Abstract
The ethanol extract of the leaf of Musa paradisiaca and its aqueous fraction were investigated for antibacterial potentials against Gram-positive and Gram-negative organisms of clinical importance. Phytochemical screenings showed the presence of secondary metabolites namely tannin, flavonoids, alkaloids among others. When the extracts were subjected to susceptibility test, results showed that while both the ethanol extract and its aqueous fraction showed activity against the test organisms, the aqueous fraction showed better antibacterial activity. The minimum inhibitory concentration of the aqueous fraction ranged from 3.125 to 25 mg/ml. Acute toxicity test of the aqueous fraction using Swiss Albino mice gave an LD50 of 489.9 mg/kg body weight which indicates a relative toxicity of the extract. The time–kill studies demonstrated a reduction in the viable cell count for the extract.
Public Interest Statement
Treatment of infectious diseases has always been a challenge to man. As scientists continue to search for new agents for the treatment of infectious diseases, plants have proved to be a reliable source. This research seeks to provide the scientific basis for the use of Musa paradisiaca (plantain) leaf for such purpose and perhaps establish it as a possible source of a new drug against some organisms. The ethanol extract of the leaf and its aqueous fraction were therefore tested against some organisms. The extract showed activity by inhibiting the growth of these organisms and causing a reduction in their viable count with time while showing only a moderate toxicity in mice. These findings provide scientific explanation for the use of this plant leaf in traditional medicines and shows that it can be exploited as a possible source of new antibiotics.
Competing Interest
The authors declare no competing interest.
1. Introduction
For a long period of time, plants have been a valuable source of natural products for maintaining human health (Karadi, Shah, Parekh, & Azmi, Citation2011). About 80% of individuals from developed countries use traditional medicine, which has compounds derived from medicinal plants (World Health Organisation, Citation1997). Many plants have been used because of their antimicrobial potentials, which are due to compounds synthesized in the secondary metabolism of the plant such as the phenolic compounds which are part of the essential oils as well as in tannins (Venkanna & Estari, Citation2012).
Thousands of plants have been studied and most have been found to possess phytochemicals which are safe and broadly effective alternatives with less adverse effect. Many beneficial biological activities such as antimicrobial, antioxidant, antidiarrhoeal, analgesic, antiulcerative, antihypertensive and wound healing activity have been reported (Agarwal et al., Citation2009; Alisi, Nwanyanwu, Akujobi, & Ibegbulem, Citation2008; Goel & Sairam, Citation2002; Osim & Ibu,Citation1991; Rabbani et al., Citation2001; Singh & Dryden, Citation1990; Yin, Quan, & Kanazawa, Citation2008).
Musa paradisiaca also known as plantain is a familiar tropical fruit and important source of food in the world and is consumed as an energy yielding food and desert (Ighodaro, Citation2012). It is extensively cultivated in the tropics and is a staple crop for over 70 million people of the Sub-Saharan Africa (Yusoff, Citation2008). Various parts of the plant such as the leaves, roots and flowers have been reportedly used for medicinal purposes. For example, the leaf juice is used in the treatment of fresh wounds, cuts and insect bites (Onyenekwe, Okereke, & Owolewa, Citation2013). The sap of the plant has been used as a remedy for epilepsy, hysteria and in dysentery and diarrhoea, the roots as anthelmintic, flowers as astringent and fruits as mild laxatives (Okareh, Adeolu, & Adepoju, Citation2015). A cold infusion of the root has been used to treat venereal diseases and anaemia. In addition, the fruit has been reportedly used as antiscorbutic, aphrodisiac and diuretic (Gill, Citation1992). Plantain has been reported to have a high fibre content, and thus is capable of lowering cholesterol and helps to relieve constipation and hence prevention of colon cancer. In Nigeria, traditional medicine practitioners have used the leaf decoction of plantain and banana for treatment of typhoid fever, diarrhoea, malaria, stomach ache or ulcers (Apata, Citation1979; Okigbo & Omodamiro, Citation2006). Okorondu, Akujobi, and Nwachukwu (Citation2012) have reported the antifungal properties of the peel and stalk extracts of M. paradisiacal. Karadi et al. (Citation2011) and Karuppiah and Mustaffa (Citation2013) have reported the activity of the leaf extract against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa while Sahaa, Acharyaa, Shovon, and Royd (Citation2011) have reported antibacterial activities of the leaf extract against Salmonella typhi, Shigella dysenteriae and Bacillus cerus.
A number of new antibiotics have been produced by pharmaceutical industries in the last 30 years. Resistance to these drugs by micro-organisms has been increased day by day (Karuppiah & Mustaffa, Citation2013). WHO 2014 report on global surveillance of antimicrobial resistance revealed that antibiotic resistance is no longer a prediction for the future; it is happening right now, across the world, and is putting at risk the ability to treat common infections in the community and hospitals. In line with this, new drugs, both synthetic and natural must be sought to treat diseases. This implies that local medicinal plants need to be screened for antimicrobial properties of their extracts against known organisms which rely on the bioactive phytocomponents present in the plants (Karadi et al., Citation2011; Okorondu, Citation2011; Okorondu, Sokari, Akujobi, & Braide, Citation2010; Veeramuthu, Muniappan, & Savarimuthu, Citation2006).
This research work is aimed at providing scientific basis for the use of this plant traditionally to treat different ailments and also provide evidence for the possible use of this plant to produce new drugs for the treatment of infections caused by micro-organisms used in this study.
2. Materials and methods
2.1. Plant collection, authentication, preparation and extraction
Fresh plantain leaves were collected from the plantain plantation of Chief E.A. Bassey in Use Ndon, Ibiono Ibom Local Government Area, Akwa Ibom state. The botanical authentication was done at the Department of Pharmacognosy, Faculty of Pharmacy, University of Uyo, Uyo. The herbarium number is UUPH 51(a).
The leaves were cut into smaller pieces for easy drying. The dried leaves were powdered using a mortar and pestle. The powdery samples were packed into screwed bottles and labelled appropriately. The ethanol extract of the leaf of M. paradisiaca was prepared by soaking 300 g of the dried powdery samples in 2,500 ml of ethanol for 48 h, during which the mixture was intermittently shaken. It was later filtered through Whatman No. 42 filter paper. The extracts were evaporated to dryness at 40°C in a water bath.
2.2. Fractionation of extract
The ethanol extract was fractionated using petroleum ether, chloroform and water according to the method of Udobi, Onaolapo, and Agunu (Citation2008). Twenty grams of the extract was dissolved in 200 ml of water before shaking vigorously in a separating flask. The mixture obtained was filtered using a filter paper to remove debris. Two hundred millilitres of petroleum ether was then added to the mixture in a separating funnel, shaken vigorously and allowed to settle. The petroleum ether layer (on top) was removed and concentrated while a further 200 ml of chloroform was added to the aqueous layer and also vigorously shaken and allowed to settle. The aqueous and the chloroform layers were further separated, the chloroform portion was concentrated to dryness by allowing to stand on the laboratory bench until all the solvent evaporated, while the aqueous layer was concentrated to dryness using the water bath at 40°C.
2.3. Phytochemical screening
The ethanol extract was screened for the presence of alkaloids, saponins, tanins, carbohydrates, flavonoids, anthraquinones, cardiac glycosides, deoxy sugar, steroids and terpenes using standard methods as described by Harborne (Citation1973), Sofowora (Citation1993), Trease and Evans (Citation1986), William, Trease, and Evans (Citation1996).
2.4. Preparation of culture media
The culture media used namely nutrient agar and nutrient broth (Oxiod, England), were prepared according to the manufacturer’s instruction, sterilized in an autoclave at 121°C for 20 min and allowed to cool to about 45°C.
2.5. Preparation of inoculum
Test inoculum was prepared according to the method of Chinweizu (Citation2010). Nutrient broth medium was used as the diluent. The Gram-positive organisms were diluted to 1:1000 while the Gram-negative organisms were diluted to 1:5000 to avoid overcrowding.
2.6. Susceptibility testing
The agar well diffusion method was used to carry out this test. 0.1 ml of 1:1000 dilution of test organism for Gram-positive and 1:5000 dilution of test organism for Gram-negative were introduced into labelled sterile Petri dishes. Twenty millilitres of the cooled nutrient agar medium was aseptically poured into each Petri dish and gently swirled to mix. The plates were allowed to set and wells were created using sterile 4-mm cork borer. Different concentrations (200–12.5 mg/ml) of the extract diluted with water was introduced into the different wells and labelled appropriately. The Petri dishes were allowed to stand for 20 min before incubation at 37°C for 24 h. The diameters of the zones of growth inhibition were measured in millimetres (mm).
2.7. Minimum inhibitory concentration
Agar dilution method was used to determine the minimum inhibitory concentration (MIC). The medium used was nutrient agar medium. 50 mg/ml of the extract was prepared in warm water. Ten millilitres of the extract (50 mg/ml) was transferred aseptically into a universal bottle containing 10 ml of warm nutrient agar prepared as double strength. The agar was mixed well and this gave a concentration of 25 mg/ml. A twofold serial dilution was carried out with 10 ml of single strength nutrient agar to have concentrations of 12.5, 6.25, 3.125 and 1.562 mg/ml, respectively. The agar was immediately transferred aseptically into labelled sterile Petri dishes and allowed to set. The plates were inoculated with a 0.1 ml each of 1:1000 dilution of test organism for Gram-positive and 1:5000 dilution of test organism for Gram-negative. Incubation was done at 37°C for 24 h. The plates were examined for the present of microbial growth. The lowest concentration of the extract that showed no visible growth of test organisms was considered as the MIC.
2.8. Acute toxicity testing
Lorke’s method (Lorke, Citation1983) was used to assess the lethal dose (LD50) of the aqueous fraction of the ethanol extract of M. paradisiaca leaf that kills 50% of the test animal population. In the first phase, nine healthy mice were divided into three groups of three animals each. The animals were fasted for 24 h. Each group of animals were administered different doses (10, 100 and 1,000 mg/kg body weight) of the plant extract. The animals were placed under observation for 24 h and monitored for mortality. The second phase involved the use of four mice which were distributed into four groups of one animal each. The animals were administered different doses (200, 400, 600, 800 mg/kg body weight) of the plant extract according to the specifications of the method. They were then observed for 24 h and mortality taken note of. All experimental protocols were in compliance with the Faculty of Pharmacy University of Uyo ethics on research in animals as well as internationally accepted principles for laboratory animal use and care.
2.9. Time–kill test
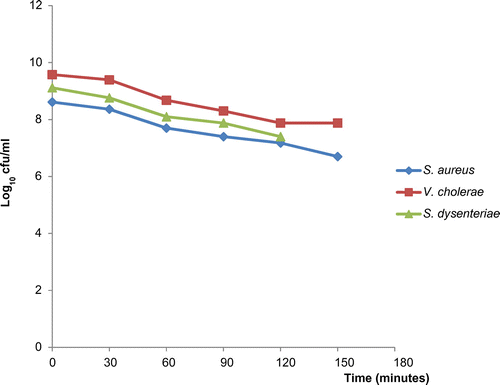
The time–kill test was carried out according to the method of Coudron and Stratton (Citation1995). This test is used to assess the in vitro reduction of a microbial population of test organisms after exposure to a test material. The organisms used were S. aureus, Vibrio cholerae and S. dysenteriae. Three test tubes containing 8 ml of sterile nutrient broth each were labelled according to the organisms; one test tube was used as control. One millilitre of the aqueous fraction of the ethanol extract of M. paradisiaca leaf was introduced into the test tubes in concentrations that gave a final concentration corresponding to the MIC of each organism, 3.125 mg/ml for S. aureus and S. dysenteriae, 12.5 mg/ml for V. cholerae. One millilitre of a standardized inoculum of overnight broth culture was introduced into each of the test tube. The test tubes were incubated at 37°C with shaking. At 30 min intervals, 0.1 ml was withdrawn from each test tube and diluted to 102 dilution factor. 0.1 ml of this dilution was aseptically transferred into sterile Petri dishes containing nutrient agar medium in triplicates. The plates were incubated at 37°C for 24 h. After the incubation period, the number of colony on each plate was enumerated and the colony forming units calculated, expressed in log10. This was used to generate a time–kill curve for each organism by plotting the log10 colony forming unit against time.
3. Results
3.1. Phytochemical screening
The phytochemical screening showed the presence of some secondary metabolites which includes alkaloids, saponins, tannins, cardiac glycosides, terpenes, deoxy sugar, flavonoids and carbohydrates. The result is presented in Table .
Table 1. Result of the phytochemical screening of the ethanol leaf extract of Musa paradisiaca
3.2. Susceptibility testing
Microbial susceptibility test with the crude ethanol extract and the aqueous fraction of M. paradisiaca leaf showed zones of growth inhibitions whose diameters were measured in millimetres (mm) and presented in Tables and , respectively.
Table 2a. Growth inhibition zones of different concentrations of the crude ethanol extract of M. paradisiaca leaf
Table 2b. Growth inhibition zones of different concentrations of the aqueous fraction of the ethanol extract of M. paradisiaca leaf
3.3. Minimum inhibitory concentration
The results obtained showed the minimum concentration of the aqueous fraction of the ethanol extract of M. paradisiaca leaf that inhibits the growth of the different micro-organisms as presented in Table .
Table 3. MIC of the aqueous fraction of the ethanol extract of M. paradisiaca leaf
3.4. Acute toxicity testing
The concentration of the aqueous fraction of the ethanol extract of M. paradisiaca leaf that killed 50% of mice, expressed as LD50 is presented in Table .
Table 4. Acute toxicity test of the aqueous fraction of the ethanol extract of M. paradisiaca leaf
3.5. Time–kill test
The mean of the number of colonies obtained per plate in respect to the time interval was used to calculate the colony forming unit, cfu/ml of the isolates and log10 cfu/ml.
4. Discussion
The ability of plants to synthesize a wide variety of chemical compounds that are used to perform important biological functions have been the basis for the use of plants to effectively treat human diseases (Harborne, Citation1973). Even though pharmaceutical industries have produced a number of new antibiotics in the last four decades, resistance to these drugs by micro-organisms has increased (Karadi et al., Citation2011) and therefore there is need for research on plant materials with antimicrobial properties.
In this regard, M. paradisiaca, commonly known as plantain was studied to extract the active components of the leaf, carry out phytochemical screening of the crude extract and identify the secondary metabolites present. The antibacterial activities of these metabolites against selected Gram-positive and Gram-negative clinical isolates were further studied.
Bioactive compounds found to be present in this plant include alkaloids, saponins, tannins, cardiac glycosides, terpenes, deoxy sugar, flavonoids and carbohydrates as presented in Table . The findings of this study is consistent with reports of the presence of these phytochemicals in various parts of the M. paradisiaca plant as documented by Akpuaka and Ezem (Citation2011) and Akpabio, Udiong, and Akpakpan (Citation2012). These phytochemicals have been reported to exert multiple biological and pharmacological effects (antibacterial, antihypertensive, antidiabetic and anti-inflammatory activities) (Ighodaro, Citation2012). The presence of these bioactive substances in M. paradisiaca leaves therefore suggests that the leaves possess valuable medicinal potential that can be explored.
The results of the antimicrobial screening of the crude ethanol extract and the aqueous fraction of M. paradisiaca leaf against five human pathogenic microbes showed that both extracts were less effective when compared to a reference antibiotic, cefuroxime. S. aureus, Bacillus subtilis, P. aeruginosa, V. cholerae and S. dysenteriae were comparatively more inhibited by both extracts (Tables and ). The aqueous fraction of the ethanol extract exhibited higher antimicrobial activities compared to the crude ethanol extract (Tables and ).This is because the secondary metabolites being polar compounds like water have been pooled into the aqueous fraction during fractionation. S. aureus had a wider zone of inhibition than others, indicating a possibility in the use of the plant to treat infections due to the organism which are on the increase. The antimicrobial properties of plant extracts had been attributed to the presence of alkaloids and flavonoids (Okorondu et al., Citation2012) which have been found present in this plant. Alkaloids, flavonoids and tannins have been known to show medicinal activity as well as exhibiting physiological activity (Sofowora, Citation1993). Tannins in plants have been shown to confer antidiarrhoeic properties on plants (Asquith & Butler, Citation1986). This is consistent with the traditional use of the leaf of M. paradisiaca for the treatment of diarrhoea (Onyenekwe et al., Citation2013) and is evident in the activity of the extract against S. dysenteriae and V. cholerae which causes diarrhoea. Flavonoids are known to have antioxidant effects, the presence of these phytochemicals in the leaf of M. paradisiaca confers medicinal properties on the plant and this explains the use of this plant for treatment of different ailments (Kim, Kim, Kim, Oh, & Jung, Citation1994). Okorondu et al. (Citation2012) reported that alkaloids are phytochemical components with bitter taste and possess antimicrobial properties. The microbes against which the extracts were effective are pathogens already implicated in the aetiology and severity of human diseases. Thus, the plant extract may be useful in pharmaceutical and medical formulations. Therefore, further purification of the extract and formulation into antibiotics is suggested.
The lowest concentration of the plant extract required for inhibiting the growth of test organisms (MIC) was considered. The MIC values of the aqueous fraction against the test micro-organisms presented on Table , ranged from 3.125 mg/ml for S. aureus and S. dysenteriae to 25 mg/ml for P. aeruginosa.
The safety of the aqueous fraction of the ethanol extract of M. paradisiaca leaf was evaluated using Lorke’s method of determination of acute toxicity (Lorke, Citation1983). The median lethal dose that killed 50% of the test animal, LD50 was 489.9 mg/kg body weight. Ngulde, Tijjani, Ihopo, and Ya’uba (Citation2013) states that any extract with LD50 greater than 5,000 mg/kg is considered as safe. Therefore, the aqueous fraction of the ethanol extract of M. paradisiaca leaf can be said to be moderately toxic being less than 5,000 mg/kg.
The in vitro time–kill regimes of the aqueous fraction of the ethanol extract of M. paradisiaca against S. aureus, V. cholerae and S. dysenteriae was assessed using standard microbiological procedures. The results are presented in Table . The extract demonstrated a bacteriostatic activity showing reduction in bacterial count due to growth inhibition since the MIC was used. The log reduction in viable cell count in the time–kill assay within 2 h 30 min ranged from 8.613 to 6.699 log10 for S. aureus, 9.576 to 7.875 log10 for V. cholerae and 9.114 to 7.398 log10 for S. dysenteriae. The downward steep of the time–kill curves (Figure ) generated shows that the log reduction of microbial population is proportionate with time of exposure. This time–kill studies corroborates the reported efficacies of preliminary antibacterial study of selected plant extracts as reported by Oladosu, Isu, Ibrahim, and Ibrahim (Citation2010) and this supports the folkloric uses of these plants in the treatment of different ailments among the traditional people.
Table 5. Results of the time–kill test of the aqueous fraction of the ethanol extract of M. paradisiaca leaf against S. aureus, V. cholerae and S. dysenteriae
5. Conclusion
The results of this study indicate that the crude and the aqueous fraction of the ethanol extract of M. paradisiaca leaf possess antibacterial activity. The aqueous fraction showed greater activity than the crude extract against the test organisms. The Gram-positive organisms responded better than the Gram-negative, this may be due to the difference in the morphology of the cell wall, especially the presence of Gram-negative cell envelope. From the MIC values obtained from this study, it can be concluded that the concentration of M. paradisiaca leaf extract required to arrest the growth of test organisms is relatively less. The time–kill curves clearly show the ability of the extract to reduce microbial population exponentially with time. As such, the antibacterial activity of M. paradisiaca can be exploited for the treatment of infections caused by the test organism at relatively low doses to avoid toxicity.
Additional information
Funding
Notes on contributors
Chinweizu Ejikeme Udobi
Chinweizu Ejikeme Udobi, PhD, is a senior lecturer in the pharmacy school of the University of Uyo where he teaches pharmaceutical microbiology and related courses. Among other areas, his research interest includes the development of novel antimicrobial agents from natural sources. Together with members of his research team, Ejikeme has published extensively on the potentials of most plants of tropical Africa for use in the development of new anti-infective agents in these days of continuous development of resistance by organisms. Ememobong Gideon Asuquo is an active member of Ejikeme’s research team.
References
- Agarwal, P. K., Singh, A., Gaurav, K., Goel, S., Khanna, H. D., & Goel, R. K. (2009). Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian Journal of Experimental Biology, 47, 322–340.
- Akpabio, U. D., Udiong, D. S., & Akpakpan, A. E. (2012). The physicochemical characteristics of plantain (Musa paradisiaca) and banana (Musa sapientum) pseudo stem wastes. Advances in Natural and Applied Sciences, 6, 167–172.
- Akpuaka, M. U., & Ezem, S. N. (2011). Preliminary photochemical screening of some Nigerian dermatological plants. Journal of Basic Physiological Research, 2(1), 1–5.
- Alisi, C. S., Nwanyanwu, C. E., Akujobi, C. O., & Ibegbulem, C. O. (2008). Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (var Sapientum). African Journal of Biotechnology, 7, 1821–1825.10.5897/AJB
- Apata, L. (1979). The practice of herbalism in Nigeria. In A. Sofowora (Ed.), African medicinal plants (pp. 13–19). Ile Ife: Proceedings of a Conference, University of Ife Press.
- Asquith, T. N., & Butler, L. G. (1986). Interactions of condensed tannins with selected proteins. Phytochemistry, 25, 1591–1593.10.1016/S0031-9422(00)81214-5
- Chinweizu, E. U. (2010). Parkia biglobosa (African Locust Bean): The medicinal tree of the future. Saarbrucken: Lap Lambert Academic Publishing/AG and Co. Saarbrucken.
- Coudron, P. E., & Stratton, C. W. (1995). Use of time-kill methodology to assess antimicrobial combinations against metronidazole-susceptible and metronidazole-resistant strains of Helicobacter pylori. Antimicrobial Agents and Chemotherapy, 39, 2641–2644.10.1128/AAC.39.12.2641
- Gill, L. S. (1992). Ethnomedicinal uses of plants in Nigeria (pp. 169–170). Benin City: University of Benin Press.
- Goel, R. K., & Sairam, K. (2002). Anti-ulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasma, Asparagus racemosus and Zingiber officinale. Indian Journal of Pharmacology, 34, 100–110.
- Harborne, J. B. (1973). Phytochemical methods: A guide to modern techniques of plant analysis. London: Chapman and Hall Limited.
- Ighodaro, O. M. (2012). Evaluation study on Nigerian species of Musa paradisiaca peels: Phytochemical screening, proximate analysis, mineral composition and antimicrobial activities. Researcher, 4, 17–20.
- Karadi, R. V., Shah, A., Parekh, P., & Azmi, P. (2011). Antimicrobial activities of Musa paradisiaca and Cocos nucifera. International Journal of Research in Pharmaceutical and Biomedical Sciences, 2, 264–267.
- Karuppiah, P., & Mustaffa, M. (2013). Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pacific Journal of Tropical Biomedicine, 3, 737–742.10.1016/S2221-1691(13)60148-3
- Kim, S. Y., Kim, J. H., Kim, S. K., Oh, M. J., & Jung, M. Y. (1994). Antioxidant activities of selected oriental herb extracts. Journal of the American Oil Chemists’ Society, 71, 633–640.10.1007/BF02540592
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Archives of Toxicology, 54, 275–287.10.1007/BF01234480
- Ngulde, S. I., Tijjani, M. B., Ihopo, J. M., & Ya’uba, A. M. (2013). Antitrypanasomal potency of methanol extract of Cassia arereh Delile root bark in albino rats. International Journal of Drug Research and Technology, 3(1), 1–7.
- Okareh, O. T., Adeolu, A. T., & Adepoju, O. T. (2015). Proximate and mineral composition of plantain (Musa paradisiaca) wastes flour; a potential nutrient source in the formation of animal feeds. African Journal of Science and Technology, 6, 53–57.
- Okigbo, R. N., & Omodamiro, O. D. (2006). Antibacterial effects of leaf extracts of pigeon pea (Cajanuscajan (L) Mill sp.) on some human pathogens. Journal of Herbs, Spices and Medicinal Plants, 12, 117–127.
- Okorondu, S. I. (2011). Evaluation of the antifungal properties of picralima nitida seed extracts. International Journal of Natural and Applied Sciences, 7, 41–46.
- Okorondu, S. I., Sokari, T. G., Akujobi, C. O., & Braide, W. (2010). Phytochemical and antibacterial properties of Musa paradisiaca stalk plant. International Journal of Biological Science, 2, 128–132.
- Okorondu, S. I., Akujobi, C. O., & Nwachukwu, I. N. (2012). Antifungal properties of Musa paradisiaca (plantain) peel and stalk extracts. International Journal of Biological Science, 6, 1527–1534.
- Oladosu, P., Isu, N. R., Ibrahim, K., & Ibrahim, J. A. (2010). Ethnobotanical survey and preliminary evaluation of some selected medicinal plants used in the treatment of tuberculosis in some parts of Northern Nigeria. ZUMA Journal of Applied Sciences, 8, 1–4.
- Onyenekwe, P. C., Okereke, O. E., & Owolewa, S. O. (2013). Phytochemical screening and effect of Musa paradisiaca stem extrude on rat haematological parameters. Current Research Journal of Biologicl Sciences, 5, 26–29.
- Osim, E. E., & Ibu, J. O. (1991). The effect of plantains (Musa paradisiaca) on DOCA-induced hypertension in rats. Pharmaceutical Biology, 29, 9–13.10.3109/13880209109082841
- Rabbani, G. H., Teka, T., Zaman, B., Majid, N., Khatun, M., & Fuchs, G. J. (2001). Clinical studies in persistent diarrhea: Dietary management with green banana or pectin in Bangladeshi children. Gastroenterology, 121, 554–560.10.1053/gast.2001.27178
- Sahaa, K., Acharyaa, S., Shovon, S. S. H., & Royd, P. (2011). Medicinal activities of leaves of Musa sapientum var. sylvesteris in vitro. Dhaka: Department of pharmacy, East West University.
- Singh, Y. N., & Dryden, W. F. (1990). The augmenting action of banana tree juice on skeletal muscle contraction. Toxicon, 28, 1229–1236.10.1016/0041-0101(90)90122-N
- Sofowora, A. (1993). Medicinal plants and traditional medicine in Africa (pp. 191–289). Ibadan: Spectrum Books.
- Trease, G. E., & Evans, W. C. (1986). Textbook of Pharmacognosy (13th ed.) (pp. 343–383). London: Bailliere Tindal.
- Udobi, C. E., Onaolapo, J. A., & Agunu, A. (2008). Antibacterial activities and bioactive components of the aqueous fraction of the stem bark of Parkia biglobosa (Jacq) (Mimosacea). Nigerian Journal of Pharmaceutical Science, 7, 76–82.
- Veeramuthu, D., Muniappan, A., & Savarimuthu, I. (2006). Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complementary and Alternative medicine, 6, 35.
- Venkanna, L., & Estari, M. (2012). In vitro antimicrobial activity of some medicinal plants used by tribes in Warangal district (Andhra Pradesh) India. Biology and Medicine, 4, 85–88.
- William, C. E., Trease, G. E., & Evans, E. C. (1996). Pharmacognosy (14th ed.). WB, Saunders Limited: London.
- World Health Organisation. (1997). Monongraph on selected medicinal plants. Geneva: WHO VBC/97.1.
- Yin, X., Quan, J., & Kanazawa, T. (2008). Banana prevents plasma oxidative stress in healthy individuals. Plant Foods for Human Nutrition, 63, 71–76.10.1007/s11130-008-0072-1
- Yusoff, N. A. B. (2008). Correlation between total phenolics and mineral content with antioxidant activity and determination of bioactive compounds in various local bananas (Musa spp.) Penang: Universiti Sains Malaysia.