBN 978-81-8177-036-3.
- Smith, K. J., Warnock, D. W., Kennedy, C. T. C., Johnson, E. M., Hopwood, V., van Cutsem, J., & Vanden Bossche, H. (1986). Azole resistance in Candida albicans. Medical Mycology, 24, 133–144.10.1080/02681218680000201
- Stephenson, J. (1997). Investigators seeking new ways to stem rising tide of resistant fungi. JAMA: The Journal of the American Medical Association, 277, 5–6.10.1001/jama.1997.03540250013006
- Turk, H., Yilmaz, M., Tay, T., Turk, A. O., & Kivanc, M. (2006). Antimicrobial activity of extracts of chemical races of the lichen Pseudoevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetoric acid constituents. Zeitschrift für Naturforschung C, 61, 499–507.
- Veinović, G., Cerar, T., Strle, F., Lotrič-Furlan, S., Maraspin, V., Cimperman, J., & Ružić-Sabljić, E. (2013). In vitro susceptibility of European human Borrelia burgdorferi sensu stricto strains to antimicrobial agents. International Journal of Antimicrobial Agents, 41, 288–291.10.1016/j.ijantimicag.2012.11.016
- White, T. C., Oliver, B. G., Graser, Y., & Henn, M. R. (2008). Generating and testing molecular hypotheses in the dermatophytes. Eukaryotic Cell, 7, 1238–1245.10.1128/EC.00100-08
- Wingfield, A. B., Fernandez-Obregon, A. C., Wignall, F. S., & Greer, D. L. (2004). Treatment of tinea imbricata: A randomized clinical trial using griseofulvin, terbinafine, itraconazole and fluconazole. British Journal of Dermatology, 150, 119–126.10.1111/bjd.2004.150.issue-1
- Yilmaz, M., Turk, A. O., Tay, T., & Kivanc, M. (2004). The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (-)-Usnic acid, Atranorin, and Fumaroprotocetraric acid constituents. Zeitschrift für Naturforschung C, 59, 249–254.