?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Caffeic acid (CA) is a well-known natural compound used for both health supplement and metabolic diseases. In the present study, single, easy, and quick isolation procedure of caffeic acid was described from rosmarinic acid. A quick crystal formation also was described for highly hygroscopic CA which was spontaneous and stable with lower hygroscopic nature. In addition, the produced crystal CA was highly stable and showed neuroprotective activity with <6 μg/ml in R28 cell line and cytotoxic activity with >100 μg/ml in U87 MG which revealed its dual biological effect for the treatment of Alzheimer’s disease and cancer, respectively.
Public Interest Statement
The emerging growing interest to design the formulation in improving drug’s shelf life is becoming viable to eradicate their differential efficacy. In the current study, a kind of viable natural solution of increasing shelf-life of the chemical compound and/or drug was described that showed worth even the smallest risk of differential efficacy in clinical set. The formulation scientist’s genuine interest should look after the drug’s behavior in different states of them under clinical use.
1. Introduction
Caffeic acid (CA) is an interesting small molecule due to its chemical stability and antioxidant power (Chen & Ho, Citation1997; Gulcin, Citation2006; Hong, Yang, Nam, Koo, & Lee, Citation2015). It is a potent molecule which started a rational-based drug discovery for wide range of therapeutic application (Rezg et al., Citation2015; Rosendahl et al., Citation2015). Chemical structure of caffeic acid is delicate to drive any kind of desired scaffold hope. In our lab, we routinely check the phytochemicals and computationally design their newer derivatives which ultimately may become suitable candidates for the treatment of Alzheimer’s disease and metabolic disorder like cancer. Earlier studies on caffeic acid showed its mild stimulant effect (Liszt, Eder, Wendelin, & Somoza, Citation2015; Shin et al., Citation2015; Toth et al., Citation2015). The past studies also showed its cytoprotective effect (Lee et al., Citation2015; Nunes et al., Citation2015), antiangiogenic effect, and moderate toxicity (Akyol et al., Citation2015; Kosova, Kurt, Olmez, Tuğlu, & Arı, Citation2015) that can be affected due to the stability of the compounds. This earlier observation postulates that caffeic acid can show its dual biological efficacy due to half-life, solubility, stability, and physicochemical properties of the compound. Caffeic acid is highly hygroscopic and degrade at room temperature and in the presence of light. To determine the stability impact of the CA in biological activity is essential to assess the therapeutic efficacy. There is no evidence yet to explain the dual biological efficacy of the CA yet. The hygroscopic nature has greater impact for the CA bioactivity which might lead to the dual efficacy of this compound. In the present study, our objective was to stabilize the CA using natural ways and to find the reason of the dual efficacy of the same compound.
2. Experimental
2.1. Crystallography
All reagents were purchased from Fluka™, Aldrich™, and JT Baker™, USA. All solvents were used as received without further purification. The isolated caffeic acid was dissolved by adding methanol dropwise and kept under vacuum hood for two weeks at room temperature. After slow evaporation, faint yellow microcrystals were obtained. The crystals were filtered off and washed by cold methanol at room temperature. The microcrystals solution of Dimethylformamide (DMF) was slowly evaporated to determine the crystal’s chemical structure suitable for X-ray diffraction studies. Crystal data were obtained on a Bruker SMART APEXII CCD area-detector diffractometer using graphite monochromated MoKα radiation (λ = 0.71073 Å) at 100 K. A crystal with dimension 9 × 8 × 31 mm was used. The data were collected and reduced using Cambridge online crystallography program and Crystallo Chemcraft win version 1.8. The structure of all compounds was solved using the Crystallo Chemcraft win version 1.8 program package, and refined using the Crystallo Chemcraft win version 1.8 program package. The molecular graphics were created using Crystallo Chemcraft win version 1.8.
2.2. Cell culture and reagents
The rat retinal cell line R28 was purchased from Kerafast, USA, and human glioblastoma cell line U87-MG was bought from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified eagle medium (DMEM) (GIBCO, USA) supplemented with 10% heat inactivated fetal bovine serum (HIFBS) (GIBCO), 100 units/mL penicillin–streptomycin (GIBCO), and 2 mM glutamine (GIBCO) in a humidified incubator with 5% CO2 at 37°C. Rosmarinic acid (RA) was purchased from Sigma (Germany). MTT, (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), trypsin, and phosphate-buffered saline (PBS) were purchased from Sigma. Matrigel matrix (10 mg/mL) was obtained from BD Bioscience (USA). About 10 mg of powdered RA (96% purity) was dissolved in sterile, cell-cultured tested DMSO (Sigma, USA) to prepare a 10 mg/mL stock solution, which was stored in a moisture controlled environment at RT. Other chemicals used were of analytical grade.
2.3. In vitro cytotoxicity and cytoprotection
The effects of CA on R28 cell and U87MG toxicity were determined using the MTT at 0.004 μg/ml (i.e. 20 μl from 5 mg/ml stock solution) cell proliferation assay. Briefly, R28 and U87MG cells (70% confluent) were seeded in 96-well plates and cultured overnight followed by treatment with different concentrations of CA (6.25, 12.5, 25, 50, 100, and 200 μg/mL). Cells were incubated for 48 h at 37°C and 5% CO2, and cell proliferation and cytotoxicity were calculated using a regression equation (below). After 48 h of treatment, MTT solution (20 μl) was added to each well and the mixture was re-incubated for 4 h. Absorbance of the samples then was read at 490–570 nm, and percentage proliferation and cytotoxicity (percent inhibition (i.e. IC50)) were calculated as follows:
The % inhibition was plotted against the concentration tested using Microsoft Excel, and the IC50 was calculated using the non-linear regression equation. Effect of cytoprotective activity of the crystal CA was determined using serum deprivation assay. Cells were treated with and without serum and after 48 h of treatment and MTT assay was conducted as discussed above. Percent proliferation was determined according to earlier study of Khan et al. (Citation2016).
2.4. Stability study
The thermal, chemical, biological, and environmental stability of the CA was determined according to earlier described study by Khan et al. (Citation2016). In brief, thermal stability was determined by HPLC after treating the caffeic acid (50 mg of powder and crystal) at 60°C in the oven for 48 h. For chemical stability study, caffeic acid was treated with LiOH and quantity of caffeic acid was determined by mass balance and HPLC. Conversely, HCl and Krebs-Heneseleit bicarbonate buffer (pH 7.4) treated caffeic acid at 37°C for 2 h was quantified by HPLC peak and time. To determine the biological stability, caffeic acid (10 mM of powder and crystal) was treated by human serum which was then deproteinized using 500 μL of acetonitrile containing 0.1% trifluoroacetic acid and filtered off to determine the quantity in HPLC. The HPLC method was validated according to earlier described method by Khan et al. (Citation2016).
3. Results and discussion
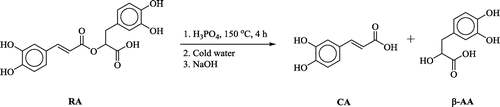
Keeping in view the literature information of CA, we successfully hydrolyzed the rosmarinic acid (Scheme ) in the presence of acid as catalysis (polyphosphoric acid) and obtained crystals of CA (He, Li, & You, Citation2015). The reaction was carried out by mixing 1 equivalent of RA with 15 equivalents of H3PO4 to get a thick paste. The paste was heated to 150°C and poured into a large volume of stirring cold water. The solution was then neutralized by sodium hydroxide and the white precipitates were collected. Recrystallization by methanol provided colorless crystals of CA. The melting point of the crystals was found to be more than 300°C. The expected component β-aryllactic acid (β-AA) could not be isolated from the reaction mixture (Scheme ). The crystals of CA so obtained were exposed to X-ray crystallographic machine. The molecular structure of CA along with the atomic numbering scheme and its packing in the crystal lattice are given in Figure (A) and (B).
Figure 1. (A) The molecular structure of caffeic acid (CA) draw at 50% probability (B) shows the molecular packing.
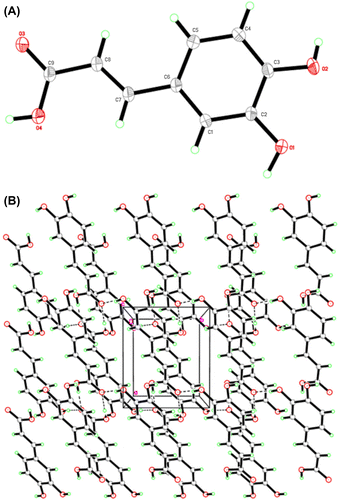
Figure 2. In vitro toxic effects of CA in the R28 cell lines (A, B) and U87 MG cells (C, D). The IC50 of CA was 109.52 μg/ml (F) and 532.83 μg/ml (G) for R28 and U87 MG cells, respectively. The R28 cells were not affected and proliferated at lowest dose (6.25 μg/ml) of caffeic acid (E, H) in serum insult assay.
The summary of the crystal data of CA is provided in the Table . Table S1 shows the selected bond lengths (Å) and angles (°), see supplementary file. The compound crystallizes into a monoclinic crystal system with space group P21/n. According to the molecular structure obtained from X-ray crystallographic technique, the CA contains a carboxylic group connected to a benzene ring containing two hydroxy groups through an alkyl chain (C6–C7–C8), see Figure (A). The molecular structure of the CA can be divided into two planar moieties that are connected by a single C–C bond. The first plane, the pyrocatechol plane, includes OH, OH, and benzene ring, whereas the second plane, the (E)-but-2-enoic acid plane, includes CO, OH, and C7–C8. Dihedral angle between these two planes is 122.55(3)°. In CA, the C7–C8–benzene torsion angle is 178.94(15)°. In the crystal lattice, the packing of the molecules is stabilized by intermolecular hydrogen bondings. Further stabilization is provided by C-H–π and π–π interactions.
Table 1. Crystal data and structure refinement parameters for CA
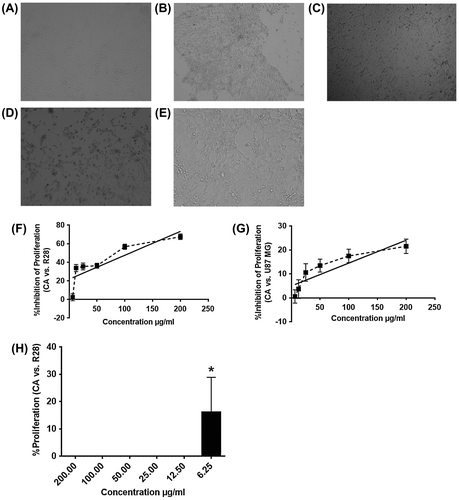
The ring and (E)-but-2-enoic acid are not in the same plane. The reason behind this is the packing effects where the molecules have low π–π stacking interactions. Figure (B) illustrates the crystal packing pattern, which shows that the molecules are connected through the C–O–H bonding in a three-dimensional network. The details of bond angles, lengths and torsions were presented in the Figure .
Figure 3. The molecular structure of CA labeled with: (A) bond angles, (B) bond lengths, and (C) torsion angles based on the crystallographic details.
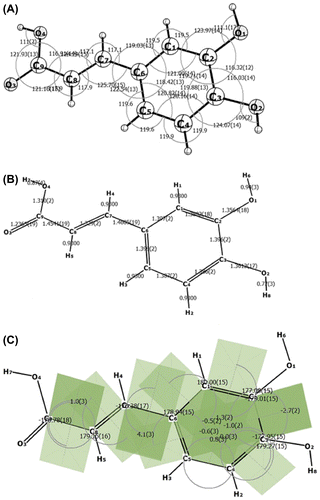
The crystals of CA were evaluated for the safety in rat neurosensory retinal (R28), and human glioblastoma U87MG cells by inducing serum insult. The R28 retinal precursor cell line is useful to determine the retinal cell behavior including differentiation, neuroprotection, cytotoxicity, light stimulation, retinal gene expression, and neuronal function. R28 cell line is nontumorigenic and the toxic effect on this cell line refers to the injury and inflammation of retina. R28 cells and U87MG cells were treated with 200 μg/mL to 6.25 μg/mL of CA for 48 h. Cell viability was measured using MTT assay. The cell viabilities of R28 cells, and U87MG cells treated with CA were significantly different (p > 0.05) from that of untreated controls. The IC50 values were found in R28 as 109.53 μg/ml (Figure (A), (B), and (F)) and in U87MG as 532.84 μg/ml (Figure (C), (D), and (G)). There was significant difference (p > 0.05) between viabilities of R28 and U87 MG cells treated with CA. This report suggests that CA, at the given concentrations or above the dose normally used in clinical practice, is toxic to rat neurosensory retinal, and human glioblastoma cells in vitro. The reason for this dual effect of crystal CA may be due to stability and solubility parameters. The effect of crystal CA with 6.25 μg/mL had obvious cytoprotective effect which was slightly different with the CA contained moisture (Figure (E) and (H)). In each case of stability study, caffeic acid was determined by HPLC before and after treatment of chemical, thermal, biological, and environmental factors. The elution time of caffeic acid was 3–5 min that revealed the affected stability for its solid composition of powder and crystal form. The crystal form of the caffeic acid was more stable than powdered form that the elution time was constant for crystal but it was deviated with multiple peaks at different elution time for powdered form which might be the reason of moisture content (Figures S1 and S2).
4. Conclusion
The biological activity of caffeic acid is dependent on its delicate dose and stability which determines its efficacy to be nutrient or toxic. The lower dose of the stable CA showed cytostatic properties while the dose above 100 μg/ml could be cytotoxic based on the cell origin. The activity of CA is dependent on the stability and kind of cells origin that less sensitive cell such as highly survival capacity of cells might not be affected by the CA in compared to delicate cells such as U87 MG. CA crystallography provides more information for the resolution of atomic structure, stability impact and their study in the cellular environment. The further impact of the stability of CA will be explored more in future studies.
Funding
The entire study is supported by Universiti Sains Malaysia (USM) under the Research University (RU) [grant number 1001/PKIMIA/811217].
Supplementary material
The supplementary material for this paper is available online at http://dx.doi.10.1080/23312025.2016.1243460.
Supplementary_Material.docx
Download MS Word (69.4 KB)Acknowledgments
We are grateful to the EMAN LAB researcher who helped us during the analysis and experimentation. We are thankful to the USM doctoral fellowship scheme 2015–2016.
Additional information
Notes on contributors
Md Shamsuddin Sultan Khan
The authors are working to find out the differential efficacy of a drug and that solution. This kind of problem is seen during the early development of the drug in clinical case and thereby their failure of approval and marketing. Here, authors are trying to discuss the physical development of a candidate drug which might at least reduce risk of potency of a drug in different clinical cases.
References
- Akyol, S. , Akbas, A. , Butun, I. , Toktas, M. , Ozyurt, H. , Sahin, S. , & Akyol, O. (2015). Caffeic acid phenethyl ester (CAPE) as a remedial agent for reproductive functions and oxidative stress-based pathologies of gonads. Journal of Intercultural Ethnopharmacology , 4 , 187–191.10.5455/jice.
- Chen, J. H. , & Ho, C. T. (1997). Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. Journal of Agricultural and Food Chemistry , 45 , 2374–2378.10.1021/jf970055t
- Gulcin, I. (2006). Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology , 217 , 213–220.10.1016/j.tox.2005.09.011
- He, X. , Li, Z. , & You, Q. (2015). Use of polyphosphoric acid in the hydrolysis of chromone esters. Synthetic Communications , 32 , 709–714.
- Hong, Y. J. , Yang, S. Y. , Nam, M. H. , Koo, Y. C. , & Lee, K. W. (2015). Caffeic acid inhibits the uptake of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by inducing the efflux transporters expression in caco-2 cells. Biological & Pharmaceutical Bulletin , 38 , 201–207.10.1248/bpb.b14-00495
- Khan, M. S. S. , Salam, M. A. , Haque, R. S. M. A. , Abdul Majid, A. M. S. , Abdul Majid, A. S. B. , Asif, M. , ... Tabana, Y. M. (2016). Synthesis, cytotoxicity, and long-term single dose anti-cancer pharmacological evaluation of dimethyltin(IV) complex of N (4)-methylthiosemicarbazone (having ONS donor ligand). Cogent Biology , 2 , 1154282.10.1080/23312025.2016.1154282
- Kosova, F. , Kurt, F. , Olmez, E. , Tuğlu, I. , & Arı, Z. (2015). Effects of caffeic acid phenethyl ester on matrix molecules and angiogenetic and anti-angiogenetic factors in gastric cancer cells cultured on different substrates. Biotechnic & Histochemistry , 91 , 38–47. doi:10.3109/10520295.2015.1072769
- Lee, S.-Y. , Ku, H.-C. , Kuo, Y.-H. , Yang, K.-C. , Tu, P.-C. , Chiu, H.-L. , Su, M.-J. (2015). Caffeic acid ethanolamide prevents cardiac dysfunction through sirtuin dependent cardiac bioenergetics preservation. Journal of Biomedical Science , 22 (1), 1–11.
- Liszt, K. I. , Eder, R. , Wendelin, S. , & Somoza, V. (2015). Identification of catechin, syringic acid, and procyanidin B2 in wine as stimulants of gastric acid secretion. Journal of Agricultural and Food Chemistry , 63 , 7775–7783.10.1021/acs.jafc.5b02879
- Nunes, S. , Madureira, R. , Campos, D. , Sarmento, B. , Gomes, A. M. , Pintado, M. , & Reis, F. (2015). Therapeutic and nutraceutical potential of rosmarinic acid-cytoprotective properties and pharmacokinetic profile. Critical Reviews in Food Science and Nutrition . doi:10.1080/10408398.2015.1006768
- Rezg, R. , Mornagui, B. , Santos, J. S. O. , Dulin, F. , El-Fazaa, S. , El-Haj, N. B. , ... Gharbi, N. (2015). Protective effects of caffeic acid against hypothalamic neuropeptides alterations induced by malathion in rat. Environmental Science and Pollution Research , 22 , 6198–6207.10.1007/s11356-014-3824-5
- Rosendahl, A. H. , Perks, C. M. , Zeng, L. , Markkula, A. , Simonsson, M. , Rose, C. I. , ... Jernstrom, H. (2015). Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clinical Cancer Research , 21 , 1877–1887.10.1158/1078-0432.CCR-14-1748
- Shin, H. S. , Satsu, H. , Bae, M.-J. , Zhao, Z. , Ogiwara, H. , Totsuka, M. , & Shimizu, M. (2015). Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chemistry , 168 , 167–175.10.1016/j.foodchem.2014.06.100
- Toth, B. , Bartho, L. , Vasas, A. , Sandor, Z. , Jedlinszki, N. , Pinke, G. , Hohmann, J. (2015). Dual excitatory and smooth muscle-relaxing effect of Sideritis montana extract on guinea-pig ileum. Natural Product Communications , 10 , 487–490.