Abstract
Methotrexate (MTX) has been widely used as an anticancer drug. It acts as a folic acid analog, inhibits purine and pyrimidine synthesis, which accounts for its efficacy in the therapy of cancer as well as for some of its toxicities. The present study is an attempt of modulating MTX-induced toxicities using aqueous extract of Morinda citrifolia L. (Noni) as a nutritional supplement. Hematological parameters such as RBC, WBC, and platelet count that decreased (p < 0.05) after methotrexate injection was found to have been restored in rats co-treated with Noni. Enhanced levels of lipid peroxides (p < 0.05) in animals administered with MTX showed significant revision after co-administration of Noni. Alterations of other biochemical constituents in blood like glucose, urea, uric acid, and cholesterol (p < 0.05) were also reversed to near normal levels in animals co-treated with Morinda citrifolia L. The study provides preliminary evidence that Morinda citrifolia L. extract can ameliorate MTX mediated side effects.
Public Interest Statement
Methotrexate (MTX) (4-amino-10-methylpteroylglutamic acid) is a potent antineoplastic agent used to treat choriocarcinoma, leukemia, osteosarcoma, non-Hodgkins lymphoma, breast cancer, and lung cancer. It is also involved in the treatment of non-cancerous conditions such as rheumatoid arthritis, psoriasis, immunological abnormalities, and systemic inflammation. Low-moderate to high doses of MTX causes various side effects and may lead to conditions such as liver cirrhosis or fibrosis. It has also been shown that MTX administration has severe side effects on the hematopoietic system. This work is to highlight the importance of Morinda citrifolia L. fruit extract supplementation as an antioxidant in chemotherapy to re-establish the levels of antioxidant and to strike a balance between the oxidant and antioxidant levels thus preventing the enormous toxicity observed due to this drug. Combinational chemotherapy gives an insight for an effective treatment to cancer patients helping them exhibit minimum levels of the deleterious drug-induced side effects.
Competing Interests
The authors declare no competing interest.
1. Introduction
Methotrexate (MTX), a structural analog of folic acid, a potent inhibitor of enzyme dihydrofolate reductase is widely used as a chemotherapeutic agent for leukemia and other malignancies (Faten, Ibrahim, & Khaled, Citation2013). Nowadays, it is also used for sarcoidosis, inflammatory bowel diseases, vasculitis, arthritis, and severe refractory asthma (Patel & Ghodasara, Citation2014). However, its use is limited due to high incidence of serious dose-dependent toxicity, including hepatotoxicity, renal damage, bone marrow suppression, and gastrointestinal mucosal inflammation (Mohamed Akram, Citation2006; Ramadan, Citation2008).
Low-moderate to high doses of MTX causes various side effects and may lead to conditions such as liver cirrhosis or fibrosis (Katherine, Anjali, & Judith, Citation2010). It has also been shown that MTX administration has severe side effects on the hematopoietic system. MTX induces oxidative stress by increasing ROS production which is implicated in tissue injury (Viswa, Premila, & Bina, Citation2007). Further, associated increase in oxidative stress may play an important role in the pathophysiology of drug-induced side effects (Rakesh & Neeta, Citation2013).
Fruits of Morinda citrifolia L., also known as Noni, have been used as herbal medicines by ancient Hawaiians as remedies for various ailments. It has been reported to have a broad range of therapeutic effects, including antibacterial, antiviral, antifungal, antitumor, analgesic, hypotensive, anti-inflammatory, and immune enhancing effects (Khuntia & Panda, Citation2010). Empirical use of medicine derived from plants has been widely disseminated since ancient times to treat a wide range of diseases. Noni juice in particular has been used in the treatment of diabetes, heart disease, high blood pressure, and kidney and bladder disorders (Ajadi, Adenubi, & Thomas, Citation2011). A number of major chemical compounds and antioxidants like, alkaloids, flavonoids, tannins, and phenols have been identified in the leaves, roots, and fruits of Noni plant. Daily intake of Noni has reported to reduce free radical-induced oxidative damage and the consequent lipid peroxidation, and it improves the quality of life of patients undergoing chemotherapy. It has been reported to have a broad range of health benefits for cancer, infection, arthritis, diabetes, asthma, hypertension, and pain (Wang, West, & Jenesen, Citation2002). Concurrent use of Morinda citrifolia L. extract with chemotherapy can reduce the toxicity and increase the efficacy of the drug. Increased serum levels of lipid peroxides have been reported to decrease after noni administration and protects fall in leukocyte count, hemoglobin level, and mean osmotic fragility of erythrocytes (Bhakti & Thankamani, Citation2016). This study is a preliminary evaluation of the hematological and biochemical profile of rats treated with methotrexate and to determine the extent to which of Morinda citrifolia L., treatment could ameliorate the detrimental effects induced by MTX.
2. Materials and methods
Drugs and chemicals: methotrexate injection Folitrax-15 IP (15 mg/ml) was purchased from Ipca Pharmaceuticals. The fruits of Morinda citrifolia were purchased from Abirami Botanical, Tamil Nadu, India. Specimen was authenticated from St. Xaviers College, Department of Botany, Mumbai as Morinda citrifolia L. belonging to family Rubiaceae with Blatter Herbarium specimen number 108. The fruits were air-dried for 2 days and ground to powder. All other chemicals were of high analytical grade from SRL, Mumbai, India and Merck India Ltd, Mumbai, India and solvents were of Qualigen finechemicals, Mumbai, India.
The aqueous extract of Noni was prepared by cold maceration of 250 g of the shade-dried fruit powder in 500 ml of distilled water which was allowed to stand overnight. It was boiled for 5–10 min till the volume was reduced to half its original volume. The solution was then cooled, filtered through eight-layered muslin cloth, concentrated, Whatman, dried in vacuum (yield 36 g) and the residue stored in a refrigerator at 2–8°C for subsequent use (Bhakti & Thankamani, Citation2015).
Animal model: adult male albino rats of Wistar strain (100 ± 20 g) were obtained from Bharat Serum Pvt. Ltd, Thane, Navi Mumbai, India. The animals were maintained under standard conditions of temperature (25 ± 2°C), light (12 h light/12 h dark), and humidity. They were divided into groups as given in Table . They were fed standard rat pelleted diet obtained from Lipolin, India and water ad libitum. Experimental animals were handled according to the Institutional legislation, regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Table 1. Design of treatment protocol
Experimental Design: following the acclimatization period of one week, a pilot study was conducted with 5 mg/kg/bw of methotrexate (ip) diluted in autoclaved distilled water twice weekly for 30 days. It was found to be highly toxic as there was extensive weight loss, loss of appetite, and diarrhea. Animals died within two weeks, hence the dose of methotrexate was modified to 1 mg/kg/bw of methotrexate ip twice weekly for 30 days and 5 mg/kg/bw of concentrated Noni extract diluted in distilled water. Rats were randomly divided into four groups consisting of six animals each (Table ).
At the end of the experimental period, the animals were killed by cervical decapitation. Blood was collected in EDTA for plasma and without anticoagulant for serum. Blood was processed further for RBC and WBC counts (Raghuramulu, Madhavan, & Kalyansundaram, Citation1983) differential count (Dacie & Lewis, Citation1984), platelet count (Samuel, Citation1986), hemoglobin, (Varley, Citation2005), glucose (Trinder, Citation1969) and urea (Varley, Citation2005). The serum levels of protein (Reinhold, Citation1953), lipid peroxides (Yagi, Citation1984), uric acid, creatinine, bilirubin, and cholesterol (Varley, Citation2005) were also determined.
Statistical analysis: the results are expressed as mean ± standard deviation (SD) for six animals in each group. The statistical evaluation of all data was done using analysis of variance (ANOVA). p-value < 0.05 was considered statistically significant. It was complemented with unpaired Student’s t-test. Data were analyzed by SPSS software, version 19 (Chicago, IL, USA).
3. Results and discussion
There were prominent, distinctive clinical signs, mortality, or morbidity observed in Group II during the experimental period. Control rats receiving Morinda citrifolia L. alone (Group III) did not show any significant change when compared with control rats (Group I), indicating that Morinda citrifolia L. does not have any adverse effect. Table shows the results of the complete blood count. There is a significant decrease in number of RBCs (p < 0.05) and WBCs (p < 0.01) accompanied with a decrease in the levels of hemoglobin. Among the WBCs, the significant decrease is in the number of neutrophils. It is well known that myelosuppression and pancytopenia are characteristic features of chemotherapy. Actively proliferating progenitor cells in the bone marrow are sensitive to anticancer agents and are subjected to an irreversible removal process. According to clinical studies (Patel & Ghodasara, Citation2014) of methotrexate, the principal dose-limiting toxicities are neutropenia, thrombocytopenia, and anemia. In the present study, hematological effects of MTX including decreased RBC, WBC, and platelets are in agreement with the results of above clinical studies. Premature death of RBCs as a result of oxidative injury can also contribute to the reduction in RBC count and a decrease in hemoglobin. Pretreatment with Morinda citrifolia L. showed beneficial effect by restoring the levels of RBCs, WBCs, and hemoglobin suggesting the ameliorative effect of Morinda citrifolia L. in preventing MTX-induced bone marrow suppression. Glucose levels in blood of MTX-treated rats increased by 25 percent (Table ). Insulin is essential for maintaining blood sugar levels and decreased availability of insulin due to MTX-induced toxicity to the pancreas may contribute to the observed hyperglycemia. This hypoinsulinemia leads to less utilization of glucose by the cells and hence an increase in blood sugar (Singh & Marar, Citation2011).
Table 2. Effect of methotrexate and Morinda citrifolia L. fruit extract on complete blood count
Table 3. Effect of methotrexate and Morinda citrifolia L. fruit extract treatment on serum biochemical parameters of rats
Hepatotoxicities induced by MTX may also lead to increase in glucose levels since liver is also actively involved in glucose metabolism (Mohamed Akram, Citation2006).
MTX also causes increase in lipid peroxidation which has been reported to inactivate enzymes involved in glycolysis. Morinda citrifolia L. co-treated animals did not show hyperglycemia. Dietary supplementation of Morinda citrifolia L. has been reported to reduce blood sugar levels (Ajadi et al., Citation2011). Further protection rendered by Morinda citrifolia L. against lipid peroxidation could help in restoring activities of the enzymes (Table ).
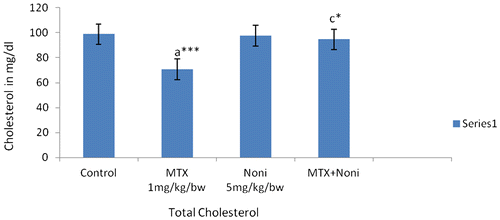
A significant decrease in serum cholesterol levels was seen in MTX-treated rats as shown in Figure . This might be correlated to malabsorption, maldigestion, and diarrhea observed in animals intoxicated with MTX. Further exocrine pancreatic insufficiency and hepatotoxicosis induced by MTX may also be a contributory factor. Supplementation of Morinda citrifolia L. in the diet could render protection to the gastrointestinal system and thus normalize the levels of cholesterol in serum.
Figure 1. Effect of methoterexate and Morinda citrifolia L. fruit extract on levels of serum cholesterol.
There is an increase in the serum levels of creatinine, bilirubin, urea, and uric acid in rats treated with MTX (Table ). An increase in the levels of urea (p < 0.05) and uric acid (p < 0.01) was also reported in animals injected with MTX (Kevin & Moder, Citation1995). Similarly, serum creatinine and urea levels were considerably increased (p < 0.01) than normal which indicated nephrotoxicity (Patel & Ghodasara, Citation2014). Moderate rise in urea is an indicator of increased protein breakdown associated with cytotoxicity of drugs. Uric acid is the end product of purine metabolism and it increases consistently with cell death induced by MTX. Our results reveal that blood creatinine, bilirubin, protein, urea, and uric acid returned to near-normal levels in animals supplemented with Morinda citrifolia L. These results further confirm the hepatoprotective nature of Noni (Seshachary & Satyavati, Citation2014).
Membrane lipids succumb easily to deleterious actions of reactive oxygen species. In present study, the increased levels of lipid peroxides in serum of Group II animals reflect the oxidative stress (Table ). Treatment with Morinda citrifolia L. protects the cells through attenuation of lipid peroxidation, as evident from the decreased levels of serum lipid peroxides in Group IV animals. Noni has been reported to possess antioxidant activity (Katherine et al., Citation2010) and its co-administration may have helped decrease oxidative stress.
4. Conclusion
The present investigation reveals that Morinda citrifolia L. fruit juice can ameliorate toxicities induced by MTX. Often, argument concerned with supplementation of antioxidants in chemotherapy arises. The basis of this disagreement is that many of the chemotherapeutic drugs induce the formation of oxygen-derived free radicals, the effect of which would be blocked by antioxidants. The anticancer effect of methotrexate however, does not depend on the formation of free radicals. Therefore, administration of antioxidants might reduce the side effects of methotrexate without compromising its efficacy. Further studies assessing the potential usefulness of Morinda citrifolia L. treatment in MTX-induced toxicities on other organs and organ systems are required which may provide an effective way to improve their therapeutic efficacy.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Bhakti A. Mhatre
Bhakti A. Mhatre is presently perusing PhD in Biotechnology from School of Biotechnology and Bioinformatics, D.Y. Patil University CBD Belapur, Navi Mumbai, Maharashtra, India. Mhatre is presently working on anticancer properties of Morinda citrifolia L. fruit extract. The use of chemotherapeutic agents in the treatment of cancer has hampered and complicated by toxic side effects. Many types of chemotherapy destroy cancer cells by generating free radicals which can cause cellular damage. Unfortunately, these free radicals are not discriminatory in their destructive action leading to undesirable side effects and sometimes even new cancers. Therefore, administration of antioxidants might reduce the side effects of camptothecin without compromising its efficacy. Further studies assessing the potential usefulness of Morinda citrifolia L. fruit extract treatment in methotrexate-induced toxicities on other organs and organ systems are warranted which may provide an effective way to improve their therapeutic efficacy.
References
- Ajadi, T., Adenubi, T., & Thomas, C. (2011). Protective effect of Tahitian Noni juice on the reproductive functions of Male Wistar Rats traeted with cyclophosphamide. Iranian Journal of Pharmacology and Therapuetics, 10, 39–43.
- Bhakti, M., & Thankamani, M. (2015). Vetting and comparative analysis of antioxidants and phytochemicals from methanolic, aqueous and a popular commercial fruit juice (Noni) Morinda Citrifolia L. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 6, 884–890.
- Bhakti, M., & Thankamani, M. (2016). Ameliorativeeffect of Morinda citrifolia L. extracton Imatinib induced toxicities: In vitro studies on human RBC’s. Journal of Cell and Tissue Research, 16, 5507–5510.
- Dacie, J., & Lewis, S. (1984). Practical Hematology (pp. 84–86). NewYork, NY: Churchill-Lingstone Edinburgh.
- Faten, R. A., Ibrahim, A. E., & Khaled, A. E. (2013). Protective and modulatory effects of Curcumin and L-Carnitine against Methotrexate-induced Oxidative stress in albino rats. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 4, 744–754.
- Katherine, S., Anjali, G., & Judith, A. S. (2010). Evaluation of the hepatic metabolism and antitumor activity of Noni juice (Morinda citrifolia L.) in combination with chemotherapy. Journal of the Society for Integrative Oncology, 8, 89–94.
- Kevin, G., & Moder, M. (1995). Malignancies and the use of Methotrexate in rheumatoid arthritis. America Journal of Medicines Hematologic , 99, 276–281.
- Khuntia, T., & Panda, D. (2010). Evaluation of antibacterial, antifungal and anthelmintic activity of Morinda citrifolia L. (Noni). International Journal of PharmTech Research, 2, 1030–1032.
- Mohamed Akram, A. M. (2006). Histological and histochemical studies on the effects of methotrexate on the liver of adult male albino rat. International Journal of Morphology, 24, 417–422.
- Patel, N. N., Ghodasara, D. (2014). Subacute toxicopathological studies of methotrexate in Wistar rats. Dave Veterinary World, 223, 489–495.
- Raghuramulu, N., Madharan, N., Kalyansundaram, S. (1983). A manual of laboratory techniques (Vol. 78, pp. 257–258). Hyderabad: Silver prints.
- Rakesh, K., & Neeta, S. (2013). Morinda citrifolia (Noni) alters oxidative stress marker and antioxidant activity in cervical cancer cell lines. Asian Pacific Journal of Cancer Prevention, 14, 4603–4606.
- Ramadan, A. M. (2008). Curcumin attenuates methotraxate-induced hepatic oxidative damage in Rats. Journal of the Egyptian National Cancer Institute, 20, 141–148.
- Reinhold, J. (1953). Standard methods of clinical chemistry (Vol. 1, pp. 88–97). M. Reiner (Ed.), NewYork, NY: Academic Press.
- Samuel, K. M. (1986). Notes on clinical lab techniques (168). Madras: MGK Iyyer and Sons Publishers.
- Seshachary, A. K., & Satyavati, D. (2014). Chemoprotective effect of ethanolic extract of Morinda citrifolia against Cisplatin induced nephrotoxicity. The Pharma Innovation , 3, 84–91.
- Singh, K., & Marar, T. (2011). Acute toxicity of Camptothecin and influence of α-Tocopherol on haematological and biochemical parameters. Journal of Cell and Tissue Research, 11, 2833–2837.
- Trinder, P. (1969). Analytical Clinical Biochemistry, 6, 24–25.
- Varley, H. (2005). Practical Clinical Biochemistry (4th ed.). New Delhi: CBC Publisher and distributor.
- Viswa, K. K., Premila, A., & Bina, I. (2007). Alteration in antioxidant defense mechanisms in the small intestines of methotrexate treated rat may contribute to its gastrointestinal toxicity. Cancer Therapy, 5, 501–510
- Wang, M.-Y., West, B. J., & Jenesen, C. J. (2002). Morinda citrifolia (Noni): A literature review andrecent advances in Noni research. Act a Pharmacolog ica Si nic, 23, 1127–2141.
- Yagi, K. (1984). Assay for plasma lipid peroxides. Methods in Enzymology, 109, 328–331.
