Abstract
Clove (Syzygium aromaticum) is a popular medicinal plant which has been traditionally used in India as spice and medicine to counter various ailments. However, the stress modulatory and antiaging potential of this plant is yet to be characterized. Therefore, the present study evaluates the effect of clove oil (CO) on oxidative stress, lifespan, mobility, and the expression of aging-related proteins using Caenorhabditis elegans model system. The CO (10 ppm) was found to extend mean lifespan in worms by 21.4% (p < 0.001) under normal and by 63% (p < 0.0001) under juglone-induced oxidative stress conditions. The extension of mean lifespan in mev-1 mutant and elevated expression of gst-4 and sod-3 confirmed stress modulatory effects of CO. Additionally, the CO reduced intracellular ROS and Aβ1–42 proteotoxicity. Altogether, the present study unravels the anti-aging and stress modulatory potential of CO and suggests CO as a potential pharmaceutical entity in modulating aging process.
Public Interest Statement
Spices and herbs have been part of various food preparations since ancient time till today. These spices contribute to various beneficial therapeutic effects in human. Clove is one of these spices/herbs which has proven efficacy in treating dental problems, inflammation, and neurodegenerative disorders. Therefore, present study evaluates effect of clove oil on aging and age-related neurodegenerative Alzheimer’s disease using a free living soil nematode Caenorhabditis elegans organism model which shares genetic similarity with human. Clove oil was found to improve health span and lifespan of C. elegans in dose-dependent manner. The study unravels potential of clove oil in delaying aging and age-related pathologies.
Competing Interests
The authors declare no competing interest.
1. Introduction
Ayurveda, the word derived from Sanskrit itself means “the scripture of longevity”. The practice finds its foundation in holistic view of treatment which cures human diseases all the way through the establishment of equilibrium between elements of human life, the body, the mind, and the soul. The scientific investigation concerning the best known of these traditionally practiced herbs is need of the hour and therefore, we investigated anti-aging and stress modulating potential of widely used popular medicinal and aromatic plant clove (Syzygium Aromaticum). This popular spice has proven efficacy in treating dental problems, inflammation, and neurodegenerative disorders (Adams, Gmünder, & Hamburger, Citation2007; Chaieb et al., Citation2007). Despite its well-known therapeutic potential, the plant is yet not fully explored against aging disorders. Aging is a multifaceted phenomenon and a time-dependent decline in physiological functions which is universal to every living organism (Kenyon, Citation2010). The hallmark of cell senescence is the onset of various age-related afflictions including neurodegeneration, cardiovascular disease, and cancer (Guarente & Kenyon, Citation2000). The main goal of gerontological research is to identify pharmacological molecules that can delay age-related diseases and maintain vitality of later life in humans. A number of earlier reports have established that active constituents of different plant extracts are efficient in reversing aging, extending lifespan, and improving stress tolerance (Arya, Dwivedi, & Subramaniam, Citation2009; Asthana et al., Citation2015; Kampkötter et al., Citation2008). Thus, a proper understanding of clove oil (CO) on aging and longevity modulation is still a unfolded mystery and should be investigated. As an organism, soil nematode Caenorhabditis elegans ages and shares similar aspects of aging like human (Kenyon, Citation2010). Further, the advantageous characteristics of this model organism are attributed to its genetic pliability, invariant and fully described developmental program, well characterized genome, ease of maintenance, short and fertile life cycle, and small body size (Kenyon, Citation2010). C. elegans elegans shares more than 80% gene homology with human and protein network regulating aging is conserved between worm and human which makes this nematode model a powerful tool for the screening of lifespan modulators (Kenyon, Citation2010). The present investigation attempts to determine the effects of CO on oxidative stress, lifespan, mobility, neurotoxicity, and the expression of aging-related proteins.
2. Experimental
2.1. Plant material and GC/MS analysis
The Syzygium aromaticum buds were procured from a local market. The volatile oil was obtained by conventional hydrodistillation of the buds of S. aromaticum in a clevenger-type apparatus. The GC and GC/MS analyses were performed using previously described standard methods (Adams, Citation2012; Kollmannsberger & Nitz, Citation1994; Srivastava, Srivastava, & Syamsundar, Citation2005). The GC and GC/MS analyses resulted in the identification of major constituent as eugenol (65%, Figure S1).
2.2. Caenorhabditis elegans maintenance and egg preparation
The wild-type C. elegans Bristol strain N2, TK22, mev-1 (kn1), CL4176 (dvIs27 [myo3::Aβ let 3ʹUTR (pAF29); pRF4 (rol6 (su1006)]), CL 2006 (dvIs2 [pCL12 (unc-54/human Aβ peptide 1–42 minigene) + pRF4]) was used in this experiment. Animals were maintained on nematode growth medium (NGM) and fed with OP-50 strain Escherichia coli at 20°C (unless otherwise indicated). A synchronized culture was obtained by sodium hypochlorite treatment (50% sodium hypochlorite [12% Cl]; 2.5 M sodium hydroxide), which kills adult worms, but not their eggs (Brenner, Citation1974). The strain used in this experiment was obtained from the Caenorhabditis Genetics Centre, University of Minnesota, and Minneapolis, USA.
2.3. Lifespan analysis
Age-synchronized N2 worms were used for lifespan assay (Pant et al., Citation2014). Worms were synchronized by alkaline hypochlorite treatment. The isolated eggs were allowed to hatch on NGM plates previously spotted with or without different concentrations of CO different doses (1, 10, 100 ppm) till L4 stage. About 25–30 L4 molts were then transferred to NGM plates previously spotted with corresponding test concentration and 50-μM FUdR (Sigma-Aldrich) to block progeny development. Worms were then observed daily for survival and transferred to fresh plates after every 3–4 days to avoid contamination and to assure the presence of the compound throughout experiment. The experiment was terminated when all worms were scored as dead or censored. Three independent trials were performed for all treatments, and the data shown represent three replicates with similar effects on longevity.
2.4. Stress assays
The effect of CO on stress response of worm was assessed by exposing treated worms to juglone--induced oxidative stress and heat-induced thermal stress (Pant et al., Citation2014). For assessing thermotolerance, age-synchronized N2 worms were raised on treatment (CO) and control plates at 20°C and shifted to 35°C as day-2 adults. The survival of worms was scored by touch provoke method (Lithgow, White, Melov, & Johnson, Citation1995). Furthermore, oxidative stress resistance was assessed by exposing age-synchronized CO and EU pre-treated and control day-2 adult worms to lethal dose (250 μM) of juglone (5-Hydroxy-1,4-napthoquinone, Sigma-Aldrich). The survival was scored every hour after the treatment. The experiments were done in three replicates independent of each other.
2.5. Measurement of body size
The fourth larval stage (L4) worms exposed onto a bacterial lawn spot of an NGM plate containing different doses (1, 10, 100 ppm) of CO and control plates without the phytomolecule which were incubated for 24 h were directly picked for body size measurement (Pant et al., Citation2014). The body sizes of more than 20 animals in the photo pictures with a scale were randomly measured using the Leica Application Suite V3 software (version 3.4.0). The experiment was performed independently thrice.
2.6. Brood size assay
Synchronous wild-type hermaphrodites were cultured at 20°C for 3 days until they reached the L4 stage. One worm each (three replicates of each treatment) was then transferred and maintained on a separate NGM plate with (10, 100 ppm) of CO or without treatment and incubated at 20 ± 0.5°C in incubator. Individual worms were transferred daily; the progeny were left to develop for 3 days before counting (Pant et al., Citation2014). After three days, the progeny were counted under stereoscopic microscope.
2.7. Measurement of aging phenotype
2.7.1. Assessment of feeding behavior
The progression in age is correlated to decline in pharyngeal pumping rate. The effect of CO on feeding behavior was evaluated by counting the pharyngeal contractions and relaxation. Pharyngeal pumping is defined as number of contractions (i.e. backward grinder movements in the terminal bulbs). This assay was performed with day-2 and day-4 adult worms on approximately 40 worms at regular interval of 20 s at room temperature (Pant et al., Citation2014). The p-value was calculated using Assistat 7.7 beta statistical assistance software. The assay was performed three times independently.
2.7.2. Lipofuscin assay
The effect of CO treatment on age pigment lipofuscin was evaluated using previously described method (Pant et al., Citation2014). The day-4 adult worms (n = 20) were randomly selected from each treatment group (CO and control) and observed for autoflorescence. The lipofuscin levels were quantified by determining the average pixel intensity in each worm using Image-J software (NIH). The p-value was calculated using Assistat 7.7 beta statistical assistance software. The assay was performed thrice independently.
2.8. Measurement of intracellular ROS in C. elegans
The intracellular ROS levels were determined according to Smith and Luo, Citation2003 with minor modifications. Adult day-4 worms treated with or without CO were used for intracellular ROS determination. Worms were collected in 300 μl of 0.1% PBST and equally timed homogenization and sonication. The homogenized samples were transferred to 96 well plate and prior to reading, 15 μl of 10 mM H2DCF-DA was added to each well. Fluorescent readings were measured using Spectra Max M2 multimode microplate reader, (Molecular Devices) at 485 nm excitation and 530 nm emission. Observations were recorded at every 20 min for 2 h and 30 min at 37°C. The test was performed three times independently.
2.9. Worm paralysis assays
The CL4176 strain [dvIs2 [pCL12 (unc54/human Aβ minigene) + pRF4] containing a heat-sensitive mutation developed to express human amyloid β1–42 (Aβ1–42) present in the muscle tissue was maintained on NGM plates at 16°C (Dostal, Roberts, & Link, Citation2010). Previous to the beginning of the experiment, C. elegans were age synchronized at 16°C on CO pre-treated NGM plates. L1 worms from F2 generation were transferred to control or treatment plates and allowed to mature gravid adult stage to lay eggs. After reaching the L3 stage, the incubation temperature of the plates was increased from 16°C to 25°C, in order to induce the expression of Aβ1–42. The evaluation of the mobility of worms was started 18–20 h after increasing the incubation temperature in 2 h increments until all worms were paralyzed. The worms are considered paralyzed if they failed to respond to prodding and demonstrated “halos” of cleared bacteria around their heads (indicative of insufficient body movement to access food), eggs accumulation close to the body. The experiment was performed in three independent trials.
2.10. In vivo gene expression studies in transgenic C. elegans
The in vivo expression of stress response genes sod-3 and gst-4 was quantified with the aid of transgenic GFP reporters CF1553 (muIs84) and CL2166 (dvIs19) using fluorescence microscopy. The treated/untreated worms were photographed individually by piptetting onto 2% agarose pad on glass slide. The worms were anaesthetized with 1% sodium azide prior to microscopy. The assay was performed according to previously described methods (Pant et al., Citation2014) using a GFP filter (with excitation at 365 nm and emission at 420 nm) using a fluorescence microscope DMI 3000 B (Leica, Wetzlar, Germany) at 20X. The fluorescence levels were quantified using Image-J software (NIH).
2.11. Statistical analysis
Experiments were performed at least in triplicate. Significant differences between the lifespan of treated and control worms under normal/stressed conditions were determined using Kaplan-Meir survival assay in Med Calc software version 12.7.7.0. Data are presented as mean ± SD, and student’s t-test or one-way ANOVA analysis was used to determine the statistical significance between experimental groups. Statistical significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
3. Results and discussion
3.1. Clove oil promotes healthy lifespan in C. elegans
An increment in aging world population and age-related ailments have sidetracked the aging research towards dietary interventions promoting longevity with maintenance of vitality of later life. Aging is a global phenomena accompanied with decline in ability of an organism to maintain cellular homeostasis with time (Kenyon, Citation2010). The significant progress in field of gerontological sciences has focused research towards aging and factors modulating lifespan, but mechanisms extending lifespan of an organism still remain elusive. Therefore, we evaluated the age defying effects of CO which is commonly used in traditional Ayurvedic medicine, dentistry, and food preparations for its potential health benefits in humans. The N2 wild-type worms were exposed to different doses of CO (1, 10 and 100 ppm) from early stages of lifespan and maximal lifespan extension of 21.4% (p < 0.0001) was recorded in 10 ppm followed by 14.35% (p < 0.0001) in 100 ppm and 6.99% (p < 0.0001) in 1 ppm (Figure (a), Table ). The extension in lifespan by different interventions is oftenly associated with decline in feeding behavior, growth, and fertility of an organism (Gruber, Tang, & Halliwell, Citation2007; Kenyon, Citation2010). The extension in mean lifespan in exchange of health span and vitality is not the prime goal of aging research. Therefore, we evaluated effect of CO on pharynx pumping rate, growth and fecundity of worm. The non-significant difference between treated (CO) and untreated (control) was observed suggesting lifespan extension with maintenance of health span (Figure S2(A)–(C)). The present findings are supported by previous studies where lifespan extension is mediated by various natural herbs and molecules.
Figure 1. Effect of CO on lifespan in wild type and mutant strain of C. elegans at 20°C. (a) The maximum extension of 21.4% (p < 0.0001) in lifespan was recorded in 10 ppm CO followed by 14.35% (p < 0.0001) in 100 ppm and 6.99% (p < 0.0001) in 1 ppm; (b) the percentage mean survival in 10 ppm CO-treated mev-1 (kn1) mutants is enhanced by 30.94% (p < 0.0001) in comparison to control. The data were analyzed using the Kaplan–Meir survival analysis in Medcalc 12.7.7.0 software. Effect of CO on stress level; (c) CO (10 ppm) treatment significantly reduced the juglone sensitivity in pre-treated worms compared to untreated control worms mediating improved survival under oxidative stress conditions; (d) CO (10 ppm) treatment significantly enhanced mean lifespan of worms in comparison to control under thermal stress conditions. The data are statistically analyzed using ANOVA in ASSISTAT 7.7 beta statistical assistance software. Differences between the data were considered significant at p ≤ 0.05.
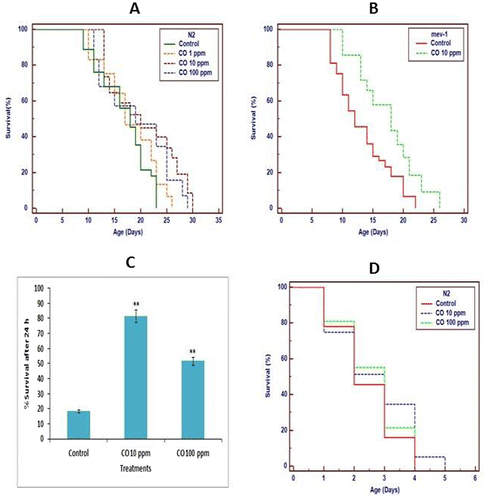
Table 1. Lifespan analysis of wild-type N2 and mev-1 mutant at 20°C
3.2. CO reduces intestinal lipofuscin autoflorescence
The lifespan progression is marked with increment in oxidative stress and aggregation of byproducts of macromolecular damage which causes stress in an organism (Garigan et al., Citation2002). The accumulation of auto fluorescent intestinal age pigment lipofuscin is a biomarker of aging in worms. The aggregation of lipofuscin protein is an aging biomarker. The age-related stress is directly linked with lipofuscin aggregation. The significant decline in intestinal lipofuscin was observed in CO-treated worm by 15.78% (p = 0.0019). Whereas, significant increase was recorded in control worms (Figure S2(D)–(F)). The significant decline in lipofuscin aggregation suggests that CO can maintain function of primary-targeted organ with progression in aging process.
3.3. CO confers stress tolerance and attenuates ROS level in C. elegans
The increment in stress and intracellular ROS levels leads to decline in mean survival in organisms (Abdollahi, Moridani, Aruoma, & Mostafalou, Citation2014; Epel & Lithgow, Citation2014). The progression in lifespan is accompanied by cellular metabolic decline and disruption of normal cellular redox function which hampers cellular macromolecular organization (Kenyon, Citation2010). Therefore, the present study evaluates effect of CO on stress level and intracellular ROS level in worms. The CO pre-treated worms demonstrated enhanced mean survival by 63% (p < 0.0001) in 10 ppm under intracellular ROS generator juglone-induced oxidative stress (Figure (c)). Additionally, enhanced survival was observed in CO (10 ppm)-treated worms by 10.87% (p = 0.0119) under thermal stress condition (Figure (d)). To investigate whether the increment in mean survival under normal and oxidative stress conditions is associated with reduction in ROS level, we employed DCF-DA method using whole live nematode for quantification of in vivo ROS. The decline of 26% (p = 0.047) in ROS level was observed in CO (10 ppm)-treated worms in comparison to untreated control worms (Figure S3). Furthermore, CO-treated mev-1 mutant worm demonstrated prolonged lifespan by 30.94 (p < 0.0001) in comparison to untreated control suggesting stress alleviating potential of CO (Figure (b), Table ). Altogether, the results suggest CO exposure mediates increment in stress tolerance level compared to untreated control. The results are supported by previous findings where enhanced stress tolerance was found to extend mean lifespan in various organisms.
3.4 CO-delayed β-amyloid proteotoxicity in C. elegans
The extension in lifespan is of little benefit if it doesn’t revive overall health of living organism. The progression in age is accompanied by an increment in oxidative stress which has implication in age-related pathologies like neurodegeneration (Shaw, Werstuck, & Chen, Citation2014). The age-related pathologies elicit protein aggregation and misfolding (Dostal & Link, Citation2010). The disruption of protein homeostasis leads to accumulation of insoluble proteins aggregate such as β-amyloids which is associated with Alzheimer’s associated neurotoxicity and cell death (Dostal & Link, Citation2010). The β-amyloids aggregation is target of various therapeutic studies as it is a marker of AD progression (Gutierrez-Zepeda, Santell, Wu, Brown, & Wu, Citation2005). Therefore, we exploited C. elegans transgenic model of human proteotoxic disease CL4176 (dvIs27 [myo3::Aβ let 3ʹUTR (pAF29); pRF4 (rol6(su1006))]), which expresses an aggregating amyloid β1–42 peptide in muscle tissue (Dostal & Link, Citation2010; Link, Citation1995, Citation2006). When this transgenic strain is subjected to temperature up shift from 16 to 26°C, worms express β-amyloid protein aggregates in body muscles and followed by paralysis. We observed delayed paralysis in CO-treated worms in comparison to untreated control worms (Figure (a)). The decline in percentage of paralyzed worms was observed in CO-treated worms as compared to control. The CO treatment decreased the proportion of paralyzed worms due to β-amyloid aggregates followed by increment in mean survival in CL4176 worms. In addition to that CO treatment demonstrated decline in ROS level in β-amyloid expressing transgenic strain CL2006 in comparison to untreated control CL2006 worms (Figure (b)). In C. elegans, increment in oxidative stress level strongly correlates with Aβ toxicity, and it has been shown that a number of natural products that reduce ROS are neuroprotective (Dostal et al., Citation2010). Altogether, CO rescued paralysis phenotype and reduced ROS level in Alzheimer’s worm model suggesting CO can ameliorate detrimental effects of β-amyloid induced proteoxicity.
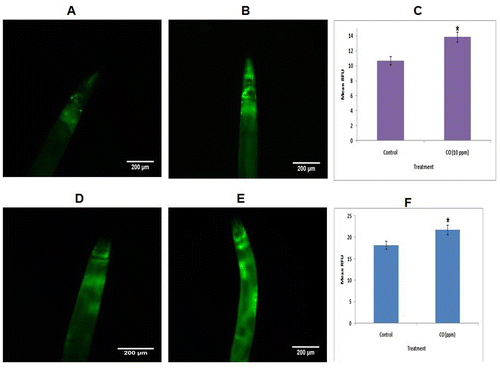
3.5. CO alters expression of stress response genes
The aging phenomenon is followed by elevation in oxidants production and decline in antioxidant enzymes which leads to compromised cellular redox homeostasis (Kenyon, Citation2010). The CO treatment lead to decline in oxidative stress, ROS level and age-related β-amyloid with extension in lifespan. Furthermore, the transgenic strains stably expressing SOD-3 and GST-4 were evaluated for change in expression on CO treatment. CO exposure up regulated the expression of sod-3 and gst-4 (Figure ). The CO-treated worms demonstrated elevation in SOD-3 expression by 29.7% (p = 0.0222) in comparison to control. Additionally, up regulation in GST-4 expression was observed on CO treatment by 19.38% (p = 0.001) in comparison to untreated control worms. Altogether, extension in mean lifespan and decline in cellular stress can be attributed to up regulation of antioxidant genes sod-3 and gst-4.
Figure 2. CO treatment-attenuated Aβ-induced paralysis in C. elegans CL4176 and intracellular ROS mediated oxidative stress due to Aβ proteotoxicity in CL2006 transgenic strains. (a) CO (10 ppm) treatment was able to rescue worms from Aβ-induced paralysis thereby enhancing mean lifespan in worms in comparison to control; (b) CO (10 ppm) treatment reduced Aβ proteotoxicity in CL2006 transgenic worms by reducing intracellular ROS mediated oxidative stress. The data are statistically analyzed using ANOVA in ASSISTAT 7.7 beta statistical assistance software. Differences between the data were considered significant at p ≤ 0.05.
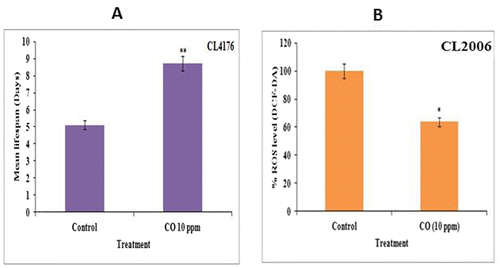
Figure 3. Effect of CO on stress response gene sod-3. (a) Control sod-3::gfp transgenic strain of C. elegans (n = 89); (b) 10 ppm CO-treated sod-3::gfp transgenic strain; (c) Quantification of SOD-3::GFP expression; (d) Control gst-4::gfp; (e) 10 ppm CO-treated gst-4::gfp transgenic strain of C. elegans ; (f) Quantification of GST-4::GFP expression. Scale bar = 200 μm. Scale bar = 200 μm. The data are statistically analyzed using ANOVA in ASSISTAT 7.7 beta statistical assistance software. Differences between the data were considered significant at p ≤ 0.05.
4. Conclusion
The present study suggests that CO modulates stress level, which leads to lifespan extension and alleviation of Aβ proteotoxicity in C. elegans. The CO exposure prolonged lifespan with alleviation of stress and age-related Aβ-proteotoxicity which can be attributed to up regulation of stress response gene gst-4 and sod-3. The neuroprotective and lifespan prolonging effects demonstrated by CO can be subjected to future investigations. The neuromodulatory and longevity promoting effects are of medical interest because of its impact on age-related pathologies like AD. Thus, EU might facilitate the development of targeted therapy for AD and several age-related disorders.
Funding
The authors are highly grateful to the Caenorhabditis Genetics Centre (CGC), Minneapolis, MN, USA, which is funded by the NIH Office of Research infrastructure Programs [grant number P40 OD010440], National Centre for Research Resources (USA), for providing the C. elegans strains.
Supplementary_Files.doc
Download MS Word (515.5 KB)Acknowledgments
The authors are also thankful to Director, CSIR-CIMAP, Lucknow and the Head of Department of Biotechnology and Botany, Kumaun University for their valuable support.
Additional information
Notes on contributors
Aakanksha Pant
Aakanksha Pant has published peer-reviewed articles in the area of aging, stress biology, dietary interventions regulating aging and age associated disorder. She has studied human homologous Caenorhabditis elegans model system for deciphering role of various signaling pathway in regulating aging phenomenon. The author has keen interest in identifying plant-based natural bioactive molecule which will be able to elicit anti-aging effects and can be utilized for formulating therapies for various stress and age disorders.
References
- Abdollahi, M., Moridani, M. Y., Aruoma, O. I., & Mostafalou, S. (2014). Oxidative stress in aging. Oxidative Medicine Cellular Longevity, 2014, 1–2.10.1155/2014/876834
- Adams, M., Gmünder, F., & Hamburger, M. (2007). Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. Journal of Ethnopharmacology, 113, 363–381.10.1016/j.jep.2007.07.016
- Adams, R. P. (2012). Identification of essential oils by ion trap mass spectroscopy. San Diego, CA: Academic Press.
- Arya, U., Dwivedi, H., & Subramaniam, J. R. (2009). Reserpine ameliorates Aβ toxicity in the Alzheimer’s disease model in Caenorhabditis elegans. Experimental Gerontology, 44, 462–466.10.1016/j.exger.2009.02.010
- Asthana, J., Pant, A., Yadav, D., Lal, R. K., Gupta, M. M., & Pandey, R. (2015). Ocimum basilicum (L.) and Premna integrifolia (L.) modulate stress response and lifespan in Caenorhabditis elegans. Industrial Crops and Products, 76, 1086–1093.10.1016/j.indcrop.2015.08.032
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics, 77, 71–94.
- Chaieb, K., Hajlaoui, H., Zmantar, T., Kahla-Nakbi, A. B., Rouabhia, M., Mahdouani, K., & Bakhrouf, A. (2007). The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytotherapy Research, 21, 501–506.10.1002/(ISSN)1099-1573
- Dostal, V., & Link, C. D. (2010). Assaying β-amyloid toxicity using a transgenic C elegans model. Journal of visualized experiments, (44), e2252–e2252.
- Dostal, V., Roberts, C. M., & Link, C. D. (2010). Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans Model of β-amyloid peptide toxicity. Genetics, 186, 857–866.10.1534/genetics.110.120436
- Epel, E. S., & Lithgow, G. J. (2014). Stress biology and aging mechanisms: Toward understanding the deep connection between adaptation to stress and longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 69, S10–S16.10.1093/gerona/glu055
- Garigan, D., Hsu, A. L., Fraser, A. G., Kamath, R. S., Ahringer, J., & Kenyon, C. (2002). Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics, 161, 1101–1112.
- Gruber, J. A. N., Tang, S. Y., & Halliwell, B. (2007). Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Annals of the New York Academy of Sciences, 1100, 530–542.10.1196/annals.1395.059
- Guarente, L., & Kenyon, C. (2000). Genetic pathways that regulate ageing in model organisms. Nature, 255–262.10.1038/35041700
- Gutierrez-Zepeda, A., Santell, R., Wu, Z., Brown, M., & Wu, Y. (2005). Soy isoflavone glycitein protects against beta-amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neuroscience, 6–54.
- Kampkötter, A., Timpel, C., Zurawski, R. F., Ruhl, S., Chovolou, Y., Proksch, P., & Wätjen, W. (2008). Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 149, 314–323.10.1016/j.cbpb.2007.10.004
- Kenyon, C. J. (2010). The genetics of ageing. Nature, 464, 504–512.10.1038/nature08980
- Kollmannsberger, H., & Nitz, S. (1994). The flavour-composition of supercritical gas extracts: III. Clove (Syzygium aromaticum) [Ueber die aromastoffzusammensetzung von hochdruck-extrakten: III. Gewuerznelken (Syzygium aromaticurn)]. Chemie, Mikrobiologie, Technologie der Lebensmittel, 16, 112–123.
- Link, C. D. (1995). Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proceedings of the National Academy of Sciences, 92, 9368–9372.10.1073/pnas.92.20.9368
- Link, C. D. (2006). C. elegans models of age-associated neurodegenerative diseases: Lessons from transgenic worm models of Alzheimer’s disease. Experimental Gerontology, 41, 1007–1013.10.1016/j.exger.2006.06.059
- Lithgow, G. J., White, T. M., Melov, S., & Johnson, T. E. (1995). Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proceedings of the National Academy of Sciences, 92, 7540–7544.10.1073/pnas.92.16.7540
- Pant, A., Saikia, S. K., Shukla, V., Asthana, J., Akhoon, B. A., & Pandey, R. (2014). Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Experimental Gerontology, 57, 81–95.10.1016/j.exger.2014.05.007
- Shaw, P. X., Werstuck, G., & Chen, Y. (2014). Oxidative stress and aging diseases. Oxidative Medicine and Cellular Longevity, 2014, 569146.
- Smith, J. V., & Luo, Y. (2003). Elevation of oxidative free radicals in Alzheimer's disease models can be attenuated by Ginkgo biloba extract EGb 761. Journal of Alzheimer's Disease, 5, 287–300.
- Srivastava, A. K., Srivastava, S. K., & Syamsundar, K. V. (2005). Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour and Fragrance Journal, 20, 51–53.10.1002/(ISSN)1099-1026
