 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Swift quarantine offers some hope in the fight against Ebola but its implementation faces some resistance in many settings. Hence, it is critical to explore whether introducing pre-exposure vaccination in an area where quarantine for the exposed and infected is already practiced would benefit the community with regard to controlling Ebola virus disease and vice versa. We present a mathematical model that explores the potential role of pre-exposure vaccination and quarantine in the fight against Ebola. Threshold parameter of the model is computed and rigorously analysed. Sensitivity analysis is carried out in an effort to understand the effects of constituent parameters on the threshold parameter. The results indicate that pre-exposure and quarantine are able to reduce the disease threshold parameter suggesting they offer hope of Ebola virus disease control.
Public Interest Statement
Currently there is no vaccine against Ebola epidemics, in this manuscript the potential benefits of a yet to be developed; pre-exposure vaccine against Ebola is explored using a mathematical model. The manuscript will also explore other possible intervention strategies (quarantine and quick and safe burial of the infected dead).
Competing Interests
The authors declare no competing interests.
1. Introduction
The current Ebola virus disease outbreak in west Africa threatens the global human health hence it urgently needs to be controlled. Since its discovery in 1976 in Zaire—now the Democratic Republic of Congo (CDC, Citation2014)—more than 25 outbreaks of Ebola virus disease have been recorded (Camacho et al., Citation2014). From 11 January 2014, there have been 21,296 probable, confirmed and suspected Ebola cases, and 8,429 deaths, (WHO, Citation2014a) with a case fatality ratio of about 70%. So far, quarantine is one of the intervention strategies that is widely employed to bring the epidemic under control. While quarantine has proved successful in some settings it remains controversial in others. This then calls for the need to explore other intervention strategies.
Currently, there is no vaccine for Ebola virus licenced for human use but some advances made and efficacy studies in non-human primates on several platforms have been encouraging (Centre for Infectious Disease Research and Policy [CIDRAP], Citation2015; Feldmann et al., Citation2007; Sullivan, Sanchez, Rollin, & Yang, Nabel, Citation2000; Sullivan et al., Citation2003). It is worth reporting that very little progress has been made in developing treatment interventions for Ebola virus infections (Bray & Paragas, Citation2002; Feldmann, Jones, Schnittler, & Geisbert, Citation2005; Geisbert & Hensley, Citation2004). Currently, there are a number of vaccines at different stages of development. These include, but are not limited to: (a) cAd3-ZEBOV (also known as the NIAID/GSK Ebola vaccine or cAd3-EBO Z), a GlaxoSmithKline (GSK) vaccine which began in 2011 with clinical trials currently under way in USA, Europe and Africa (CIDRAP, Citation2015). (b) Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus (rVSV-ZEBOV), a live-virus replication competent vaccine and some experts have raised concern about viral shedding which could pose a threat to livestock and possibly humans (CIDRAP, Citation2015). However, animal studies have not demonstrated VSV shedding post-vaccination (Geisbert & Fieldmann, Citation2004) and rVSV vaccines on non-human primates have demonstrated efficacy against infection, both pre- and post-exposure (Geisbert & Fieldmann, Citation2004). Phase 1 clinical trials of this vaccine were stopped when 20% of the participants had experienced joint symptoms (WHO, Citation2014b). (c) Ad26.ZEBOV/MVA-BN-filo, which is currently under phase 1 clinical trials in UK (CIDRAP, Citation2015).
Following up from previous mathematical studies on Ebola (Camacho et al., Citation2014; Fasina et al., Citation2014; Ndanguza, Tchuenche, & Haario, Citation2013 and other references cited there in) we explore the role of dual intervention strategies, that is pre-exposure vaccination and quarantine, in the control of Ebola virus disease. While we acknowledge the fact that to date no vaccine has been licenced for human use, efforts are underway to develop novel pre-exposure drugs. This justifies the need to develop a mathematical model that gives insight into the potential role of pre-exposure vaccination in the fight against Ebola. Here we present such a model that allows us to test the impact of pre-exposure vaccination in the presence of quarantine or quarantine in the presence pre-exposure vaccination. To our knowledge, this is the first time these intervention strategies have been explored simultaneously.
2. Model description
The population is divided into the following classes: unvaccinated susceptibles , vaccinated susceptibles
, exposed and not yet detected individuals
, exposed and quarantined individuals
, infectious not yet quarantined
, quarantined infectives
, recovered individuals R(t), dead and not yet buried D(t). The total human population is given by
Unvaccinated and vaccinated susceptibles are infected with Ebola virus at rates and
, (
accounting for reduction in susceptibility to infection for the vaccinated), respectively, to enter the exposed class
. Here,
(1)
(1)
where is the community contact rate,
is the funeral contact rate,
accounts for the reduction in infectivity for those not yet displaying the clinical signs of the disease. Unvaccinated susceptibles are vaccinated at a rate
and for the vaccinated the vaccine wanes at a rate
. Individuals in
-class are detected and put on quarantine at a rate
to enter the
-class. Individuals in the
and
classes develop clinical Ebola symptoms at a rate
to enter the
and
-classes, respectively. Infectives not yet quarantined
are quarantined at a rate
to enter the
-class. Those
and
-classes die at a rate
due to the disease. However, for Those who die in class
and
they enter the class of the dead D(t) and are buried at a rate
. It is important to note that some infectives (
and
) recover at a rate
to enter the recovered class R(t). Unless otherwise stated, values used in the analysis and simulations are given in Table .
Table 1. Default (baseline) model parameters used in the analysis and simulations
The following system of equations describe the model.(2)
(2)
The threshold parameter of model system (Equation 2) that governs the spread of Ebola is given by(3)
(3)
where which is a threshhold parameter which determines whether or not the disease (Ebola) will invade the host population the presence of pre-exposure vaccination for the uninfected and quarantine for the infected. If is less than unity then Ebola will be under control and if it is not then there will be an outbreak of it.
and
represent the contribution of infected corpses and the infected humans to Ebola epidemics.
2.1. Analysis of the threshold parameter, 

In the absence of any intervention strategy we have(4)
(4)
2.1.1. Effects of pre-exposure vaccination
If pre-exposure vaccination is the only intervention then(5)
(5)
Subtracting Equation (5) from Equation (4) we obtain(6)
(6)
Thus, the limiting value of when there is no intervention is greater than the limiting value of
when pre-exposure vaccination is the only intervention (
. Thus, pre-exposure vaccination reduces
. A reduction in
translates to a decrease in disease prevalence. This result points to need to expedite research on the development of pre-exposure vaccines in order for the world to defeat the spread of the deadly Ebola virus.
2.1.2. Effects of quarantine
If quarantine is the only intervention then(7)
(7)
Subtracting Equation (7) from Equation (4) we obtain(8)
(8)
Thus, the limiting value of when there is no intervention is greater than the limiting value of
when quarantine for the exposed and infected individuals is the only intervention (
. Thus, quarantine reduces
. A reduction in
translates to a decrease in disease prevalence. From this result we can conclude that even as the only intervention strategy, quarantining the exposed and infected individuals has the potential to control this deadly infection. However, it’s implementation faces some resistance challenges. There is need to understand if introducing pre-exposure vaccination in an area where quarantine for the exposed and infected is already practiced would benefit the community with regard to controlling Ebola virus disease. Comparing
and
we have
(9)
(9)
Thus Equation (9) is positive suggesting that introducing pre-exposure vaccination in an area where quarantine for the exposed and infected individuals is already present will be beneficial to the community. Furthermore, we explore the implications of introducing quarantine for exposed and the infected individuals in an area where pre-exposure vaccination is practiced. Comparing
and
we have
(10)
(10)
Thus, the limiting value of when pre-exposure vaccination is the only intervention is greater than
(
. Thus, quarantine reduces
. This result suggests that introducing quarantine in an environment in which pre-exposure vaccination is in place will assist in reducing Ebola transmission in the community. Finally, we explore the implications of introducing pre-exposure vaccination in an environment where quarantine for the exposed and infected is in place. Comparing Comparing
and
we have
(11)
(11)
The fact that the limiting value of when quarantine is the only intervention is greater than
(
, suggests that introducing pre-exposure in an environment in which quarantine was already would be beneficial to the community.
3. Sensitivity analysis
There are many types of sensitivity analyses that may be used to assess uncertainty associated with a cost–benefit study which includes partial derivatives, a sensitivity index, a relative deviation ratio, partial rank correlation coefficients, rank regression coefficients and the squared-ranks test among others. However, in this manuscript, we are to use Latin Hypercube Sampling and Partial Rank Correlation Coefficients (PRCCs) with 1,000 simulations per run to account for the effects of variations in to its constituent parameters.
Figure 1. Partial rank correlation coefficients showing the effect of parameter variations on using ranges in the table. Notes: Parameters with positive PRCCs will increase
when they are increased, whereas parameters with negative PRCCs will decrease
when they are increased.
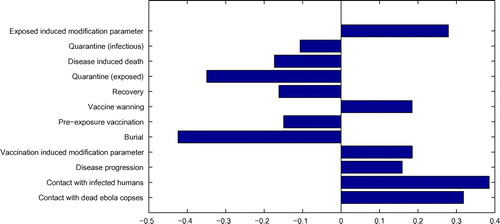
PRCCs illustrate the degree of the effect that each parameter has on the outcome. Figure illustrates the PRCCs using as an output variable. The parameters with the greatest effect on the outcome are burial and quarantine for of the exposed suggesting that their increase will have a positive impact in controlling the spread of Ebola. This result calls for quick and safe burial of all corpses suspected to have been killed by Ebola virus. Furthermore, pre-exposure vaccination is also shown to have an effect on the outcome, its increase results in a decrease in the threshold parameter,
. A decrease in
suggests a decrease in disease prevalence.
Figure 2. Monte Carlo simulations of 1,000 sample values for six illustrative parameters (,
,
,
,
and
) chosen via Latin hypercube sampling.
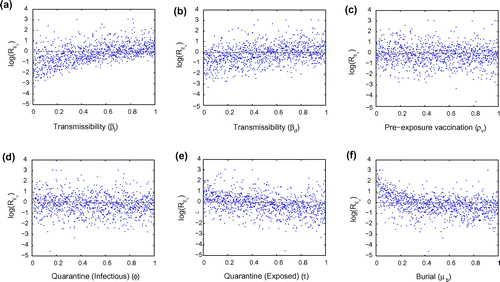
Figure illustrates the effect that varying six sample parameters will have on . Results from Figure show that increase in quarantining the exposed (
) and quick and safe burial of corpses who died due to Ebola (
) reduces
to levels below unity. A reduction in
to levels below unity is a sufficient condition for disease control. Results from Figure (a) and (b) show that Ebola transmission is mostly driven by live infectious cases as opposed to infected corpses.
4. Discussion
A mathematical model is developed to explore the potential impact of the “new” pre-exposure vaccine and quarantine of the infected and the exposed in controlling the spread of Ebola virus. Analysis of the threshold parameter () suggests that pre-exposure vaccination or quarantine for the exposed/ infected can single handedly afford to control Ebola virus transmission under control. Furthermore, analysis of the threshold parameter (
) shows that quarantine (pre-exposure vaccination) coupled with pre-exposure vaccination (quarantine) does better than either of the single strategies. Sensitivity analysis (PRCCs and Monte Carlo simulations) show that the threshold parameter (
) is a decreasing function of quarantine (for the exposed/infectious), pre-exposure vaccination and quick and safe burial of the infected corpses. This is in agreement with the analytic results obtained, that is sensitivity analysis and analytic results go hand in hand. Against this background, there is need for researchers to strengthen their research towards producing a novel pre-exposure vaccine which when used together with other conventional intervention strategies like will be able to keep Ebola virus infections under control. However, in the current set-up there is a need to upscale quarantine as well as quick and safe burial of the corpses suspected to have died due to Ebola virus infection. The model presented in this manuscript gives insight into the dynamics of Ebola in the presence of quarantine and vaccination. The results assist decision-makers to make informed decisions regarding Ebola control. This study can be further improved by incorporating the aspects associated with the costs and benefits of exploring new pre-exposure vaccine and quarantine in the fight against Ebola.
Acknowledgements
The author thanks the handling editor and reviewers for their insighful comments which improved the manuscript.
Additional information
Funding
Notes on contributors
C.P. Bhunu
C.P. Bhunu (BSc Hons, MSc, DPhil) is a professor in the Department of Mathematics, University of Zimbabwe. He currently serves as an external examiner for Chinhoyi University of Technology, Harare Institute of Technology and Zimbabwe Open University. He was a visiting African scientist at Cambridge Infectious Disease Consortium (2010) and a visiting professor at the University of Venda (2012), respectively. He is a life member of the Clare Hall College, University of Cambridge. He also serves as an editor and reviewer of several international journals in Applied Mathematics. His research interests lie in the field of mathematical modelling of issues affecting mankind ranging from social issues to biological issues as well as the theoretical analysis of the mathematical models that arise in all these applications.
References
- Bhunu, C. P. (2015). Assessing the potential of pre-exposure vaccination and chemoprophylaxis in the control of lymphatic filariasis. Applied Mathematics and Computation, 250, 571–579.
- Bray, M., & Paragas, J. (2002). Experimental therapy of lovirus infections. Antiviral Research, 54, 1–17.
- Camacho, A., Kucharski, A. J., Funk, S., Breman, J., Piot, P., & Edmunds, W. J. (2014). Potential for large outbreaks of Ebola virus disease. Epidemics, 9, 70–78.
- Centre for Infectious Disease Research and Policy. (2015). Recommendations for accelerating the development of Ebola vaccines: Report and analysis.
- CDC. (2014). Outbreaks chronology: Ebola Hemorrhagic fever. Retrieved from http://www.cdc.gov/vhf/ebola/resourses/outbreak-table.html
- Fasina, F. O., Shittu, A., Lazarus, D., Tomori, O., Simonsen, L., Viboud, C., & Chowell, G. (2014). Transmission dynamics and control of Ebola virus disease disease in Nigeria. Rapid Communications.
- Feldmann, H., Jones, S. M., Schnittler, H. J., & Geisbert, T. (2005). Therapy and prophylaxis of Ebola virus infections. Current Opinion in Investigational Drugs, 6, 823–830.
- Feldmann, H., Jones, S. M., Daddario-DiCaprio, K. M., Geisbert, J. B., Str{\”o}her, U., Grolla, A., ... Geisbert, T. W. (2007). Effective post-exposure treatment of Ebola infection. PLOS Pathogens, 3(1), e2. doi:10.1371/journal.ppat.0030002
- Geisbert, T. W., & Hensley, L. E. (2004). Ebola virus: New insights into disease aetiopathology and possible therapeutic interventions. Expert Reviews in Molecular Medicine, 6, 1–24.
- Geisbert, T. W., & Fieldmann, H. (2004). Recombinant vesicular stomatis virus-based vaccines against Ebola and Marburg virus infections. Journal of Infectious Diseases, 204, S1075–S1081.
- Ndanguza, D., Tchuenche, J. M., & Haario, H. (2013). Statistical data analysis of the 1995 Ebola out-break in the Democratic Republic of Congo. Afrika Matematika, 24, 55–68.
- Sullivan, N. J., Geisbert, T. W., Geisbert, J. B., Xu, L., Yang, Z. Y., Roederer, M., ... Nabel, G. J. (2003). Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature, 424, 681–684.
- Sullivan, N. J., Sanchez, A., Rollin, P. E., Yang, Z. Y., & Nabel, G. J. (2000). Development of a preventive vaccine for Ebola virus infection in primates. Nature, 408, 605–609.
- WHO. (2014a). Disease outbreak news. Geneva: Author.
- WHO. (2014b). Third teleconference on vaccine clinical trials design for Guinea, Liberia and Sierra Leon. Geneva: Author.
