 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Marine natural products have been increasingly found to be a promising source of drug candidates for fighting human diseases. The present study was carried out to assess the antimicrobial properties of a brown alga, Turbinaria ornata. Hexane, dichloromethane, methanol, and water extracts were tested against 23 micro-organisms including Gram-positive and negative bacteria, yeasts, and fungi. The disk diffusion method was employed followed by modified resazurin microtitre assay (REMA). The results obtained from modified REMA using both methods of colorimetric and fluorometric were compared. The best antimicrobial activity was recorded in dichloromethane extract for disk diffusion. Further, modified REMA showed inhibition in Bacillus cereus, Bacillus subtilis, Staphylococcus aureus ATCC 25923, S. aureus, Staphylococcus epidermidis, Staphylococcus saprophyticus, Enterococcus faecalis, Pseudomonas aeruginosa ATCC 27853, Candida parapsilosis ATCC 22019, Candida guilliermondii ATCC 6260, and Saccharomyces cerevisiae. Both methods of modified REMA were substantially in agreement with each other based on Cohen’s kappa statistical analysis (κ value = 0.712; p < 0.0005). Our findings suggested that T. ornata dichloromethane extract has the potential to be used as a source of antimicrobial compounds.
Public Interest Statement
Micro-organisms constantly evolve to adapt to new environments. As such, antimicrobial resistance is the ability of micro-organisms to grow in the presence of a chemical (drug) that would normally kill them or limit their growth. As a result of antimicrobial resistance, existing antibiotics become less and less effective in eliminating infections caused by these micro-organisms. One of the ways to address this issue is to develop new antibiotics. This paper discusses the potential of sourcing antimicrobial compounds from a marine alga, Turbinaria ornata. To test the antimicrobial properties of the marine alga’s extract, a chemical indicator, resazurin was used. There are two methods of using resazurin to indicate antimicrobial properties; the authors used both methods and tried to analyze if the two methods were agreeable to each other.
Competing Interests
The authors declare no competing interest.
1. Introduction
Great interest has been developed in sourcing natural products from the marine environment due to the ocean’s unique biodiversity (Baker, Chu, Oza, & Rajgarhia, Citation2007). Algae biosynthesize compounds called secondary metabolites in order to adapt to surrounding environment and to protect themselves against predators or pathogens (Lane et al., Citation2009). In addition, algae were reported to possess various biological properties such as antioxidant, anti-inflammatory, antitumor, antimicrobial, and anticoagulant (Ananthi et al., Citation2010; Aravindan, Delma, Thirugnanasambandan, Herman, & Aravindan, Citation2013; Manivannan, Karthikai Devi, Anantharaman, & Balasubramanian, Citation2011; Wang, Zhang, Zhang, Hou, & Zhang, Citation2011).
Antimicrobial resistance is a worldwide hazard and new resistance mechanisms have been emerging and spreading globally. Antimicrobial resistance results in prolonged illness, higher health costs, and higher risks of death (World Health Organization, Citation2015). One of the ways to address the issue of antimicrobial resistance is to actively encourage innovations and development of new antimicrobials. There is a great potential for sourcing antimicrobials from brown algae such as Colpomenia sinuosa, Dictyota dichotoma, D. dichotoma var. implexa, Petalonia fascia, and Scytosiphon lomentaria which possessed antimicrobial properties against Bacillus subtilis and Staphylococcus aureus. Compounds identified from these macroalgae included hydrocarbons, terpenes, acids, phenols, sulfur-containing compounds, and aldehydes (Demirel, Yilmaz-Koz, Karabay-Yavasoglu, Ozdemir, & Sukatar, Citation2009). Compounds such as phytol, neophytadiene, fucosterol, palmitoleic, and oleic acids had been identified in Himanthalia elongate and Synechocystis sp. These compounds were active against Escherichia coli and S. aureus (Plaza et al., Citation2010).
Turbinaria ornata is a brown alga under the family Sargassaceae. It is known to produce phenolic compounds such as phlorotannins (Girija, Hemalatha, Saranya, Parthiban, & Anantharaman, Citation2013) which were active against S. aureus, methicillin-resistant S. aureus (MRSA), Salmonella spp. and E. coli (Eom, Kim, & Kim, Citation2012). Using agar well diffusion method, Jeyaseelan (Citation2012) reported that direct ethanol extract and sequentially extracted acetone and ethanol extracts of T. ornata were found to inhibit growth of E. coli and S. aureus. Furthermore, methanolic extract of T. ornata contained phenolic compounds which exhibited activities against nine Gram-positive and negative bacteria based on agar well diffusion test (Vijayabaskar & Shiyamala, Citation2011).
Conversely, it is also possible to detect antimicrobial activity in lipophilic extracts of algae. Metabolites with high lipophilicity have the advantage of being able to remain potent for longer periods as they diffuse slowly into seawater (Cortés et al., Citation2014). Therefore, four different solvents (dichloromethane, hexane, methanol, and water) with the potential to extract both hydrophilic and lipophilic compounds were employed in the extraction of T. ornata. These extracts were tested for antimicrobial activity against a total of 23 micro-organisms consisting of Gram-positive, Gram-negative bacteria, yeasts and fungi. This study utilized the modified resazurin microtitre assay (REMA) where methods of visual observation (colorimetric) and emission intensity (fluorometric) were compared.
2. Results and discussion
2.1. Results for disk diffusion assay
Both hexane and dichloromethane extracts showed growth inhibition in all seven strains of Gram-positive bacteria tested (Table ). Dichloromethane extract had stronger antimicrobial activity in Bacillus cereus, B. subtilis, S. aureus ATCC 25923, and Staphylococcus epidermidis compared to hexane extract. For dichloromethane extract, except for S. aureus ATCC 25923 and S. aureus, in which inhibition started at 10 mg/mL, the rest of the strains were inhibited at the lowest extract concentration of 3 mg/mL. Methanol extract only exhibited moderate zones of inhibition at higher extract concentrations of 50 mg/mL in strains of B. cereus, B. subtilis, S. aureus ATCC 25923, and Staphylococcus saprophyticus and 100 mg/mL in S. aureus. As for water extract, no antimicrobial activity was detected in all strains of Gram-positive bacteria.
Table 1. Antimicrobial activity of T. ornata extracts against Gram-positive bacteria by disk diffusion method
Out of the six Gram-negative bacteria tested, Pseudomonas aeruginosa was the only Gram-negative bacteria inhibited by dichloromethane extract starting at concentration of 50 mg/mL with zone of inhibition 7.00 ± 0.00 mm (Table ). Methanol extract only inhibited growth of Enterobacter spp. at 100 mg/mL with zone of inhibition recorded as 8.33 ± 0.58 mm. There was no inhibition observed in all Gram-negative bacteria using the hexane extract as well as water extract.
Table 2. Antimicrobial activity of Turbinaria ornata extracts against Gram-negative bacteria by disk diffusion method
Dichloromethane extract caused growth inhibition in three strains of yeasts, namely Candida guilliermondii ATCC 6262, Candida parapsilosis ATCC 22019, and Saccharomyces cerevisiae (Table ). Hexane extract inhibited S. cerevisiae as well, but only at higher extract concentrations (≥25 mg/mL) and smaller zones of inhibition were observed as compared to dichloromethane extract. C. parapsilosis ATCC 22019 was the only yeast inhibited by methanol extract, again at higher extract concentrations (≥50 mg/mL) and smaller zones of inhibition compared to dichloromethane extract. The growth of all strains showing positive results was inhibited by the extracts in a concentration-dependent manner. All four extracts were not active against the three strains of fungi tested (Aspergillus niger ATCC 46404, Aspergillus fumigatus, and Penicillium chrysogenum).
Table 3. Antimicrobial activity of T. ornata extracts against yeasts by disk diffusion method
2.2. Results for modified resazurin microtitre assay
The results obtained in REMA are tabulated in Table . The organisms tested were most susceptible to dichloromethane extract. Percentage viability of microbial cells obtained from fluorometric REMA for each extract of T. ornata is illustrated in Figures .
Table 4. Antimicrobial activity of T. ornata extracts against bacteria and yeasts using REMA
Figure 1. Percentage viability of microbial cells tested using different concentrations of Turbinaria ornata hexane extract in fluorometric REMA.
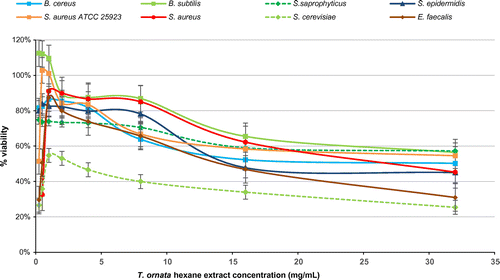
Figure 2. Percentage viability of microbial cells tested using different concentrations of Turbinaria ornata dichloromethane extract in fluorometric REMA.
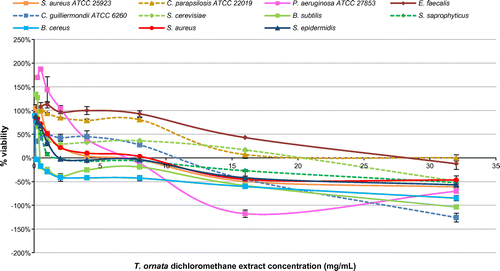
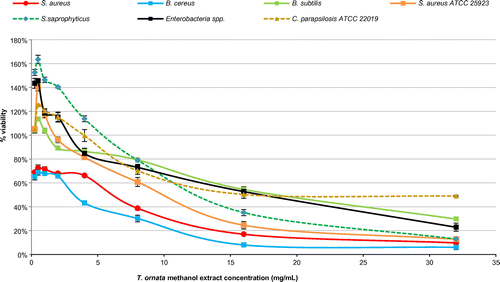
Figure 3. Percentage viability of microbial cells tested using different concentrations of Turbinaria ornata methanol extract in fluorometric REMA.
Cohen’s κ was conducted using SPSS (IBM Corporation) to evaluate the extent of agreement between readings obtained from the experiment using the methods of colorimetric and fluorometric REMA. The κ value obtained was 0.712 with p < 0.0005, indicating there was substantial agreement between the two methods following the suggestions for kappa-statistic interpretation by Landis and Koch (Citation1977). Since p < 0.0005, the κ coefficient was statistically significantly different from zero.
2.3. Discussion
In this study, it was observed that dichloromethane was the best solvent for extracting the effective antimicrobial compounds from T. ornata when this extract was tested against the selected strains of microbes compared to hexane, methanol, and water extracts. Demirel et al. (Citation2009) utilized the three solvents (hexane, dichloromethane, methanol) in extraction of five brown algae; the dichloromethane extracts were found to have more potent antimicrobial activity then the hexane and methanol extracts at extract concentrations of 1.0 and 1.5 mg/disk. However, it was found in the present study that at extract concentrations of 1.0 mg/disk, T. ornata dichloromethane extract possessed superior activity than that of the dichloromethane extracts of brown algae reported by Demirel et al. T. ornata dichloromethane extract exhibited zone of inhibition of 18.67 mm in B. subtilis, while Demirel et al. recorded zone of inhibition of only 6.5–7.5 mm in B. subtilis ATCC 6633.
Positive results in Gram-positive bacteria were expected as they lack an outer membrane (lipopolysaccharide layer) which is present in Gram-negative bacteria (Nikaido, Citation2003). The limited susceptibility in Gram-negative bacteria could thus be attributed to limited outer membrane permeability and presence of porins in the membrane which narrows penetration of the extract (Delcour, Citation2009; Nikaido, Citation2003). Interestingly, dichloromethane extract exhibited positive effect against P. aeruginosa which lacks general diffusion porins and possesses “slow porins” instead (Nikaido, Citation2003). P. aeruginosa has an outer membrane with decreased permeability and has a more effective drug efflux mechanism compared to the other Gram-negative bacteria (Nikaido, Citation2003). In fact, P. aeruginosa is less susceptible to most antibiotics than Enterobacteriaceae (Nikaido, Citation2003).
In the present study, T. ornata water extract did not show any antimicrobial activity although inhibitory effect against S. aureus was reported by Zubia, Payri, and Deslandes (Citation2008). In addition, Vijayabaskar and Shiyamala (Citation2011) reported that methanol extract of T. ornata inhibited four Gram-negative bacteria, namely E. coli, K. pneumoniae, P. vulgaris, and P. aeruginosa. However, this was not observed in the present study; the only Gram-negative bacterium inhibited was Enterobacter spp. These discrepancies might be attributed to the different source of T. ornata used. Seasonal and geographical variations may alter algal production of antibacterial substances resulting in different antimicrobial activities (Moreau, Pesando, Bernard, Caram, & Pionnat, Citation1988; Stirk, Reinecke, & van Staden, Citation2007; Vidyavathi & Sridhar, Citation1991). Discrepancies might also result from the different assays used; agar well diffusion method (Berghe & Vlietinck, Citation1991) was used in Vijayabaskar and Shiyamala’s (Citation2011) study. The strains of the particular bacteria species employed were different too. Adherence to established guidelines such as that of CLSI might help to reduce conflicting reports on antimicrobial activities in the future.
The inhibition observed in selected strains of yeasts suggested that hexane, dichloromethane, and methanol extracts of T. ornata contained antifungal substances. Brown algae were known to have antifungal activities against Candida species (Khaled, Hiba, & Asma, Citation2012) and S. cerevisiae (Sridhar, Kumar, Babu, Aruna, & Mansuya, Citation2010). The underlying antifungal mechanisms are yet to be completely elucidated. However, purified phlorotannins from brown seaweeds were suggested to have an effect on ergosterol and respiration in yeasts (Lopes, Pinto, Andrade, & Valentão, Citation2013).
The use of REMA is preferable over agar disk diffusion method since it is more sensitive and accurate. Although RPMI 1640 medium is recommended by CLSI for susceptibility testing of yeast and fungi, Mueller Hinton broth (Oxoid, England) was used in this study and had comparable results as evidenced by the achievement of the same MIC value for positive antibiotic control for the yeast control strain C. parapsilosis ATCC 22019 (for amphotericin B, MIC value obtained in this study was 1 ± 0 μg/mL which was within CLSI standard’s range of 0.5–4 μg/mL for 48 h incubation). In dichloromethane extract, MIC values obtained from colorimetric and fluorometric REMA were the same (Table ), with exception for the strains S. aureus ATCC 25923, P. aeruginosa ATCC 27853, C. guilliermondii ATCC 6260, C. parapsilosis ATCC 22019, and S. cerevisiae. For the case of S. aureus ATCC 25923, colorimetric measurement with the unaided eye might be a shortcoming as the color of the well could not be distinguished clearly between blue and purple. A purple color would be regarded as a trailing result, where some metabolic activities and a longer incubation time would cause purple color to change to pink (Monteiro et al., Citation2012).
In some of the MIC values obtained, fluorometric REMA readings were lower than colorimetric REMA readings. In dichloromethane extract, for the case of P. aeruginosa ATCC 27853, the colorimetric method revealed an MIC value of 32 mg/mL. However, percent viability calculations showed an MIC value of 8 mg/mL. This could be perhaps explained by the drawback of using the modified REMA. In modified REMA, the antibiotic (extract), strain, and resazurin were added at the same time before a certain period of incubation. It was possible that in modified REMA, living microbial cells produced enough reduced resofurin during the incubation time to give a highly fluorescent pink color, although most of the cells were dead at the time of reading (O’Brien, Wilson, Orton, & Pognan, Citation2000). This would mean an overestimation of survival. In order to improve the accuracy of the assay, the classic REMA method where resazurin was added after the incubation time could be utilized. Alternatively, it might be of use to employ a slower reacting indicator to allow ample time for the extract to react with the bacterial cells (Gabrielson et al., Citation2002).
For the yeast strain S. cerevisiae, there was a discrepancy between the colorimetric and fluorometric measurements using dichloromethane extract. MIC value was taken at 0.5 mg/mL during visual observation. However, it was not expected that fluorometric measurements revealed an MIC value at 32 mg/mL. MIC is a quantitative endpoint measurement and there are various factors controlling the ultimate endpoint (Othman et al., Citation2011). Further validation of the MIC value could be done by performing the Minimum Bactericidal Concentration (MBC) test.
No prior literature has made a comparison between colorimetric and fluorometric modified REMA. The use of resazurin as a colorimetric indicator in addition to CLSI’s protocol of microdilution assay was first described by Tiballi, He, Zarins, Revankar, and Kauffman (Citation1995) and was found to be simple, sensitive, rapid, robust, and reliable in testing antimicrobial properties of natural products (Sarker, Nahar, & Kumarasamy, Citation2007). This study is the first to compare between colorimetric and fluorometric REMA utilizing kappa statistics. Based on the results obtained, the colorimetric method is reliable and could be used in resource-limited laboratories where a microplate reader is not available.
3. Experimental
3.1. Solvent extraction
T. ornata (Turner) J. Agardh was collected from Pulau Kerindingan, Semporna on 4 October 2012 and transported to the IMU Research laboratory. The seaweed sample was authenticated by Prof. Phang Siew Moi, University of Malaya (Voucher No.: PSM12862). The samples were cleaned, rinsed with sterile distilled water, oven-dried at 4°C, and powdered in a mixer grinder. Samples were sequentially extracted by soaking in various solvent (Merck, USA) systems started with hexane, followed by dichloromethane and methanol. Each solvent extraction process was conducted for three days. The extracts were then concentrated using rotary evaporator. For the water extraction, the samples were soaked with ultrapure water for three days prior to freeze-drying (Labconco, USA). All extracts were kept in a desiccator with silica gel until use.
3.2. Test micro-organisms
All micro-organisms were obtained from the culture collections of Institute of Medical Research, Kuala Lumpur, Malaysia and the American Type Culture Collection (ATCC, USA). In this study, the seven Gram-positive bacteria screened were Bacillus cereus, B. subtilis, S. aureus ATCC 25923, S. aureus, S. epidermidis, Staphylococcus saprophyticus, and Enterococcus faecalis. Six Gram-negative bacteria used were E. coli ATCC 25922, Enterobacter spp., Klebsiella pneumoniae, P. aeruginosa ATCC 27853, Proteus vulgaris, and Proteus mirabilis. The yeast strains employed were Candida albicans ATCC 60193, Candida glabrata ATCC 2001, Candida parapsilosis ATCC 22019, Candida tropicalis ATCC 201380, C. guilliermondii ATCC 6260, Candida lusitaniae ATCC 34449, and S. cerevisiae. Three fungi A. niger ATCC 46404, A. fumigatus, P. chrysogenum were also tested. All bacteria were maintained on Tryptone Soy Agar (Oxoid, UK) and incubated for 18 h while yeasts and fungi were cultured on Malt Extract Agar (Oxoid, UK) and incubated for 48 h. Overnight fresh microbial cultures were used to prepare inoculum suspensions for the following antimicrobial studies.
3.3. Disk diffusion antimicrobial assay
All four extracts were preliminarily screened for antimicrobial activity using the Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) M02-A11 guidelines (CLSI, Citation2012a). Gentamicin (10 μg/disk) (Oxoid, England) was used as positive control for bacterial strains while amphotericin B (10 μg/disk) (Sigma–Aldrich, USA) was used as positive control for yeasts and fungi. Dimethylsulfoxide (DMSO) 99.5% (Sigma-Aldrich, USA) which was used to dissolve the hexane, dichloromethane, and methanol extracts served as negative control. Ultrapure water served as negative control in the case of water extract. Mueller-Hinton agar (MHA) was used for bacteria while MHA supplemented with 0.5 μg/mL methylene blue dye and 2% glucose was used for yeasts and fungi for a more defined zone of inhibition. The agar plates were incubated for 18 h at 37°C for bacteria and 48 h at 37°C for yeasts and fungi. The antimicrobial activity was assessed by measuring the diameter of zone of inhibition around sterile filter paper disks (6 mm diameter; Oxiod, England) impregnated with 20 μL of algal extracts at concentrations of 100, 50, 25, 15, 10, 5, and 3 mg/mL. All tests were performed in at least duplicates.
3.4. Modified resazurin microtitre assay
The strains of microbes found to have inhibition in the disk diffusion method were subjected to broth microdilution test to determine the minimum inhibitory concentration (MIC). The MIC assay was performed in accordance to CLSI guidelines (M07-A9 guideline for bacteria; M27-A3 guideline for yeasts; M38-A2 guideline for filamentous fungi) with modification by the addition of resazurin dye (Sigma-Aldrich, USA) at a final concentration of 0.002% (CLSI, Citation2008a, Citation2008b, Citation2012b). Mueller-Hinton broth was used in replacement of Roswell Park Memorial Institute (RPMI) 1640 medium.
The hexane extract was dissolved in Tween 80 (Sigma-Aldrich, USA). The dichloromethane extract was dissolved well in phosphate buffered saline (PBS) (pH7.2–7.6) (MP Biomedicals, USA) with sonication for more than 30 min with continuous stirring prior to use. DMSO with a final well concentration of 6.25% was used to dissolve the methanol extract. The following were placed in each well of 96-well plates: 50 μL of test algal extract for final well concentrations ranging from 0.25–32 mg/mL, 40 μL of microbial cells suspended in 2.5X Mueller-Hinton broth adjusted to 0.5 MacFarland to achieve a final bacteria concentration of 5 × 105 cfu/mL or final yeast/fungi concentration of 0.5–2.5 × 103 cfu/mL, and 10 μL of sterile resazurin of final concentration 0.002% (w/v). Gentamicin (Bioplus, USA) served as positive control for bacteria while for yeasts and fungi, amphotericin B (Sigma-Aldrich, USA) was used. Tween 80, PBS and DMSO (6.25%) were used as negative controls for hexane, dichloromethane, and methanol extracts, respectively. All experiments were performed in at least duplicates. Plates were incubated at 37°C for 18 h (bacteria) and 48 h (yeasts and fungi). The MICs were determined by colorimetric and fluorometric methods. By visual inspection, the lowest concentration at which the color of resazurin changed from blue to pink was taken as the MIC value. The MIC value was also determined by measuring fluorescence at wavelength of emission 590 nm and excitation 560 nm using a microplate reader (Tecan Infinite 200 PRO). Percentage viability for microbial cells was then calculated after background fluorescence correction with MIC value taken as the well yielding % viability of less than 10% (IC90). The formula to calculate % viability was:
where extract: extract, bacterial/yeast/fungi suspension, resazurin; color control: extract, Mueller Hinton broth, resazurin; growth control: Tween 80/PBS/ DMSO, bacterial/yeast/fungi suspension, resazurin; sterility control: Tween 80/PBS/DMSO, Mueller-Hinton broth, resazurin
3.5. Statistical analysis
Cohen’s κ was conducted using SPSS (IBM Corporation) to evaluate the extent of agreement between readings obtained from the experiment using the methods of colorimetric and fluorometric REMA, following the suggestions for kappa-statistic interpretation by Landis and Koch (Citation1977).
4. Conclusion
The results reported herein indicated that there was presence of antimicrobial activity in T. ornata hexane, dichloromethane, and methanol extracts. In particular, dichloromethane extract had the most potent antimicrobial activity, suggesting the extract’s potential as an antimicrobial agent. However, this study only served as preliminary screening. In the sequence of this work, active compounds could be isolated from dichloromethane T. ornata extract. The MICs of the active compounds could then be identified to provide lead in drug discovery. Both colorimetric and fluorometric REMA were compared and the two methods were found to be substantially agreeable to each other (κ value = 0.712 with p < 0.0005).
| Non-standard abbreviations | ||
| ATCC | = | American type culture collection |
| CLSI | = | Clinical and Laboratory Standards Institute |
| IC90 | = | concentration giving 90% inhibition |
| MBC | = | Minimum Bactericidal Concentration |
| MHA | = | Mueller-Hinton agar |
| MIC | = | minimum inhibitory concentration |
| MRSA | = | Methicillin-resistant S. aureus |
| REMA | = | modified resazurin microtitre assay |
| RPMI | = | Roswell Park Memorial Institute |
Funding
This work was supported by the International Medical University, Malaysia [grant number BP1–01/11(14)2014].
Acknowledgements
The authors would also like to thank Mr. Lee Ser Yong for helping with the water extraction of T. ornata and assays. We also appreciate the kindness of Dr. Chong Chun Wie and Ms. Tsen Min Tze for providing assistance in statistical analysis.
Additional information
Notes on contributors
Kar-Yee Tye
The main focus of our research is the exploration of natural resources for drugs and natural products. Natural products have played important roles in drug development for medicine and health. We are involved in sourcing plant and algal bioactive compounds with properties such as antioxidative, anti-neuroinflammatory, antifungal, antimicrobial as well as neuroprotective. Our research scopes include toxicology and neurodegenerative disorders. In addition, we are also involved in various molecular studies to understand the biological effects of the compounds using both animal models and in vitro cell-based assays.
References
- Ananthi, S., Raghavendran, H. R., Sunil, A. G., Gayathri, V., Ramakrishnan, G., & Vasanthi, H. R. (2010). In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga). Food and Chemical Toxicology, 48, 187–192.10.1016/j.fct.2009.09.036
- Aravindan, S., Delma, C. R., Thirugnanasambandan, S. S., Herman, T. S., & Aravindan, N. (2013). Anti-pancreatic cancer deliverables from sea: First-hand evidence on the efficacy, molecular targets and mode of action for multifarious polyphenols from five different brown-algae. PLoS One, 8, e61977.10.1371/journal.pone.0061977
- Baker, D. D., Chu, M., Oza, U., & Rajgarhia, V. (2007). The value of natural products to future pharmaceutical discovery. Natural Product Reports, 24, 1225–1244.10.1039/b602241n
- Berghe, V. A., & Vlietinck, A. J. (1991). Screening methods for anti-bacterial and antiviral agents from higher plants. Methods in Plant Biochemistry, 6, 47–68.
- Clinical Laboratory Standards Institute. (2008a). Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, CLSI document M27-A3. ISBN 1-56238-666-2.
- Clinical Laboratory Standards Institute. (2008b). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, CLSI document M38-A2. ISBN 1-56238-668-9.
- Clinical and Laboratory Standards Institute. (2012a). Performance standards for antimicrobial disk susceptibility tests; approved standard, CLSI document M02-A11. ISBN 1-56238-781-2.
- Clinical and Laboratory Standards Institute. (2012b). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard (p. 32). ISBN 1-56238-783-9.
- Cortés, Y., Hormazábal, E., Leal, H., Urzúa, A., Mutis, A., Parra, L., & Quiroz, A. (2014). Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta: Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica, agents that cause diseases in salmonids. Electronic Journal of Biotechnology, 17, 126–131.10.1016/j.ejbt.2014.04.005
- Delcour AH. 2009. Outer membrane permeability and antibiotic resistance. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1794, 808–816.10.1016/j.bbapap.2008.11.005
- Demirel, Z., Yilmaz-Koz, F. F., Karabay-Yavasoglu, U. N., Ozdemir, G., & Sukatar, A. (2009). Antimicrobial and antioxidant activity of brown algae from the Aegean sea. Journal of the Serbian Chemical Society, 74, 619–628.10.2298/JSC0906619D
- Eom, S. H., Kim, Y. M., & Kim, S. K. (2012). Antimicrobial effect of phlorotannins from marine brown algae. Food and Chemical Toxicology, 50, 3251–3255.10.1016/j.fct.2012.06.028
- Gabrielson, J., Hart, M., Jarelöv, A., Kühn, I., McKenzie, D., & Möllby, R. (2002). Evaluation of redox indicators and the use of digital scanners and spectrophotometer for quantification of microbial growth in microplates. Journal of Microbiological Methods, 50, 63–73.10.1016/S0167-7012(02)00011-8
- Girija, K., Hemalatha, A., Saranya, C., Parthiban, C., & Anantharaman, P. (2013). Extraction and isolation of phlorotannins from brown seaweed Turbinaria ornata (Turner) J. Agardh and its antioxidant activity. International Journal of Bioassays, 2, 1185–1189.
- Jeyaseelan, E. (2012). Antibacterial activity of some selected algae present in the costal lines of Jaffna peninsula. International Journal of Pharmaceutical & Biological Archive, 3, 352–356.
- Khaled, N., Hiba, M., & Asma, C. (2012). Antioxidant and antifungal activities of Padina pavonica and Sargassum vulgare from the Lebanese Mediterranean coast. Advances in Environmental Biology, 6, 42–48.
- Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174.10.2307/2529310
- Lane, A. L., Nyadong, L., Galhena, A. S., Shearer, T. L., Stout, E. P., Parry, R. M., ... Kubanek, J. (2009). Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proceedings of the National Academy of Sciences, 106, 7314–7319.10.1073/pnas.0812020106
- Lopes, G., Pinto, E., Andrade, P. B., & Valentão, P. (2013). Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS One, 8, e72203.10.1371/journal.pone.0072203
- Manivannan, K., Karthikai Devi, G., Anantharaman, P., & Balasubramanian, T. (2011). Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters, Gulf of Mannar. Asian Pacific Journal of Tropical Biomedicine, 1, 114–120.10.1016/S2221-1691(11)60007-5
- Monteiro, M. C., de la Cruz, M., Cantizani, J., Moreno, C., Tormo, J. R., Mellado, E., ... Vicente, F. (2012). A new approach to drug discovery: High-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. Journal of Biomolecular Screening, 17, 542–549.10.1177/1087057111433459
- Moreau, J., Pesando, D., Bernard, P., Caram, B., & Pionnat, J. (1988). Seasonal variations in the production of antifungal substances by some dictyotales (brown algae) from the French Mediterranean coast. Hydrobiologia, 162, 157–162.10.1007/BF00014538
- Nikaido, H. (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiology and Molecular Biology Reviews, 67, 593–656.10.1128/MMBR.67.4.593-656.2003
- O’Brien, J., Wilson, I., Orton, T., & Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry, 267, 5421–5426.10.1046/j.1432-1327.2000.01606.x
- Othman, M., Loh, H., Wiart, C., Khoo, T. J., Lim, K. H., & Ting, K. N. (2011). Optimal methods for evaluating antimicrobial activities from plant extracts. Journal of Microbiological Methods, 84, 161–166.10.1016/j.mimet.2010.11.008
- Plaza, M., Santoyo, S., Jaime, L., García-Blairsy Reina, G. G., Herrero, M., Señoráns, F. J., & Ibáñez, E. (2010). Screening for bioactive compounds from algae. Journal of Pharmaceutical and Biomedical Analysis, 51, 450–455.10.1016/j.jpba.2009.03.016
- Sarker, S. D., Nahar, L., & Kumarasamy, Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods, 42, 321–324.10.1016/j.ymeth.2007.01.006
- Sridhar, S., Kumar, J. S., Babu, S., Aruna, P., & Mansuya, P. (2010). Pharmacognostical and antifungal activity of selected seaweeds from Gulf of Mannar region. Recent Research in Science and Technology, 2, 115–119.
- Stirk, W. A., Reinecke, D. L., & van Staden, J. (2007). Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. Journal of Applied Phycology, 19, 271–276.10.1007/s10811-006-9134-7
- Tiballi, R. N., He, X., Zarins, L. T., Revankar, S. G., & Kauffman, C. A. (1995). Use of a colorimetric system for yeast susceptibility testing. Journal of Clinical Microbiology, 33, 915–917.
- Vidyavathi, N., & Sridhar, K. (1991). Seasonal and geographical variations in the antimicrobial activity of seaweeds from the Mangalore coast of India. Botanica Marina, 34, 279–284.
- Vijayabaskar, P., & Shiyamala, V. (2011). Antibacterial activities of brown marine algae (Sargassum wightii and Turbinaria ornata) from the Gulf of Mannar biosphere reserve. Advances in Biological Research, 5, 99–102.
- Wang, J., Zhang, Q., Zhang, Z., Hou, Y., & Zhang, H. (2011). In-vitro anticoagulant activity of fucoidan derivatives from brown seaweed Laminaria japonica. Chinese Journal of Oceanology and Limnology, 29, 679–685.10.1007/s00343-011-0181-9
- World Health Organization. (2015). Antimicrobial resistance fact sheet N 194.
- Zubia, M., Payri, C., & Deslandes, E. (2008). Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). Journal of Applied Phycology, 20, 1033–1043.10.1007/s10811-007-9303-3
Appendix
Table A1. Cross-tabulation table of output for Cohen’s kappa generated by SPSS
cREMA_coded * fREMA_coded Crosstabulation
Count
cREMA = colorimetric REMA
fREMA = fluorometric REMAs
Table A2. Symmetric Measures table of output for Cohen’s kappa generated by SPSS
aNot assuming the null hypothesis.
bUsing the asymptotic standard error assuming the null hypothesis.
