?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the protective effects of quercetin (QUER) in sodium azide (SAZ)-induced extra-hepatic (brain, lung, heart) oxidative stress in rats. SAZ administration significantly decreased and increased brain and lung GSH levels respectively, but was restored to levels comparable to normal control by QUER, while no significant difference was seen in heart GSH level. Brain and heart GST activities, as well as lung CAT activity were significantly reduced in SAZ-administered rats compared to normal control rats, but were significantly restored to normalcy by QUER treatment in heart only, while the significant increase in lung GST activity, as well as brain and heart CAT activities in SAZ-administered rats compared to control, were significantly attenuated by QUER treatment. A significant decrease in the brain, lung, and heart SOD activities, as well as brain and lung GPx activities in SAZ group compared to control, was recorded, but treatment with QUER significantly raised the activities of the enzymes back to normal. We therefore concluded from the findings of this study that quercetin could be a good candidate for the chemoprevention of SAZ-induced extra-hepatic oxidative stress.
Public Interest Statement
Sodium azide is a colorless, inorganic compound commonly used in our environment. It is a gas forming component in car airbag systems, an explosive, and used in organic synthesis to form azide functional group. In the field of biochemistry and biomedicine, it serves as preservative and a useful probe reagent. In hospitals, it serves as a biocide, while in agriculture, it can be used to control pest. Hence, the aim of the present study was to investigate the possible treatment of sodium azide-induced extra-hepatic oxidative stress by quercetin (a polyphenolic compound present in fruits and spices) in rats, which will further encourage the use of natural products than chemically synthesized drugs in the treatment and management of diseases.
Competing Interests
The authors declare no competing interest.
1. Introduction
Sodium azide (SAZ) is a water-soluble, colorless, and crystalline solid, manufactured as a very fine powder (Rippen et al., Citation1996). Crystalline SAZ is an explosive that can decompose on heating, emitting toxic fumes (Health & Safety Executive, Citation2000). It is used as a preservative in aqueous laboratory reagents, as a gas generating chemical in automobile airbags and seat ejectors of jet planes. It is acutely toxic, and can inhibit the activities of catalase, cytochrome oxidase, and peroxidases by binding to iron III (Fe3+) in porphyrin complexes (National Toxicology Program, Citation1991). SAZ can penetrate the blood–brain barrier (Smith, Louis, Kruszyna, & Kruszyna, Citation1991). Azide anions may be metabolized to nitric oxide (NO) in the central nervous system (CNS) (Health & Safety Executive, Citation2000). In aqueous solution, it is rapidly transformed into hydrazoic acid, responsible for the irritating effects attributed to SAZ (Graham, Citation1949; Haas & Marsh, Citation1970). SAZ is quickly absorbed from injection sites and respiratory tract (Bassendowska & Kowalski, Citation1962; Reinhardt & Brittelli, Citation1981). Exposure to SAZ canister explosion resulted in severe systemic toxicity and death (Pham, Palmieri, & Greenhalgh, Citation2001). SAZ-exposed patients developed hypotension, hypothermia, bradycardia and a profound metabolic acidosis causing death in about 12 h after exposure. NO is the metabolite mainly responsible for the toxic effects of SAZ (Eyer, Citation1994).
Medicinal properties of plants and plant derivatives have been exploited in the treatment of different ailments, and one of such is quercetin. Generally, studies on flavonoids are widely geared to focus on quercetin (Chirumbolo, Citation2010). Quercetin sources are largely abundant and easy to extract, isolate, and detect (Papiez et al., Citation2008).
Quercetin (QUER) (3, 3', 4', 5, 7-pentahydroxy flavones), a flavonoid, is a polyphenolic compound that are found exclusively in plants. These compounds are able to elicit various biological and pharmacological activities in animal cells (Alrawaiq & Abdullah, Citation2014). QUER, an abundant flavonoid in human diet, is a strong reactive oxygen species (ROS) scavenger and good metal chelator (Patra, Rautray, & Swarup, Citation2011). It is rich in phenolic hydroxyl groups that have strong antioxidant activity (Martınez-Florez, Gonzalez-Gallego, Culebras, et al., Citation2002; Tokyol, Yilmaz, Kahraman, Çakar, & Polat, Citation2006). High concentrations of QUER are found in apples, onions, potatoes, broccoli, tea, soybeans, and red wine. QUER has very potent antioxidant and cytoprotective effects in preventing endothelial apoptosis caused by oxidants (Choi, Kang, Park, et al., Citation2003; Renugadevi & Milton Prabu, Citation2010). QUER has been used to treat hepatotoxicity, liver fibrosis, and many diseases (Amália, Possa, Augusto, & Francisca, Citation2007; Gonzalez-Gallego, Sanchez-Campos, & Tunon, Citation2007; Tieppo et al., Citation2009).
The protective role of QUER against SAZ-induced hepatic and splenic oxidative stress has been reported (Somade et al., Citation2015). Therefore, we investigated the protective effects of QUER, a flavonoid and antioxidant in food, spices, wine, and fruits, on SAZ-induced oxidative stress in extra-hepatic tissues of rats.
2. Materials and methods
2.1. Test substance and kits
SAZ and QUER (≥ 98% purity) used in this study were of analytical grade, product of Sigma Chemical Co., Saint Louis, MO, USA. Creatinine, urea, uric acid, total cholesterol (T-CHOL), triacylglycerol (TG), and high density lipoprotein cholesterol (HDLC) commercial kits are product of Cypress Diagnostics, Langdorp, Belgium.
2.2. Experimental animals and study design
Twenty male wistar albino rats of an average weight of 150 g used for this study were obtained from the animal house of the College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria. They were housed in steel metal cages in the animal house of our department and were served food and water ad libitum. Permission to use the animals was approved by the Institution’s Animal Ethical Committee. The rats were divided randomly into four groups (I–IV) of five animals each. Group I animals serve as normal control, group II animals were administered a single intraperitoneal dose of 20 mg/kg SAZ for 48 h, group III animals were also administered same as group II, but treated with 100 mg/kg QUER orally for seven days, while group IV animals were administered 100 mg/kg QUER only for seven days.
We selected the dose of 100 mg/kg quercetin based on its effect in previous study (Guzy et al., Citation2003; Somade et al., Citation2015), while doses of intraperitoneal injection between 15 and 150 mg/kg of SAZ into rats caused symptoms such as bradycardia or dyspnea, salivation, paralysis, tremor, convulsions, and later death (Abbanat & Smith, Citation1964; Burger & Bauer, Citation1965).
2.3. Sample collections and preparations
At the end of the experimental period, the animals were sacrificed. They were handled and used in accordance with the international guide for the care and use of laboratory animals (National Research Council, Citation1996). Blood was collected via abdominal artery into clean heparinized tubes followed by centrifugation at 3,000 rpm for 10 min. Plasma was separated into clean 1-ml Eppendorf tubes, and stored at −18°C until when used. Also, brain, lung, and heart were harvested, they were washed in ice-cold saline (0.9% w/v) solution, blotted dry, and weighed, after which they were suspended in ice-cold 0.1 M phosphate buffer (pH 7.4) for homogenization. Homogenization is then followed by centrifugation at 5,000 rpm for 10 min. The homogenate was then used immediately for the analysis of oxidative stress parameters.
2.4. Assay for biochemical parameters
Plasma concentrations of T-CHOL, TG, HDLC, creatinine, urea, and uric acid were determined according to the methods described in Cypress Diagnostics Kits, Langdorp, Belgium.
2.5. Determination of CAT activity
Activity of catalase was determined by the method of Aebi (Citation1974). Sample (0.1 ml) was added to quartz cuvette containing 1.9 ml of 10 mM phosphate buffer (pH 7.0). Reaction was initiated by the addition of 1.0 ml of freshly prepared 30 mM hydrogen peroxide (H2O2). The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm.
2.6. Determination of SOD activity
SOD activities in harvested tissues were determined by the method of Misra and Fridovich (Citation1972). The method is based on the ability of superoxide dismutase to inhibit auto-oxidation of adrenaline to adrenochrome at alkaline pH. The unit of enzyme activity is defined as the enzyme required for 50% inhibition of adrenaline auto-oxidation.
2.7. Determination of GPx activity
GPx activities were determined by the method of Rotruck et al. (Citation1973) where the color developed was read at 412 nm.
2.8. Determination of GST activity
Activities of brain, lung, and heart glutathione S-transferase (GST) were determined by the method of Habig, Pabst, and Jakoby (Citation1974) based on enzyme-catalyzed condensation of glutathione with the model substrate, 1-chloro-2,4-dinitrobenzene. The product formed (2,4-dinitrophenylglutathione) absorbs light at 340 nm.
2.9. Determination of MDA level
MDA concentrations, a marker of lipid peroxidation (LPO) were determined by the method of Buege and Aust (Citation1978). In this procedure, 1.0 ml of the supernatant was added to 2 ml of trichloroacetic acid–thiobarbituric acid–hydrochloric acid (TCA/TBA/HCl) (1:1:1 ratio) reagent, boiled at 100°C for 15 min, and allowed to cool. Flocculent materials were removed by centrifuging at 3,000 rpm for 10 min. The supernatant was removed and the absorbance read at 532 nm against blank. MDA concentration was calculated using the molar extinction coefficient for MDA-TBA complex of 1.55 × 106 M−1 cm−1.
2.10. Determination of GSH level
Brain, lung, and heart reduced glutathione (GSH) levels were determined by the method of Moron, Depierre, and Mannervik (Citation1979), where the color developed was read at 412 nm.
2.11. Determination of total protein concentrations
Total protein concentrations in brain, lung, and heart were determined by the method of Gornall, Bardawill, and David (Citation1949).
2.12. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA), followed by least significant difference (LSD) to test for significant differences among the groups of rats using Statistical Package for Social Sciences program version 17.0. Data were expressed as mean ± standard error of mean. p values less than 0.05 were considered statistically significant.
3. Results
3.1. Results of relative brain, lung, and heart weights
Our results revealed a significant decrease (p < 0.05) in relative brain and lung weights in SAZ-administered rats compared to normal control rats, but treatment with QUER was able to restore the relative weights in both tissues (Table ), while no significant (p > 0.05) difference was seen in the relative heart weight (Table ).
Table 1. Relative organ weights of quercetin treatments in NaN3-induced toxicity in rats
3.2. Plasma total protein, CHOL, TG, HDLC, urea, uric acid, and creatinine
The significant (p < 0.05) decrease in plasma total CHOL caused by SAZ administration compared to control was not significantly increased (p > 0.05) by QUER treatment (Table ), while no significant (p > 0.05) difference was seen in plasma concentrations of TG and HDLC (Table ). Also, administration of SAZ significantly (p < 0.05) increased the plasma concentrations of protein, urea, uric acid, and creatinine, when compared with the control animals (Table ). QUER treatment was able to significantly (p < 0.05) attenuate the concentration of these parameters to levels comparable with control (Table ).
Table 2. Protection of quercetin on some plasma biochemical parameters in NaN3-induced toxicity
Table 3. Nephroprotection of quercetin in NaN3-induced renal toxicity in plasma of rats
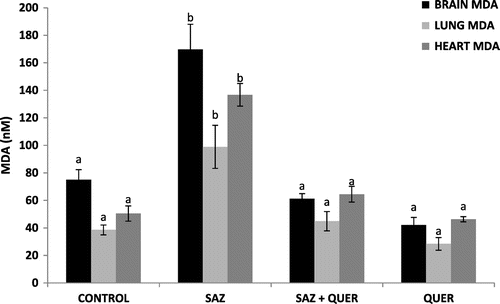
3.3. Treatment of NaN3-induced lipid peroxidation
There was a significant (p < 0.05) increase in the concentrations of brain, lung, and heart MDA in SAZ-administered rats compared to control, but treatment with QUER significantly (p < 0.05) decreased the MDA concentrations in these tissues to levels comparable to control (Figure ).
3.4. Treatment of NaN3-induced oxidative stress
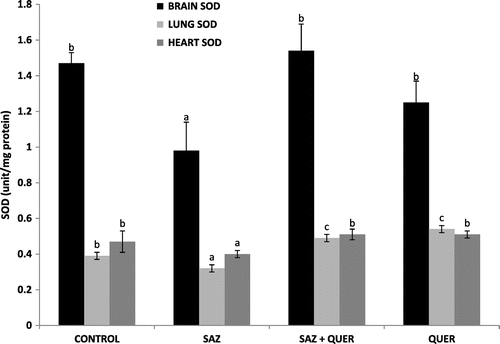
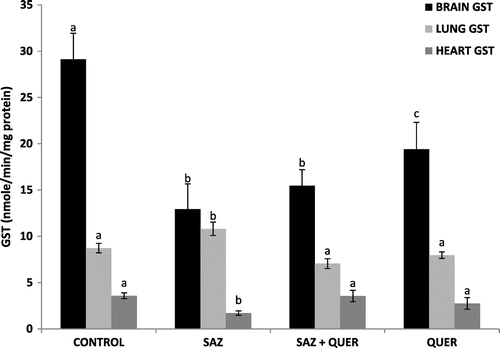
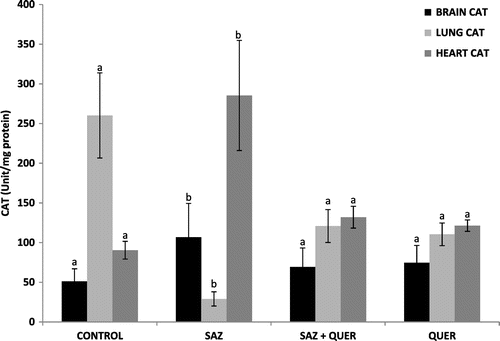
A significant (p < 0.05) decrease and increase in brain and lung GSH levels in SAZ-administered rats compared to control rats were recorded respectively, but treatment with QUER significantly (p < 0.05) increased the GSH concentration in brain, and did not significantly reduce (p > 0.05) lung GSH level, while no significant difference (p > 0.05) in heart GSH level was seen in all groups (Figure ). Activities of brain and lung GPx were significantly (p < 0.05) decreased by SAZ administration compared to control rats (Figure ), and were also significantly (p < 0.05) restored to levels comparable to control by QUER treatment (Figure ). Heart showed no significant (p > 0.05) difference in GPx activity in all the groups (Figure ). CAT activities in brain and heart (Figure ) were significantly increased (p < 0.05) following SAZ administration compared to control, but were also significantly attenuated (p < 0.05) after treatment with QUER. In the lung, the reverse is the case, as SAZ administration was accompanied by a significant decrease (p < 0.05) in CAT activity compared to control (Figure ). QUER treatment was able to restore the activity to normalcy (Figure ). SOD activities in brain, lung, and heart were significantly (p < 0.05) reduced by SAZ administration compared to control animals (Figure ), and was significantly (p < 0.05) increased back to normal by treatment with QUER (Figure ). Lastly, a significant (p < 0.05) decrease in brain and heart GST activities in SAZ-administered rats compared to control rats were recorded (Figure ). Treatment with QUER significantly (p < 0.05) increased GST activity in heart only, while the significant (p < 0.05) increase in lung GST activity compared to control rats was reduced significantly (p < 0.05) by QUER treatment (Figure ).
Figure 2. Effects of quercetin on brain, lung, and heart GSH levels in NaN3-treated rats.
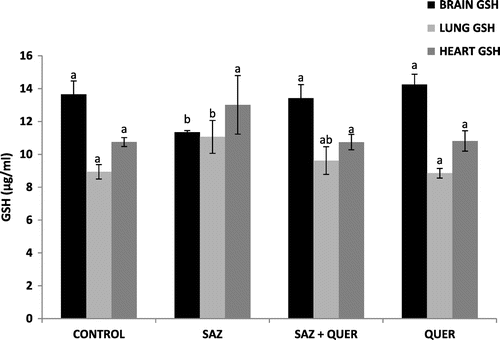
Figure 3. Effects of quercetin on brain, lung, and heart GPx activities in NaN3-treated rats.
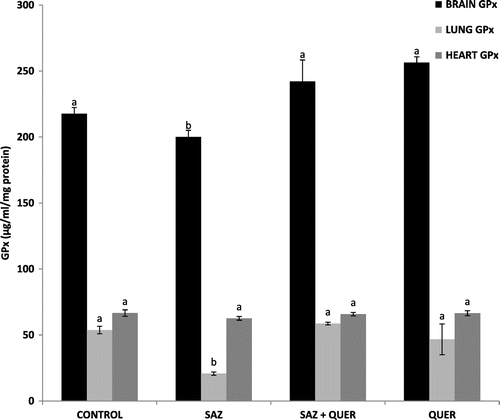
Figure 4. Effects of quercetin on brain, lung, and heart CAT activities in NaN3-treated rats.
4. Discussion
The present study has investigated the protective roles of QUER, a flavonoid, in SAZ-induced oxidative stress in rats. The significant decrease (p < 0.05) in relative brain and lung weights following SAZ administration (Table ) may indicate direct toxic effects on these tissues, while the protective effects of QUER on these tissues may be due to its antioxidant and anti-apoptotic properties (Cheng et al., Citation2009; Lugli et al., Citation2009).
The significant decrease (p < 0.05) in plasma cholesterol and non-significant decrease (p < 0.05) in plasma triglyceride (Table ) caused by SAZ administration may be due to its hypotensive properties as reported by Pham et al. (Citation2001). Also, treatment of SAZ-induced renal toxicity as marked by significant increase (p < 0.05) in the plasma concentrations of creatinine, urea, and uric acid, by QUER has confirmed the nephroprotection of this flavonoid (Table ). Our findings on renal protection of QUER are corroborated by earlier reports of Eldin, Shaheen, Abd Elgawad, and Shehata (Citation2008), Renugadevi and Milton Prabu (Citation2010), Ilić, Stojiljković, Veljković, Veljković, and Stojanović (Citation2014), and Bashir et al. (Citation2014). Also, amelioration of procarbazine-induced kidney dysfunction by QUER has been reported by Olayinka et al. (Citation2015), while the reduction in uric acid concentration following QUER treatment in high fructose induced-metabolic syndrome in rats was reported by Omar et al. (Citation2015).
Measurement of thiobarbituric acid (TBARS) is mostly used to monitor lipid peroxidation and indirectly, oxidative stress in vitro and in vivo (Beltowski, Wójcicka, Górny, & Marciniak, Citation2000). The lipid oxidation causes disruption of the bilayer and cell integrity accompanied by leakage of cellular content from the damaged organ into the blood stream (Ologundudu, Ologundudu, Oluba, et al., Citation2010). The significant increase (p < 0.05) in brain, lung, and heart levels of MDA by SAZ administration may be as a result of free radicals attack on the electron-rich membrane components, while the significant decrease in the levels of MDA in these tissues after quercetin treatments may be as a result of its antioxidant and free radical scavenging ability (Olayinka et al., Citation2015; Omar et al., Citation2015; Nirankari, Kamal, & Dhawan, Citation2016), that may have spared the cell membrane components, by donating its rich electron to the unstable, unpaired, and ravaging free radicals (Figure ). Our findings are corroborated by the reports of Dong et al. (Citation2014) who reported the protection of QUER against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage, and Ongaratti et al. (Citation2014) who documented the antioxidant and neuroprotective effect of organic and conventional white grape juices on oxidative stress induced by SAZ in cerebral cortex of rats. Also, quercetin has been reported to prevent oxidative damage in different tissues of long-term diabetic rats (Edremitlioglu, Andic, & Korkut, Citation2012).
Oxidative stress, characterized by increase in the levels of free radicals due to insufficient antioxidant defense (Mittler, Citation2002) has been shown in experimental and clinical studies to play a major role in the etiology of many diseases. Oxidative stress contributes to the pathological processes of diseases including cancer, cardiovascular diseases, rheumatoid arthritis, diabetes mellitus, and neurological disorders such as Alzheimer and Parkinson (Valko et al., Citation2007). The body has its antioxidant systems to prevent free radical production and the probable ravaging and destructive effects they can cause. The most important intracellular antioxidant defense enzymes are SOD, CAT, GPx (Altan, Dincel, & Koca, Citation2006) and non-enzymatic antioxidants such as GSH (Tomlin, Citation1994). SOD is responsible for catalytic dismutation of highly reactive and potentially toxic superoxide radicals to hydrogen peroxide (H2O2) and O2. CAT and GPx are responsible for the catalytic decomposition of H2O2 to molecular oxygen and water (Tomlin, Citation1994). The significant decrease (p < 0.05) in brain GSH level (Figure ), brain and lung GPx activities (Figure ), as well as lung CAT activity (Figure ), may be attributed to continuous generation of H2O2 following SAZ administration (Tomlin, Citation1994), as the substrate for the enzymes is H2O2, while the enzyme GPx also utilizes GSH as a substrate for detoxification of H2O2 (Tomlin, Citation1994). Furthermore, the significant decrease (p < 0.05) in brain, heart, and lung SOD activities (Figure ) as a result of SAZ exposure may be due to overwhelming generation of superoxide radical (), a substrate for the enzyme (Machado et al., Citation2015).
Also from our findings, the significant increase (p < 0.05) in lung GSH (Figure ), brain and heart CAT activities (Figure ), as well as GST activity (Figure ), following SAZ administration could be due to an adaptive response to oxidative stress-induced free radical (H2O2) generation in these tissues, probably as a result of detoxification of , as also recently reported by Machado et al. (Citation2015).
The amelioration of SAZ-induced tissue oxidative stress recorded in the study by quercetin treatments may signifies the antioxidative and tissue-protective potentials of the flavonoid (Nirankari et al., Citation2016; Olayinka et al., Citation2015), which may have spared and restored back the normal levels and activities of the endogenous antioxidant systems. The protective effects by quercetin treatment against tissue oxidative stress obtained from this study are also corroborated by findings of Guzy et al. (Citation2003), Gargouri et al. (Citation2011), Renugadevi and Milton Prabu (Citation2010), Edremitlioglu et al. (Citation2012), Bashir et al. (Citation2014), Dong et al. (Citation2014), and Ongaratti et al. (Citation2014).
We therefore concluded from the findings of this study that tissue antioxidative potentials of quercetin may be harnessed against SAZ-induced extra-hepatic oxidative stress.
Additional information
Funding
Notes on contributors
Oluwatobi T. Somade
Oluwatobi T. Somade is a biochemist, university lecturer, and researcher (Environmental Toxicology and Oncology). Current research focus is on toxicity assessments of household materials, consumables, and environmental/industrial materials.
References
- Abbanat, R. A., & Smith, R. P. (1964). The influence of methemoglobinemia on the lethality of some toxic anions. Toxicology and Applied Pharmacology, 6, 576–583.10.1016/0041-008X(64)90089-4
- Aebi, H. (1974). Catalase. In H. V. Bergmeyer (Ed.), Methods in enzymatic analysis (Vol. 2, pp. 674–684). New York, NY: Acadamic Press.
- Alrawaiq, N. S., & Abdullah, A. (2014). A review of flavonoid quercetin: Metabolism, bioactivity and antioxidant properties. International Journal of Pharm Tech Research, 6, 933–941.
- Altan, N., Dincel, A. S., & Koca, C. (2006). Diabetes mellitus ve oksidatif stres. Turkish Journal of Biochemistry, 31, 51–56.
- Amália, P. M., Possa, M. N., Augusto, M. C., & Francisca, L. S. (2007). Quercetin prevents oxidative stress in cirrhotic rats. Digestive Diseases and Sciences, 52, 2616–2621.10.1007/s10620-007-9748-x
- Bashir, S. O., Morsy, M. D., Sakr, H. F., El Refaey, H. M., Eid, R. A., Alkhateeb, M. A., & Defallah, M. A. (2014). quercetin ameliorates diabetic nephropathy in rats via modulation of renal NA+, K+-ATPase expression and oxidative stress. American Journal of Pharmacology and Toxicology, 9, 84–95.10.3844/ajptsp.2014.84.95
- Bassendowska, E., & Kowalski, Z. (1962). Investigation of the toxicity of sodium azide. Experimental investigations of acute and subacute toxic activity. Medycyna Pracy, 12, 427–442.
- Beltowski, J., Wójcicka, G., Górny, D., & Marciniak, A. (2000). The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. Journal of Physiology and Pharmacology, 51, 883–896.
- Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310.10.1016/S0076-6879(78)52032-6
- Burger, E., & Bauer, H. M. (1965). Akuter Vergiftungsfall durch versehentliches Trinken von Natriumazidlösung [Case of acute poisoning caused by accidental drinking of a sodium azide solution]. Archive Toxikology, 20, 279–283 (in German).10.1007/BF00577553
- Cheng, Y. F., Zhu, G. Q., Wang, M., Cheng, H., Zhou, A., Wang, N., ... Li, Q. L. (2009). Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP-elicited apoptosis. Neuroscience Research, 63, 52–58.10.1016/j.neures.2008.10.009
- Chirumbolo, S. (2010). The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation & Allergy - Drug Targets, 9, 263–285.10.2174/187152810793358741
- Choi, Y. J., Kang, J. S., Park, J. H., Lee, Y. J., Choi J. S., & Kang, Y. H. (2003). Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. Journal of Nutrition, 133, 985–991.
- Dong, Y., Wang, J., Feng, D., Qin, H., Wen, H., Yin, Z., ... Li, C. (2014). Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. International Journal of Medical Sciences, 11, 282–290.10.7150/ijms.7634
- Edremitlioglu, M., Andic, M. F., & Korkut, O. (2012). Quercetin, a powerful antioxidant biflavonoid, prevents oxidative damage in different tissues of long-term diabetic rats. Balkan Medical Journal, 29, 49–55.
- Eldin, A. A. K., Shaheen, A. A., Abd Elgawad, H. M., & Shehata, N. I. (2008). Protective effect of taurine and quercetin against renal dysfunction associated with the combined use of gentamycin and diclofenac. Indian Journal of Biochemistry and Biophysics, 45, 332–340.
- Eyer, P. (1994). Stickstoffwasserstoffsäure, Azide [Hydrazoic acid, azides]. In H. Marquard & S. G. Schäfer (Eds.), Lehrbuch der Toxikologie [Textbook of Toxicology] (pp. 565–566). Mannheim: B I Wissenschaftsverlag(in German).
- Gargouri, B., Mansour, R. B., Abdallah, F. B., Elfekih, A., Lassoued, S., & Khaled, H. (2011). Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids in Health Disease, 10, 149–152.10.1186/1476-511X-10-149
- Gonzalez-Gallego, J., Sanchez-Campos, S., & Tunon, M. J. (2007). Anti-inflammatory properties of dietary flavonoids. Nutricion Hospitalaria, 22, 287–293.
- Gornall, A. G., Bardawill, C. J., & David, M. M. (1949). Determination of serum protein by biuret method. Journal Biological Chemistry, 117, 751–766.
- Graham, J. D. P. (1949). Actions of sodium azide. British Journal of Pharmacology, 4, 1–6.
- Guzy, J., Kusnir, J., Marekova, M., Chavkova, Z., Dubayova, K., Mojzisova, G., ... Mojzis, J. (2003). Effect of quercetin on daunorubicin-induced heart mitochondria changes in rats. Physiology Research, 52, 773–780.
- Haas, J. M., & Marsh, W. M. (1970). Sodium azide: A potential hazard when used to eliminate interferences in the iodometric determination of sulfur. American Industrial Hygiene Association Journal, 31, 318–321.10.1080/0002889708506248
- Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
- Health & Safety Executive. (2000). Consultation document - European commission directive under the chemical agents directive 98/24/EC to establish a first consolidated list of indicative occupational exposure limit values at European community level. http://www.hse.gov.uk/consult/condocs/cd156.htm
- Ilić, S., Stojiljković, N., Veljković, M., Veljković, S., & Stojanović, G. (2014). Protective effect of quercetin on cisplatin-induced nephrotoxicity in rats. Medicine and Biology, 16, 71–75.
- Lugli, E., Ferraresi, R., Roat, E., Troiano, L., Pinti, M., Nasi, M., ... Cossarizza, A. (2009). Quercetin inhibits lymphocyte activation and proliferation without inducing apoptosis in peripheral mononuclear cells. Leukemia Research, 33, 140–150.10.1016/j.leukres.2008.07.025
- Machado, F. D., Kuo, J., Ongaratti, B. R., Medeiros, N. D., Salvador, M., Dani, C., & Funchal, C. (2015). Antioxidant and neuroprotective potential of extract of Brazilian pine Araucaria angustifolia bracts against oxidative stress induced by sodium azide in hippocampus of rats. Integrative Pharmacology, Toxicology and Genotoxicology, 1, 16–20.
- Martınez-Florez, S., Gonzalez-Gallego, J., Culebras, J. M., & Tunon, M. J. (2002). Flavonoids: Properties and antioxidizing action. Nutricion Hospitalaria, 17, 271–278.
- Misra, H. P., & Fridovich, I. (1972). The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry, 247, 3170–3175.
- Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410.10.1016/S1360-1385(02)02312-9
- Moron, M. S., Depierre, J. W., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta (BBA) - General Subjects, 582, 67–78.10.1016/0304-4165(79)90289-7
- National Research Council. (1996). Guide for the care and use of laboratory animals. Washington, DC: National Academy Press.
- National Toxicology Program. (1991). Toxicology and Carcinogenesis Studies of Sodium Azide in F344N Rats and B6C3F1 Mice (Gavage Studies) (TR 389). Research Triangle park, NC 27709: U.S. Department of Health and Human Services; Public Health Service.
- Nirankari, S., Kamal, R., & Dhawan, D. K. (2016). Neuroprotective role of quercetin against arsenic induced oxidative stress in rat brain. Journal of Environmental & Analytical Toxicology, 6(2), 359–364.
- Olayinka, E. T., Ore, A., Adeyemo, O. A., Ola, O. S., Olotu, O. O., & Echebiri, R. C. (2015). Quercetin, a flavonoid antioxidant, ameliorated procarbazine-induced oxidative damage to murine tissues. Antioxidants, 4, 304–321.10.3390/antiox4020304
- Ologundudu, A., Ologundudu, A. O., Oluba, O. M., Omotuyi, I. O., & Obi, F. O. (2010). Effect of Hibiscus sabdariffa anthocyanins on 2,4-dinitrophenyl hydrazine-induced tissue damage in rabbits. Journal of Toxicology and Environmental Health Sciences, 2, 1–6.
- Omar, A. H., Yaseen, A. A., Ghanayem, N. M., ElOdemi, M. H., Rizk, M. S., Aleskandarany, M. A., ... El-Fiky, S. R. (2015). Effect of resveratrol and quercetin on high fructose induced metabolic syndrome in rats. Nature and Science, 13, 52–63.
- Ongaratti, B. R., Machado, F. D., Medeiros, N. D., Destri, C., Silva, E. R., Quincozes-Santos, A., ... Funchal, C. (2014). Antioxidant and neuroprotective effect of organic and conventional white grape juices on oxidative stress induced by sodium azide in cerebral cortex of rats. European Journal of Nutrition & Food Safety, 4, 592–603.10.9734/EJNFS
- Papiez, M., Cierniak, A., Krzysciak, W., Bzowska, M., Taha, H. M., Jozkowicz, A., & Piskula, M. (2008). The changes of antioxidant defense system caused by quercetin administration do not lead to DNA damage and apoptosis in the spleen and bone marrow cells of rats. Food and Chemical Toxicology, 46, 3053–3058.10.1016/j.fct.2008.06.006
- Patra, R. C., Rautray, A. K., & Swarup, D. (2011). Oxidative stress in lead and cadmium toxicity and its amelioration. Veterinary Medicine International, . 10.4061/2011/457327
- Pham, T., Palmieri, T. L., & Greenhalgh, D. G. (2001). Sodium azide burn: A case report. Journal of Burn Care Rehabilitation, 22, 246–248.10.1097/00004630-200105000-00012
- Reinhardt, C. F., & Brittelli, M. R. (1981). Heterocyclic and miscellaneous nitrogen compounds: Azides. In E. Bingham, B. Cohrssen, & C. H. Powell (Eds.), Patty’s industrial hygiene and toxicology (Vol 2, pp. 2778–2784). New York, NY: Interscience.
- Renugadevi, J., & Milton Prabu, S. (2010). Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Experimental and Toxicologic Pathology, 62, 471–481.10.1016/j.etp.2009.06.006
- Rippen, H. E., Lamm, S. H., Nicoll, P. G., Cummings, L., Howearth, G., & Thayer, D. (1996). Occupational health data as a basis for process engineering changes: Development of a safe work environment in the sodium azide industry. International Archives of Occupational and Environmental Health, 68, 459–468.10.1007/BF00377870
- Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.10.1126/science.179.4073.588
- Smith, R. P., Louis, C. A., Kruszyna, R., & Kruszyna, H. (1991). Acute neurotoxicity of sodium azide and nitric oxide. Fundamental and Applied Toxicology, 17, 120–127.10.1016/0272-0590(91)90244-X
- Somade, O. T., Akinloye, O. A., Adeyeye, M. O., Fabunmi, G. D., Idowu, O. O., Badmus, F. O., & Salaudeen, B. O. (2015). Quercetin, a natural phytochemical and antioxidant protects against sodium azide-induced hepatic and splenic oxidative stress in rats. Journal of Investigational Biochemistry, 4, 69–74.10.5455/jib.
- Tieppo, J., Cuevas, M. J., Vercelino, R., Tunon, M. J., Marroni, N. P., & Gonzalez-Gallego, J. (2009). Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. Journal of Nutrition, 139, 1339–1346.10.3945/jn.109.105353
- Tokyol, Ç., Yilmaz, S., Kahraman, A., Çakar, H., Polat, C. (2006). The effects of desferrioxamine and quercetin on liver injury induced by hepatic ischaemia-reperfusion in rats. Acta Chirurgica Belgica, 106, 68–72.10.1080/00015458.2006.11679837
- Tomlin, C. D. (1994). The e-pesticide manual (10th ed.). Surrey, UK: The British Crop Protection Council.
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., & Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology, 39, 44–84.10.1016/j.biocel.2006.07.001