Abstract
Rhipsalis neves-armondii K. Schum. (Cactaceae) aerial parts preparation is used traditionally in southern Nigerian for the treatment of rheumatic disorders and cancer and so the aim of this study was to evaluate these folkloric claims. Carragenaan-induced pedal edema and cotton pellet-induced granuloma were the anti-inflammatory models employed, while the antiploliferative study was performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide] assay method on Pancreatic tumor (PANC-1) and Henrietta Lacks’ cervical (HeLa) cell lines. The dried pulverized Rhipsalis aerial parts were extracted with methanol to obtain Rhipsalis neves-armondii extract (RNE). The RNE was fractionated using chromatographic techniques to obtain the hexane fraction (HF) and ethylacetate fraction (EF). Also acute toxicity and phytochemical studies were performed using standard procedures. Results showed that the extract and fractions significantly (p < 0.05) inhibited the development of rat pedal edema. The EF (400 mg/kg) exhibited the highest percentage edema inhibition of 53.33% after 3 h, compared to 36.67% of indomethacin. Also the extract and fractions significantly (p < 0.05) suppressed granuloma formation in rats, with HF showing the highest percentage suppression of 59.4% compared to the control. The RNE exhibited potent cytotoxic effect against PANC-1 and HeLa cells with an estimated IC50 value of 30.12 and 45.1 μg/ml, respectively, and showed an LD50 greater than 5,000 mg/kg. The findings showed that the extract and fractions of Rhipsalis neves-armondii (Cactaceae) possess anti-inflammatory and antiproliferative effects.
Public Interest Statement
The use of medicinal plants in disease management is as old as man. In both developed and developing nations, extracts and preparations from medicinal plants are being used in the folkloric treatment of cancer and other diseases. Medicinal plants often serve as a reservoir for plants secondary metabolites with potent pharmacological effects from which novel compounds have been and could still be isolated and characterized for drug development and research. Several standard anti-inflammatory and anticancer agents in clinical use have their origin from plants. In chemotherapy, some naturally isolated phytoconstituents used in cancer treatment such as the taxanes, vinca alkaloids, and podophyllotoxins have their origin from plants. In Nigerian ethnomedicine, the aerial preparation of Rhipsalis neves-armondii (Cactaceae) is used in the treatment of cancer and rheumatic diseases. This study has scientifically proven the folkloric use through in vivo and in vitro models of inflammation and cancer.
Competing Interests
The authors declare no competing interest.
1. Introduction
Inflammation is an integral part of the body’s defense mechanisms. It has various components such as edema formation, leukocyte infiltration, and granuloma formation, all of which contribute to the associated symptoms and tissue injury typical of an inflammatory reaction (Mitchell, Kumar, Abbas, & Fausto, Citation2006). Acute inflammatory response is characterized by vasodilatation, exudation of plasma, release of various inflammatory mediators such as cytokines, prostaglandins, and leukocytes, while the typical features of chronic inflammation include infiltration of mononuclear cells, proliferation of fibroblasts, blood vessels, and increased connective tissue formation (Benni, Suresha, & Jayanthi, Citation2011). Studies have shown that inflammation is one of the major physiological events in cancer development (Aggarwal, Shishodia, Sandur, Pandey, & Sethi, Citation2006; Lu, Ouyang, & Huang, Citation2006). Chronic inflammatory diseases are frequently associated with increased risk of cancers and chronic inflammation has emerged as an important risk factor in the pathogenesis of cancer (Aggarwal et al., Citation2006). Globally, cancer is a leading cause of death worldwide, accounting for about 8.2 million deaths and approximately 14 million new cases in 2012 (World Health Organization, Citation2014). It is a serious health burden in both developing and civilized nations. Non-steroidal anti-inflammatory drugs (NSAIDs) are the mainstay in the management of inflammatory and other painful conditions. However, their use is often limited by associated unwanted gastro-intestinal (GI) side effects, which have been minimized since the introduction of specific cyclo-oxygenase-2 (COX-2) inhibitors (Rang, Dale, Ritter, & Flower, Citation2008). Documented experimental, epidemiologic, and clinical studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs), particularly the highly selective cyclooxygenase (COX)-2 inhibitors, have potent anticancer effects (Rayburn, Ezell, & Zhang, Citation2009; Thun, Henley, & Patrono, Citation2002). Therefore, eliminating inflammation may be a valid strategy for cancer prevention and therapy (Rayburn et al., Citation2009). Moreover, side effects associated with standard agents used in cancer chemotherapy have made the need for new and safer agents imperative. The use of herbal medicines is often sought as one of the vital means to obviate the unwanted side effects associated with standard agents. In ethnomedicine, treatment of various forms of cancer with herbal or medicinal plants preparations has been a documented regular practice (Harlev, Nevo, Solowey, & Bishayee, Citation2013). There is an increase in the use of herbal medicines and other natural products in the management of various ailments such as inflammatory disorders and cancer, both in the developed and the developing nations. It has been reported that apart from their food value, medicinal plants in the cactus genera including Rhipsalis neves-armondii K. Schum. (Cactaceae) have been used in ethnomedicine for the treatment of whooping cough, diabetes, cancer, nose bleeding, and rheumatic pains (Anderson, Citation2001). However, in Nigerian traditional medicine, preparation from the juicy aerial parts of Rhipsalis neves-armondii (Cactaceae) is used in the management of inflammatory conditions and cancer. R. neves-armondii, an epiphytic cactus herb of Brazilian origin (Anderson, Citation2001; Barthlott & Taylor, Citation1995), is commonly distributed in the tropical rainforest zone of Nigeria. It is often used in horticulture as an ornamental plant (Korotkova et al., Citation2011). This study, therefore, was aimed to investigate the anti-inflammatory and anticancer potentials of the methanol extract and fractions of the aerial parts of Rhipsalis neves-armondii in rodents and cell lines, respectively, to scientifically ascertain the folkloric use.
2. Materials and methods
2.1. Cell lines
Human embryonic kidney cells expressing SV40 Large T-antigen (293 T), Pancreatic tumor (PANC-1) cell line, and Henrietta Lacks’ cervical (HeLa) cancer cell line were propagated in D-10 medium, consisting of Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, 2 mM L-glutamine and supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. Tissue culture medium and supplements were purchased from Invitrogen (Karlsruhe, Germany). The cell cultures were maintained in a humidified 5% CO2 atmosphere at 37°C.
2.2. Animals
Adult Sprague-Dawley rats (150–250 g) and mice of either sex, obtained from the Animal House Facility of the Department of Pharmacology and Toxicology, University of Nigeria, Nsukka, were used. They were housed under standard conditions to acclimatize and fed on standard pellet and water ad libitum for 7 days. They were fasted overnight before their use for each experiment. All animal experiments were conducted in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals (Pub No. 85–23, revised 1985) and in accordance with the University of Nigeria Ethics Committee on the use of laboratory animals, registered by the National Health Research Ethics Committee (NHREC) of Nigeria, with the number; NHREC/05/01/2008B.
2.3. Preparation of plant material
Fresh succulent aerial parts of Rhipsalis neves-armondii were collected in the month of August from Nsukka, Enugu state, Nigeria. The plant was identified and authenticated by a taxonomist, Mr. Alfred Ozioko of the International Centre for Ethno-medicine and Drug Development (InterCEDD), Aku Road, Nsukka, Enugu State, Nigeria. There the voucher specimen was preserved with the number; INTERCEDD 08 11. The plant material was cut, cleaned, air-dried, and pulverized using a milling machine.
2.4. Extraction and solvent-guided fractionation of RNE
About 2.1 kg of the powdered plant material was extracted by cold maceration with methanol at room temperature for 48 h. The plant material was repeatedly washed with fresh solvent until the filtrate became clear. The filtrate was concentrated using a rotary vacuum evaporator under reduced pressure (40–50°C) to obtain 120 g (5.71% w/w) of Rhipsalis neves-armondii extract (RNE). The RNE (50 g) was subjected to solvent-guided fractionation in a silica gel (70–120 mesh size) column (60 cm in length and 7.5 cm in diameter) successively eluted with n-hexane and ethyl acetate in order of increasing polarity. The collected solvent fractions were concentrated under reduced pressure in a rotary evaporator (40–50°C) to obtain the n-hexane (HF; 29 g, 58% w/w), and the ethyl acetate (EF; 8.5 g, 17% w/w) fractions, respectively.
2.5. Phytochemical analysis
The methanol extract (RNE), n-hexane (HF), and ethyl acetate (EF) fractions were subjected to standard phytochemical analysis for identification of plants phytoconstituents using standard methods (Trease & Evans, Citation1989). Briefly, frothing test for saponins, Salkowski test for terpenoids, Liebermann–Burchard tests for steroids, ferric chloride test for tannins, Keller–Killiani test for cardiac glycosides, Dragendorff’s and Mayer’s test for alkaloids, Fehling’s test for reducing sugars, xanthoproteic test for proteins, iodine test for carbohydrates or starch, and ammonia test for detection of flavonoids were performed for qualitative identification of the phytoconstituents present.
2.6. Acute toxicity study
The acute lethal dose (LD50) of RNE was evaluated using the method described by Lorke (Citation1983). Briefly, the study was performed in two phases. In the first phase, nine mice were divided into three groups of three mice per group, and treated with the RNE at the doses of 10, 100, and 1,000 mg/kg (p.o.), respectively. The animals were observed for 24 h for signs of toxicity. In the second phase, out of the four mice used, three were treated with RNE doses of 1,600, 2,900, and 5,000 mg/kg, while the fourth mouse served as the control (5 ml/kg of distilled water). The animals were observed for 24 h.
2.7. Carragenaan-induced rat paw edema
Adult rats (120–200 g) of both sexes were distributed into five treatment groups containing six animals per group (n = 6). Group I (control) received the vehicle (5 ml/kg, 20% Tween 80 + propylene glycol (1:1) solution, p.o.), Group II received indomethacin (10 mg/kg, p.o.), while Groups III to V received 100, 200, and 400 mg/kg of the extract (RNE, p.o.), respectively. Thirty minutes later, acute inflammation was induced by sub plantar injection of 0.1 ml of 1% w/v freshly prepared carrageenan solution into the right hind paw of the rats according to the method described by Winter, Risley, and Nuss (Citation1962) and Vogel (Citation2002) with some modifications. Edema was quantified in terms of volume of the inflamed paw and measured by water displacement using an improvised plethysmometer before and at 0.5, 1, 2, 3, 4, 5, and 6 h after carrageenan injection. The same procedure was performed for fractions, HF and EF (100, 200, 400 mg/kg, p.o.). Inflammation was assessed as the difference between the volume at zero time of the treated paw and the volume at the various times after the administration of the phlogistic agent. Inhibition of edema (%) was calculated using the relation; Inhibition of edema (%) = 100[1−(a − x/b − y)]; where a = mean paw volume of treated rats at various times after carrageenan injection; x = mean paw volume of treated rats before carrageenan injection; b = mean paw volume of control rats at various times after carrageenan injection; y = mean paw volume of control rats before carrageenan injection. The area under the curve (AUC) was calculated for each of the dose levels of the extract, fractions, and indomethacin using the trapezoid rule as described by Aguwa, Ejiekpe, Okoli, and Ezike (Citation2009). The percentage levels of inhibition was obtained with the formula; Percentage Inhibition (PI) = 1−(AUCt/AUCc) × 100. where AUCt = Area under the curve of treatment groups and AUCc = Area under the curve of control group (Aguwa et al., Citation2009).
2.8. Cotton pellet granuloma test
The effect of the extract on chronic inflammation was evaluated using cotton-pellet granuloma test in rats (Swingle & Shideman, Citation1972). Sprague-Dawley rats (120–200 g) of either sex were randomly grouped into four (n = 5). The animals were anesthetized by intraperitoneal (IP) administration of ketamine (0.2 ml). Sterilized cotton pellet (100 mg) was aseptically implanted subcutaneously in the depilated neck region of each rat, and the incision sutured. Groups I and II of the animals were then treated by the oral administrations of extract (RNE) 200 and 400 mg/kg respectively, group III received indomethacin (5 mg/kg) while group IV received the vehicle (5 ml/kg), for 7 days. Animals were sacrificed by excess anesthesia on the 8th day and the cotton pellets removed surgically. Pellets were separated from extraneous tissues and dried at 60°C to a constant weight, and the weight of the granuloma tissue was recorded. Granuloma formation was evaluated by determining the average weights of the dry pellets and the percentage inhibition of granuloma formation calculated. Inhibition (%) of granuloma tissue development was calculated using the relation: Inhibition (%) = [WC−WT/WC] × 100; where WC = weight of granuloma tissue of control group; WT = weight of granuloma tissue of treated group (Mukherjee, Citation2007).
2.9. Cytotoxicity studies
The cytotoxicity assay was performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide] assay method as previously described (Romijn, Verkoelen, & Schroeder, Citation1988) on PANC-1 and HeLa cancer cell lines, while 293T cell act as control. In the MTT assay, cells were seeded onto a 96-well plate at a concentration of 104 cells/well and a volume of 100 μl per well. Different concentrations of the test extracts (31.5–1,000 μg/ml) were added to culture wells in triplicate. Culture medium without any drug was used as the “no-drug” control. After incubation at 37°C under 5% CO2 for 2 days, a solution of MTT (3 mg/ml, 50 μl per well) was added to each well and further incubated at 37°C + 5% CO2 for 4 h to allow formazan formation. Subsequently, the medium was removed and 150 μl of DMSO was used to dissolve the resulting blue formazan crystals in living cells. The optical density was determined at 550 nm using a multi-well microtiter plate reader (Tecan, Austria). Each single value of the triplicates was expressed as percent of the mean of triplicates of the “no-drug” control cultures and the mean and standard deviation of the percent values were calculated for each triplicate. The concentration of 50% cellular toxicity (IC50) of the test extracts was calculated by non-linear regression.
2.10. Statistical analysis
Data obtained were analyzed using a one-way analysis of variance (ANOVA) subjected to Dunnett multiple comparison post hoc test. The Graph Pad Prism version 5 was also used. Differences between means were accepted to be significant at p < 0.05 and the results expressed as mean ± SEM.
3. Results
3.1. Phytochemical analysis
The extract, RNE, showed the presence of alkaloids, flavonoids, terpenoids, and steroids. HF also tested positive for carbohydrates, flavonoids, resins, steroids, and terpenoids, while EF contains alkaloids, flavonoids, resins, steroids, and terpenoids (Table ).
Table 1. Phytochemical constituents of extract and fractions
3.2. Acute toxicity study
The RNE exhibited an estimated LD50 greater than 5,000 mg/kg (p. o.) and did not cause any lethality or show any signs of acute intoxication after a 48-h observation period.
3.3. Carragenaan-induced rat paw edema
The RNE, EF, and indomethacin exhibited significant (p < 0.05) inhibitions that were non-dose related but were sustained within 2–5-h post induction of edema at all doses treated compared to the control. Also the HF exhibited significant (p < 0.05) inhibitions of edema at 400 mg/kg dose (Table ). However, EF (400 mg/kg) showed the highest magnitude of anti-inflammatory activity considering its lowest AUC quantification of the global edematous response of 2.28 ml/h, while indomethacin gave 2.92 ml/h (Table ).
Table 2. Effect of extract and fractions on carrageenan-induced rat paw edema
3.4. Cotton pellet-induced granuloma test
The RNE, HF, EF, and indomethacin exhibited appreciable dose-related inhibition of granuloma tissue formation. HF (400 mg/kg) gave a significant (p < 0.05) effect with a magnitude suppression of 59.4% which was equal and comparable to effect shown by the standard agent, indomethacin. Also EF (400 mg/kg) showed 18.8% inhibition of granuloma tissue formation (Table ).
Table 3. Effect of extract and fractions on cotton pellet-induced granuloma in rat
3.5. Cytotoxicity studies
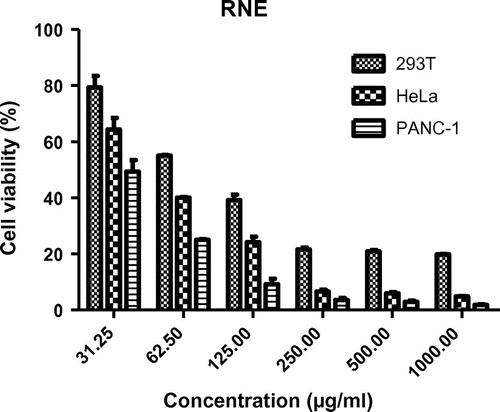
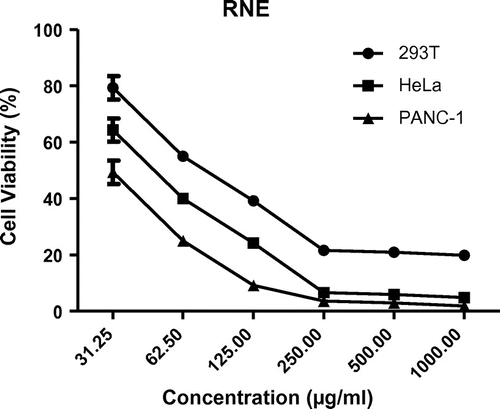
The RNE exhibited cytotoxic effect against PANC-1, HeLa, and 293 T cells with estimated IC50 values of 30.12, 45.1, and 83.5 μg/ml, respectively (Figures and ). Hence the potency of antiproliferative activity of RNE against the cells in the order of increasing activity can be shown as; PANC-1 > HeLa > 293T cells.
4. Discussion
Evaluation of the anti-inflammatory potentials of the methanol extract (RNE) and fractions of Rhipsalis neves-armondii using acute and chronic inflammation models in rats revealed that the plant possesses potent anti-inflammatory activities on both acute and chronic phases of inflammation. The RNE also exhibited potent anticancer effects against PANC-1 and HeLa cancer cells. Similarly, medicinal plants with both anti-inflammatory and anticancer activities have been reported (Siriwatanametanon, Fiebich, Efferth, Prieto, & Heinrich, Citation2010). The RNE and its fractions showed a potent suppression of systemic acute inflammation of the rat paw which was evident from 2–5-h post administration of the phlogistic agent. Carrageenan-induced paw edema is widely used for determining the acute phase of inflammation (Prakash, Prasad, Nitin, & Vijay Kumar, Citation2011). Edema formation in the paw is as a result of synergism between various inflammatory mediators that increase vascular permeability and/or the mediators that increase blood flow (Harriot, Marion, Martha, Wellford, & William, Citation2004; Lalenti, Ianaro, Moncada, & Di Rosa, Citation1995). Acute inflammation following subcutaneous injection of carrageenan into the rat paw is usually a biphasic event with the initial phase beginning at about 1 h after irritant administration and associated with massive release of mediators such as histamine and serotonin causing vasodilatation and increased permeability of capillaries, whereas the second phase occurring about 3–5 h later is being mediated by the release of bradykinin, prostaglandins, protease, and lysosomal enzymes which regulate the process of adhesion of molecules (Aruna, Vinod, & Ajudhia, Citation2010; Brooks & Day, Citation1991). Administration of carrageenan also produces accumulation of plasma fluid, at same time plasma protein exudation also takes place along with neutrophil extravasations (Chatpaliwar, Johrapurkar, Wanjari, Chakraborty, & Kharkar, Citation2002). This implies that the anti-inflammatory activity within 1-h post induction of edema may be considered to be more of antihistaminic activity, whereas suppression of inflammation after 4–6 h represents inhibition of arachidonic acid pathway. Hence, the observed anti-inflammatory effect of Rhipsalis neves-armondii suggests a possible combination of antihistaminic and arachidonate pathway inhibitory activities considering the time-course of abolishing edema events vis a vis the significant suppression of inflammation from 2- to 5-h post administration of the phlogistic agent (Vinegar, Schreibar, & Hugo, Citation1969). This has correlated with the mechanisms of action of NSAIDs via cyclooxygenase inhibition, which have also been reported to possess antiproliferative and anticancer effects (Rayburn et al., Citation2009; Thun et al., Citation2002). Epidemiological studies have shown a 40–50% reduction in mortality from colorectal cancer in individuals who take NSAIDs on regular basis compared with those not taking these agents (Aggarwal et al., Citation2006).
Granuloma tissue formation in the cotton pellet granuloma model is usually a prolonged process developing during period of several days and involving infiltration and proliferation of mononuclear inflammatory cells such as macrophages, lymphocytes, and fibroblasts which are basic sources of tissue granuloma formation. There is often the appearance of nodules of epithelioid macrophages surrounded by a collar of lymphocyte elaborating factors like IFN-γ hence granuloma tissue formation (Mitchell et al., Citation2006), with vascular proliferation attempted at healing. The cotton pellet-induced granuloma test as a model of chronic inflammation is thus indicative of the proliferative phase of inflammation since the dry weight of the cotton pellets gives a good correlation with the amount of granulomatous tissue formed (Thangam & Dhananjayan, Citation2008). Therefore, the observed anti-inflammatory effect of Rhipsalis neves-armondii extract and fractions against acute and chronic inflammatory responses may possibly be the mechanism of its antiproliferative action observed against PANC-1 and Hela cancer cells. If left untreated, chronic inflammation may often progress to pathological inflammatory conditions, leading to cancer and other disease. Key molecular players linking inflammation to cancer are cytokines (such as chemokines, IL, TNF-α), nuclear factor-kB (NF-kB), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). It has been reported that cytokine signaling could contribute to the progression of tumors by stimulation of cell growth and differentiation as well as by the inhibition of apoptosis of altered cells at the inflammatory site (Lu et al., Citation2006). However, NF-kB has been shown to be activated by inflammatory stimuli and its constitutive activation is found in cancer, hence it has long been suspected to be a critical promoter facilitating the development from inflammation into cancer (Lu et al., Citation2006). Therefore, inhibition of the effects of these compounds by NSAIDS and anticancer agents contributes to the suppression of tumor. In this study, RNE and fractions showed inhibition of granuloma tissue formation, a marker of chronic inflammation, also a possible antiproliferative mechanism of the extract and fractions, although the specific mechanism of anti-inflammatory and anticancer actions of RNE may not be ascertained at this stage of the work. Furthermore, the RNE’s IC50 value of 83.50 μg/ml, against the human 293-T cell is an indication of relative safety on human cells when compared to those of 30.12 and 45.1 μg/ml for PANC-1 and HeLa cells, respectively. In addition, EF when compared with HF, showed better anti-inflammatory effect, indicating that the active phytoconstituent(s) responsible for these activities may be polar or non lipid in nature. The EF also gave a better control by exhibiting the lowest AUC quantification of the global edematous response comparable to indomethacin. Lower AUC values indicate more concentration of the agent at the given time thereby exhibiting better anti-inflammatory effect. This is in correlation with the work reported by Aguwa et al. (Citation2009).
Qualitative phytochemical tests revealed abundant presence of alkaloids, flavonoids, terpenoids, and steroids in RNE and its fractions. However, alkaloids being a polar constituent may be among the suspected constituents responsible for the observed anti-inflammatory and anticancer effects of RNE, although at this stage of the study one will not categorically attribute the effects to any specific constituent.
5. Conclusion
Findings from this study clearly suggest that the extract and fractions of the aerial parts of Rhipsalis neves-armondii K. Schum. (Cactaceae) possess significant anti-inflammatory properties in both acute and chronic inflammation with anticancer effects. Further studies to isolate and identify the precise constituent(s) responsible for the activities are recommended.
Authors contributions
Theophine Chinwuba Akunne, Peter A. Akah and IfeomaA. Nwabunike designed the work, participated in the experiment and write up; Chukwuemeka S. Nworu performed the cytotoxic experiment during his stay in Ruhr-University Bochum, Germany, while Emeka K. Okereke, Nelson C. Okereke and Francis C. Okekecontributed in performing the experiment.
Acknowledgment
The authors appreciate the Department of Molecular & Medical Virology, Ruhr University, Bochum, Germany, for granting us the access to perform the cytotoxicity studies in their laboratory. Gratitude also goes to Mr. Alfred Ozioko of the International Centre for Ethno-medicine and Drug Development (InterCEDD), Nsukka, Nigeria for identification of the plant.
Additional information
Funding
Notes on contributors
Theophine Chinwuba Akunne
Theophine Chinwuba Akunne, PhD, is a senior lecturer and researcher in the Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka. Akunne obtained doctoral degree in Pharmacology and Toxicology, with bias in Natural Products Pharmacology. Akunne’s research interest lies in the isolation of phytoconstituents or bioactive compounds with potent anticonvulsant, anticancer, and anti-diabetic effects. Medicinal plants have been the mainstay of Complementary and Alternative Medicine (CAM) therapy in both developed and developing nations. Reduced side effects and possibility of isolation and characterization of novel compounds with clinical potentials have made natural product pharmacology very interesting, especially in the area of new drug development and research. In this study, the aerial parts of Rhipsalis nerves-armondii showed anticancer and anti-inflammatory properties, thus buttressing its use in folkloric medicine.
References
- Aggarwal, B. B., Shishodia, S., Sandur, S. K., Pandey, M. K., & Sethi, G. (2006). Inflammation and cancer: How hot is the link? Biochemical Pharmacology, 72, 1605–1621.10.1016/j.bcp.2006.06.029
- Aguwa, C. N., Ejiekpe, F. N., Okoli, C. O., & Ezike, A. C. (2009). Anti-inflammatory effects of leaf extracts of Buchholzia coriacea Engl. (Capparidaceae). NPAIJ, 5, 98–103.
- Anderson, E. F. (2001). The Cactus family (pp. 51–54). Portland, OR: Timber Press.
- Aruna, D., Vinod, G., & Ajudhia, N. K. (2010). Evaluation of anti-inflammatory activity of methanolic extract of Cassia obtusifolia seeds in Wistar rats. Journal of Chemical and Pharmaceutical Research, 2, 696–700.
- Barthlott, W., & Taylor, N. P. (1995). Notes towards a monograph of Rhipsalidaea (Cactaceae). Bradleya, 13, 43–79.
- Benni, J. M., Suresha, R. N., & Jayanthi, M. K. (2011). Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian Journal of Pharmacology, 43, 393–397.10.4103/0253-7613.83108
- Brooks, P. M., & Day, R. O. (1991). Nonsteroidal anti-inflammatory drugs: Differences and similarities. The New England Journal of Medicine, 324, 1716–1725.
- Chatpaliwar, V. A., Johrapurkar, A. A., Wanjari, M. M., Chakraborty, R. R., & Kharkar, V. T. (2002). Indian Drugs, 39, 543–545.
- Harlev, E., Nevo, E., Solowey, E., & Bishayee, A. (2013). Cancer preventive and curative attributes of plants of the cactaceae family: A review. Planta Medica, 79, 713–722.
- Harriot, M., Marion, E., Martha, A., Wellford, S., & William, A. (2004). Inflammation induced by histamine, serotonine, bradykinin and compound 48/480 in the rat. Antagonists and mechanisms of action. Journal of Pharmacology and Experimental Therapeutics, 191, 300–302.
- Korotkova, N., Borsch, T., Quandt, D., Taylor, N. P., Muller, K. F., & Barthlott, W. (2011). What does it take to resolve relationships and to identify species with molecular markers? An example from the epiphytic Rhipsalideae (Cactaceae). American Journal of Botany, 98, 1549–1572.10.3732/ajb.1000502
- Lalenti, A., Ianaro, A., Moncada, S., & Di Rosa, M. (1995). Modulation of acute inflammation by endogenous nitric oxide. European Journal of Pharmacology, 211, 177–184.
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Archives of toxicology, 54, 272–289.
- Lu, H., Ouyang, W., & Huang, C. (2006). Inflammation, a key event in cancer development. Molecular Cancer Research, 4, 221–233.10.1158/1541-7786.MCR-05-0261
- Mitchell, N. R., Kumar, V., Abbas, A. K., & Fausto, N. (2006). Robbins and cotran pathologic basis of disease (7th ed., pp. 48–85). Philadelphia, PA: Saunders Elsevier.
- Mukherjee, P. K. (2007). Quality control of herbal drugs–An approach to evaluation of botanicals (1st ed.). New Delhi: Business Horizons.
- Prakash, P., Prasad, K., Nitin, M., & Vijay Kumar, M. (2011). Evaluation of anti-inflammatory effect of Calotropis procera (AIT.) R.BR. root extract against different mediators of inflammation in albino rats. International Research Journal of Pharmacy, 2, 279–284.
- Rang, H. P., Dale, M. M., Ritter, J. M., & Flower, R. J. (2008). Drugs used in the treatment of infections and cancer. Rang and Dale’s pharmacology (6th ed., pp. 647–660). Philadelphia, PA: Elsevier.
- Rayburn, E. R., Ezell, S. J., & Zhang, R. (2009). Anti-inflammatory agents for cancer therapy. Molecular and Cellular Pharmacology, 1, 29–43.10.4255/mcpharmacol
- Romijn, J. C., Verkoelen, C. F., & Schroeder, F. H. (1988). Application of the MTT assay to human prostate cancer cell lines in vitro: Establishment of test conditions and assessment of hormone-stimulated growth and drug-induced cytostatic and cytotoxic effects. The Prostate, 12, 99–110.10.1002/(ISSN)1097-0045
- Siriwatanametanon, N., Fiebich, B. L., Efferth, T., Prieto, J. M., & Heinrich, M. (2010). Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. Journal of Ethnopharmacology, 130, 196–207.10.1016/j.jep.2010.04.036
- Swingle, K. F., & Shideman, F. E. (1972). Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. Journal of Pharmacology and Experimental Therapeutics, 183, 226–234.
- Thangam, C., & Dhananjayan, R. (2008). Anti-inflammatory potential of the seeds of Carum capticum Linn. Indian Journal of Pharmacology, 35, 388–391.
- Thun, M. J., Henley, S. J., & Patrono, C. (2002). Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. JNCI Journal of the National Cancer Institute, 94, 252–266.10.1093/jnci/94.4.252
- Trease, G. E., & Evans, W. C. (1989). Test book of pharmacognosy (11th ed., pp. 176–180), London: Brailliare Tindall and Macmillian.
- Vinegar, R., Schreibar, N., & Hugo, B. (1969). Biphasic development of carrageenan edema in rats. Journal of Pharmacology and Experimental Therapeutics, 166, 96–103.
- Vogel, H. G. (2002). Analgesic, anti-inflammatory and antipyretic activity in drug discovery and evaluation pharmacological assays (2nd ed., pp. 759–767). New York, NY: Springer.
- Winter, C. A., Risley, E. A., & Nuss, G. W. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Experimental Biology and Medicine, 111, 544–547.10.3181/00379727-111-27849
- World Health Organization. (2014). World Cancer Report.