Abstract
Numerous herbal and synthetic formulations have been used to determine the protective effects against radiation toxicity. In the present study, the radioprotective effects of aqueous leaf extract of Carica papaya (L.) and diallyl disulfide were studied in irradiated and non-irradiated groups. The optimum dose selection for papaya leaf extract and diallyl disulfide was done using Kaplan-Meier survival analysis. The mice were treated with papaya leaf extract at 5, 50, 100 and 200, 250, 500, 1,000 mg/kg body weight concentration and diallyl disulfide (DADS) at 0.5, 2, 5, 10, 20 mg/kg body weight for five consecutive days and on the 5th day one hour after dose administration they were irradiated with 10 Gy dose of lethal electron beam radiation. The mice were observed for maximum survival within 30 days post irradiation. The survival analysis from Kaplan-Meier curve indicated the optimum radioprotective dose for papaya leaf extract at 100 mg/kg body weight and for diallyl disulfide at 10 mg/kg body weight. The optimum dose was used for further studies with sublethal, lower sublethal, and adaptive split dose responses. The results indicate hematopoietic suppression in irradiated groups with sublethal, lower sublethal, and adaptive split dose groups. An increase in platelet levels and RBC levels were exhibited by papaya leaf extract pretreatment prior to irradiation with sublethal and lower sublethal doses. There was reduction in the antioxidant enzymes, total antioxidant capacity and increased lipid peroxidation seen in sublethal and lower sublethal radiation control groups. The pretreatment groups with papaya leaf extract and DADS prior irradiation have moderately enhanced the total antioxidant capacity, antioxidant enzyme levels, and reduced lipid peroxidation. There was a radio-adaptive response seen with the blood cell DNA damage in mice irradiated with an adaptive dose of 1 Gy 24 h prior to a dose of 5 Gy when compared to a single sublethal radiation dose group. The papaya leaf extract and DADS also contributed this effect and further enhanced the adaptive response.
Public Interest Statement
The study highlights the protective role of papaya leaf extract and diallyl disulfide against ionizing radiation induced tissue damages in Swiss albino mice. To understand the nature of protection offered by them we have studied at different levels like survival, antioxidant levels, changes in DNA integrity, and response at different doses. The results highlight these studies and their significance and relevance in offering the protection. The results indicate a protective role of papaya leaf extract and diallyl disulfide in improving the survival, enhancing the antioxidant levels, reducing the DNA damage, and also a radiation adaptive response in mice.
Competing Interest
The authors declare no competing interest.
1. Introduction
Carica papaya (L.)—commonly known as papaya, pawpaw has been studied extensively for its invaluable medicinal properties. Its herbal tea has shown to enhance the platelet levels in dengue patients (Kala, Citation2012). These properties can be attributed to the various bioactive components like alkaloids, flavonoids, tannins, saponins, carbohydrates, proteins, fat, and steroids.
Diallyl disulfide (DADS) is a constituent of garlic which has been studied previously for its anticancer properties (Lawson, Wang, & Hughes, Citation1991; Yan, Wang, & Barlow, Citation1992). Garlic and garlic extracts have been reported previously for their antioxidant properties and protection against free radical damage in the body (Block, Citation1985). Studies on the antioxidant properties of garlic compounds, allyl cysteine, alliin, allicin, and allyl disulfides indicated hydroxyl scavenging potential and prevent microsomal lipid peroxidation (Chung, Citation2006).
Exposure to ionizing radiations causes deleterious effects on physiology, metabolism, and behavior in almost all organisms. One of the basic mechanisms of radiation damage is the production of free radicals, leading to the formation of peroxides and oxidative reactive species. The free radicals produced may also lead to leakage of lysosomal acid phosphatases, changes in the surface properties of chromosomes leading to stickiness, breakage of double-strands of DNA, and chromosomal aberrations (Kumar et al., Citation2006). The study of these effects and novel approaches to counter them is of great importance in the field of radiation biology. In the present study, the effects of DADS—A synthetic compound and C. papaya (L.) extract—A natural herbal source were compared against different doses of electron beam radiation-induced hematological and cytogenetic changes in mice.
In the field of medicine using radiation treatment against cancers, these effects can be seen in normal tissues also. Thus, various attempts have been made to increase the radiation sensitivity of cancerous cells and or protect the normal cells from radiation. Thus, the use of chemicals along with radiation has proved to be ameliorative in cancer treatment (Upadhyay, Dwarakanath, Ravindranath, & Mathew, Citation2005). Herbal formulations which are non-toxic and inexpensive have been evaluated for their radioprotective efficacy (Suzen, Citation1999). WR-2721, also known as amifostine—a phosphorothioate is one of the best known radioprotectors which has undergone extensive preclinical testing and clinical trials for its radioprotective and chemoprotective effects (Citrin et al., Citation2010). The major reason for its success is its selective concentration in the normal tissues compared to the tumor tissue and its interaction with the DNA (Joseph, Citation1997; Yuhas, Citation1980).
2. Materials and methods
The leaves of C. papaya (Linn.) were obtained from the local plantation. The leaves were washed, dried in a hot air oven at 55°C. After drying, the leaves were powdered and stored in airtight containers. The synthetic DADS (>75% pure, HPLC graded) was obtained from TCI chemicals, Japan.
2.1. Preparation of extracts (Vennila et al., Citation2012; Zohra, Meriem, Samira, & Alsayadi Muneer, Citation2012)
The aqueous extracts of C. papaya (Linn.) were prepared by boiling approximately 5 g of powdered leaves with 100 mL distilled water for 20 min and kept overnight in a refrigerator. The extracts were filtered using Whatmann No.1 filter paper, dried, and stored in airtight containers.
2.2. Ethical clearance
The study has been ethically approved by the Institutional Animal Ethics Committee of the K S Hegde Medical Academy, Nitte University Ref. KSHEMA/IAEC/17/2013 dated 16.12.2013.
2.3. Administration and optimization of doses
The major purpose of a radioprotective drug is to enhance the life span and reduce the toxicities induced because of radiation. The major types of signs and symptoms include the gastrointestinal syndrome that occurs within 10 days after irradiation of mice to a lethal dose and the bone marrow or hematopoietic syndrome that occurs after 10 days post lethal irradiation (Ikpeme, Ekaluo, Kooffreh, & Udensi, Citation2011; Patil, Shetty, Bhide, & Narayanan, Citation2013). These syndromes are the primary cause for death and the secondary causes include severe intestinal and lung infections because of the radiation-induced immune suppression. The basis for selecting an optimum dose is to understand the minimum drug load that must be present in the system to ensure maximum survival. Hence, a series of doses ranging from the lowest to the highest non-toxic dose may be used to study the effect. The dose at which maximum survival of mice seen in a 30-day survival study post lethal irradiation is the optimum radioprotective dose.
The aqueous extract of C. papaya (L.) was chosen for the study as it was soluble and suitable for oral administration. DADS formed a colloidal suspension in water. The mice were treated with papaya leaf extract at 5, 50, 100, 200, 250, 500, 1,000 mg/kg body weight concentration and DADS at 0.5, 2, 5, 10, 20 mg/kg body weight for five consecutive days and on the 5th day one hour after dose administration they were irradiated with 10 Gy dose of lethal electron beam radiation. The mice were observed for maximum survival within 30 days post irradiation. The survival analysis from Kaplan-Meier curve indicated the optimum radioprotective dose for papaya leaf extract at 100 mg/kg body weight and for DADS at 10 mg/kg body weight.
For the sublethal, low dose and adaptive response studies, a volume of 0.1 mL/10 g of C. papaya (L.) aqueous leaf extract and DADS were administered using oral gavages to their respective groups for five consecutive days. On the 5th day, one hour after the administration of drug the mice were placed in well-ventilated perspex boxes with a dimension 3 × 6 cms. They were irradiated with a sub-lethal dose of 6 Gy electron beam radiation, 1 Gy as low dose, and 1 Gy priming dose with 5 Gy as the challenge dose for adaptive dose were given at a dose rate of 1 Gy/min, with a source to target distance of 100 cms.
The mice were dissected after 24 h of irradiation. They were anesthetized and the whole blood was collected by cardiac puncture for hematological estimations. The organs like liver, kidney, brain, and spleen were dissected and weighed. The bone marrow was removed for cytological studies.
The tissues of liver, kidney, and brain were washed with ice cold phosphate buffered saline and stored in ice cold phosphate buffered saline until processing. A 10% homogenate w/v of tissue was prepared using ice cold PBS with pH-7.4 using Remi (RQ-127A) homogenizer. The homogenized samples were then centrifuged for 20 min at 10,000 rpm at 4°C in Remi cooling centrifuge (C24BL). The supernatant was separated and used for all the estimations.
The whole blood was collected in 2% EDTA tubes and processed for hematological studies within 3 h of collection. The serum/plasma and the tissue homogenates were separated by centrifugation and stored in Panasonic (MDF-U334-PE) biomedical freezer at −30°C until further processing.
2.4. Hematological studies
The hematological studies were done using Erma veterinary blood cell counter (PCE-210VET) using the whole blood collected in 2% EDTA.
2.5. Antioxidant studies
Suitable spectrophotometric methods were used and the measurements recorded in Systronics PC-based double beam UV spectrophotometer 2202.
2.6. Total antioxidant capacity (Prieto, Pineda, & Aguilar, Citation1999)
The absorbance was read at 695 nm and the values are expressed in terms of ascorbic acid equivalents.
2.7. Superoxide dismutase (Mc Cord & Fridovich, Citation1969)
The blue-green colored solution was read at 560 nm. The activity of superoxide dismutase (SOD) was expressed in Units/mg of protein for homogenates.
2.8. Glutathione peroxidase (Hafeman, Sundae, & Houestra, Citation1974)
The absorbance was measured at 412 nm and the activity expressed in units/mg of protein for homogenates.
2.9. Catalase (Aebi, Citation1974)
The activity was expressed in units/mg of protein for homogenates and Units/mg of protein for homogenates.
2.10. Lipid peroxidation and membrane stabilization
2.10.1. Formation of malondialdehyde (Ohkawa, Ohishi, & Yagi, Citation1979)
The endpoint was measured at 535 nm and calculated using malondialdehyde standard curve.
2.11. Reduced glutathione (Moron, Depierre, & Mannervik, Citation1979)
The absorbance was read at 412 nm within 10 min and calculated using a GSH standard curve.
2.12. Genotoxicity and cytotoxicity studies
2.12.1. Comet assay (Singh, McCoy, Tice, & Schneider, Citation1988; Tice et al., Citation2000)
The slides were scored using comet score software and the parameters like tail moment, olive moment, and percent DNA in tail were considered to determine level of the genotoxicity and cytotoxicity.
2.12.2. Dose reduction factor (Nageshwar Rao, Satish Rao, Kiran Aithal, & Sunil Kumar, Citation2009)
The ratio of median lethal doses (LD50) with and without the compound gives the dose reduction factor (DRF). This factor demonstrates the radioprotective efficacy of any compound which in turn has prolonged the life expectancy in irradiated mice.
2.12.3. Statistical analysis of data
The statistical analysis of the data was done using the SPPS software. The dose optimization was done using Kaplan-Meir survival analysis. The survival studies were evaluated using probit analysis. The difference between various groups was analyzed by ANOVA with Tukey’s multiple comparison post hoc test. The results with p value less than 0.05 was considered significant.
3. Results
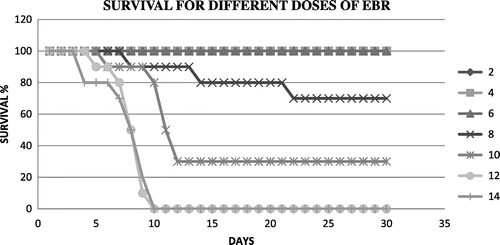
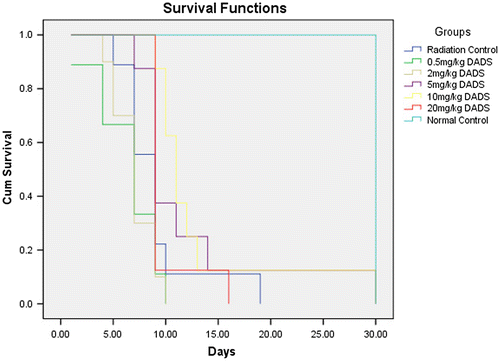
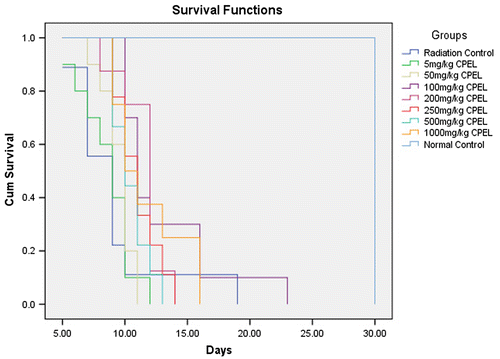
Figure shows the survival of mice at different doses of radiation in a 30 day post irradiation observation at different radiation doses. Figures and summarize the Kaplan-Meir survival analysis to extrapolate the optimum survival dose of papaya leaf extract and DADS intervention at a lethal dose EBR. An optimum radioprotective dose of 100 mg/kg body weight and a dose of 10 mg/kg body weight were shown by papaya leaf extract and DADS, respectively.
Figure 2. The maximum survival of mice at different doses of Carica papaya (L.) aqueous leaf extract.
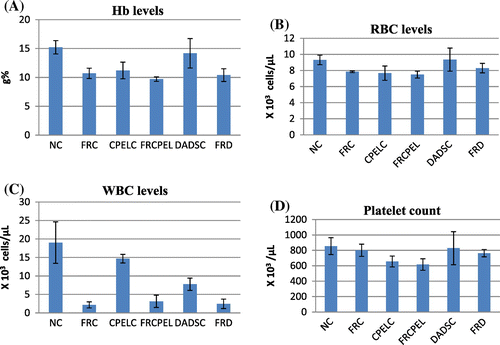
The results of hematological parameters like Hb, RBC, hematocrit, WBC, and platelets indicate a significant decrease in the irradiated groups when compared to the normal control groups (p < 0.05, p < 0.05, p < 0.05, p < 0.001, p < 0.05, respectively). Figure shows the hematological changes in control and irradiated groups with respect to papaya extract and DADS intervention. The platelet count is slightly enhanced in irradiated groups pretreated with papaya extract when compared to normal groups, but the results are of no statistical significance (p > 0.05). In low dose irradiated groups, there is a decrease in Hb, RBC, and WBC levels (p < 0.001, p < 0.05, p < 0.001, respectively). There is a moderate increase in the RBC levels in papaya leaf extract treatment prior irradiation groups (p < 0.05). In adaptive doses there is a decrease in Hb, RBC, and WBC levels in irradiated groups (p < 0.01, p < 0.05, p < 0.001, respectively). But there are no changes seen in papaya leaf extract and DADS pretreatment groups. Figures and summarize the results of the different hematological parameters in response to low dose and adaptive doses of radiation with respect to the effects of papaya leaf extract and DADS intervention.
Figure 4. The hematological parameters of control, sublethal dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
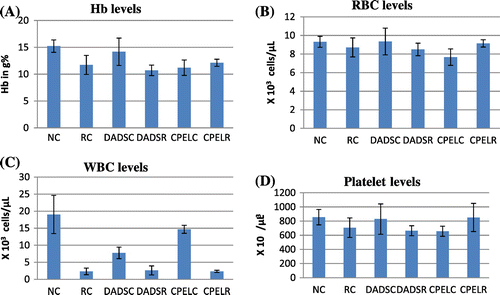
Figure 5. The hematological parameters of control, low dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
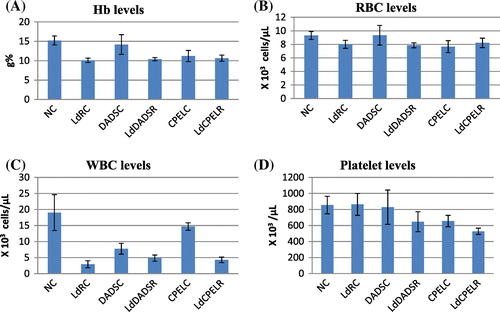
Figure 6. The hematological parameters of control, adaptive dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
In the antioxidant studies, there is a significant decrease in catalase (p < 0.01), GSH levels (p < 0.01), and total antioxidant capacity (p < 0.05) in the irradiated groups at sublethal dose. The increase in catalase was only seen in irradiated groups pretreated with DADS (p < 0.01). The GSH levels were enhanced in irradiated groups pretreated with papaya extract and DADS (p < 0.01). The total antioxidant levels were rejuvenated in irradiated groups pretreated with papaya extract (p < 0.01), but no significant changes were seen in the irradiated group pretreated with DADS. Figure summarizes the antioxidant and lipid peroxidation parameters of control and irradiated groups with respect to papaya leaf extract and DADS intervention at sublethal doses of EBR. At low and adaptive doses, the total antioxidant capacity also was reduced (p < 0.05) and near normal levels were retained in other groups but the difference was not statistically significant. The catalase levels remained unaltered in all the groups with low and adaptive radiation doses. GSH levels remained elevated in the irradiated groups pretreated with papaya extract and DADS at low radiation doses and not significant at adaptive dose. No statistically significant difference was seen in glutathione peroxidase (GPx) and SOD levels in sublethal and low doses whereas the levels of GPx was reduced in radiation control group (p < 0.01) but in the other groups the levels remained unchanged. The increased MDA levels in irradiated groups indicate increased lipid peroxidation at sublethal, low, and adaptive radiation doses (p < 0.01, p < 0.05, p < 0.05, respectively). The levels were found to be normalized in all the irradiated groups pretreated with papaya extract and DADS. In adaptive doses the MDA levels remained increased in irradiated groups pretreated with DADS and papaya extract. Figures and summarize the antioxidant and lipid peroxidation parameters in control and irradiated groups with respect to papaya leaf extract and DADS intervention at low and adaptive doses of EBR.
Figure 7. The antioxidant and lipid peroxidation parameters of control, sublethal dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
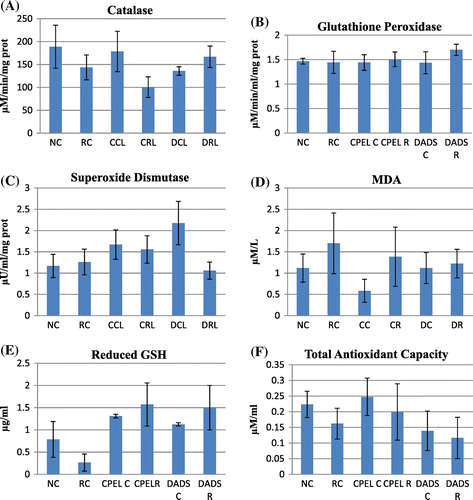
Figure 8. The antioxidant and lipid peroxidation parameters of control, low dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
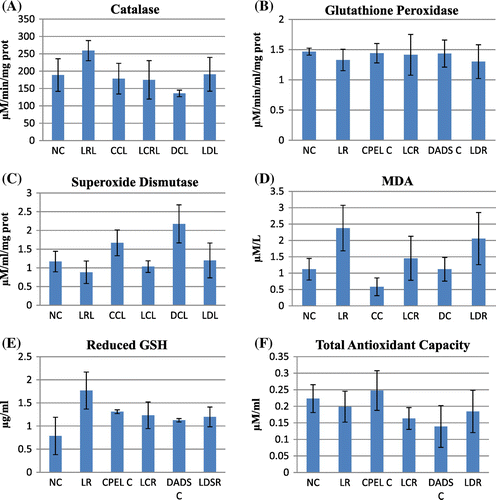
Figure 9. The antioxidant and lipid peroxidation parameters of control, adaptive dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
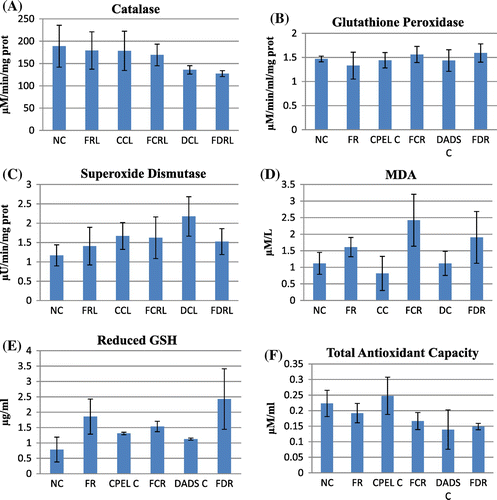
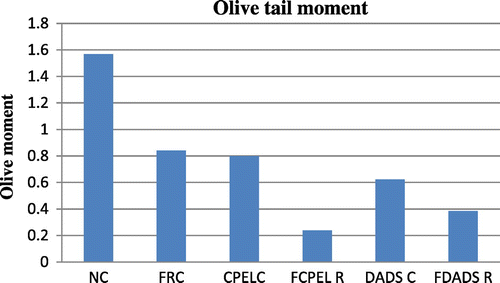
There is an overall increase in the olive tail moment (OTM) of the irradiated groups at sublethal and low doses (p < 0.01, p < 0.01, respectively) of radiation whereas there is decrease in the OTM at adaptive doses of radiation (p < 0.01). But the irradiated groups pretreated with papaya extract and DADS did not show any statistically significant results though a trend of reduction in DNA damage was observed in them when compared to the radiation control (p > 0.05). The other parameters of comet score like the percentage of DNA in tail and tail moment also showed a trend of decrease in the irradiated groups pretreated with papaya extract and DADS when compared to the radiation control, the results were not statistically significant. Figures – summarize the olive tail moment indicating DNA damage levels in control and irradiated groups with respect to papaya leaf extract and DADS intervention at sublethal, low, and adaptive doses of EBR.
Figure 10. The olive tail moment in control, sublethal dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
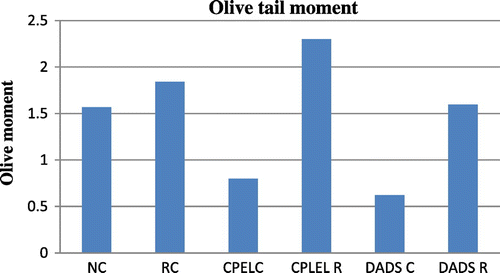
Figure 11. The olive tail moment in control, low dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
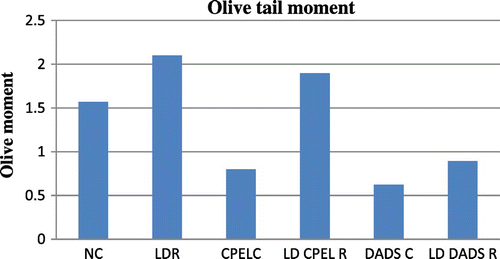
Figure 12. The olive tail moment in control, adaptive dose radiation control, and papaya and DADS pretreatment prior irradiation groups.
The DRF was calculated using the LD50 values obtained with and without the compound of interest. The LD50 without any intervention was obtained at 8 Gy and the LD50 with the papaya extract intervention shifted the LD50 to 9.0 Gy and the LD50 with DADS was found to be 10 Gy. Thus on calculating the DRF, a value of 1.12 and 1.25 was obtained for papaya leaf extract and DADS, respectively.
4. Discussion
Previous studies have shown that electron beam radiation at doses of 10 Gy, 12 Gy, and 14 Gy causes severe radiological effects in the gastrointestinal tract and bone marrow (Jagetia & Tiyyagura, Citation2002). In our study also death occurred within 15 days post radiation and maximum deaths were observed between the 9th and 14th days with 10 Gy, 12 Gy, and 14 Gy radiation. At higher doses of 12 Gy and 14 Gy there was facial edema, hypopigmentation, corneal opacity indicating loss of eyesight in mice. Reduced body weight and ruffled skin are some of the common radiological characteristics seen in mice. There was a drastic reduction in the body weights for initial 15 days post radiation. The present study reports the median lethal dose for electron beam radiation with 15 MeV electrons at a dose rate of 1 Gy/min as 8.933 Gy in Swiss albino mice.
The median lethal dose (LD50) indicating 50% survival was extrapolated as 8.933 Gy using Probit analysis. We are reporting the LD50 in the lower range compared to the previous studies for the dose rate of 1 Gy/min at 15 MeV for electron beam radiation.
The median lethal dose for DADS was found to be 75 mg/kg body weight. Previous study done by Amagase (Citation2006) has recorded the median lethal dose at 135 mg/kg body weight in females and 150 mg/kg body weight in males. The results of current study reveal diallyl sulfide to be highly toxic at those doses. From the Kaplan-Meier survival analysis the optimum radioprotective dose for DADS was found to be 10 mg/kg body weight.
Radiation-induced hematological suppression was seen at sublethal doses of 6 Gy irradiated groups. Decreases were seen in hemoglobin, RBC, total WBC count, and platelet counts in irradiated groups. Pretreatment of DADS prior irradiation did not show any ameliorative effects on hemoglobin, RBC, total WBC counts. A decrease in the total antioxidant capacity and catalase levels were seen with irradiated groups when compared to the non-irradiated groups. But no changes were seen in the levels of SOD and glutathione peroxidase levels were seen in the irradiated groups. Reduced levels of GSH and MDA levels were seen in the irradiated groups indicating increased lipid peroxidation. DADS prior treatment to radiation enhanced the levels of catalase, glutathione peroxidase and GSH. It also decreased the levels of MDA, but no changes were seen in the SOD levels and total antioxidant capacity. There was increased olive tail moment in irradiated group indicating radiation induced DNA damage, but no changes were seen with DADS pretreatment prior irradiation groups.
Similar effects were seen in the radiation studies at a lower sublethal dose of 1 Gy, where there was a decrease in the hematological parameters. Papaya leaf extract pretreatment group prior irradiation enhanced the RBC levels. Decrease in total antioxidant capacity, GSH, catalase, SOD, GPx levels, and increase in MDA levels were seen in irradiated groups and ameliorative effects were seen in the groups pretreated with papaya leaf extract and DADS.
In order to study the adaptive response, mice were irradiated initially with 1 Gy of priming dose and after 24 h a challenge dose of 5 Gy was given. There was no adaptive response seen in the hematological parameters, as there was decrease in the Hb, RBC, and WBC counts seen in irradiated groups with single sublethal dose and adaptive split dose groups. The unaltered levels of catalase, GPx, and slight increase in SOD and GSH levels provide a circumstantial evidence of dose adaptation, but decrease in the total antioxidant capacity and increase in MDA indicate deteriorating antioxidant levels. But a distinct decrease in the olive tail moment of the group irradiated with adaptive split dose on comparison with single sublethal dose indicated an adaptive response in the DNA damage levels. The DADS pretreatment prior adaptive split dose irradiation showed an enhanced adaptive response to DNA damage when compared to its respective adaptive split dose radiation control group.
Studies have shown that radiation reduces the hematological parameters like hemoglobin, red blood cell count, white blood cell count, platelet count, and inhibits hematopoiesis (Begum, Prasad, & Thayalan, Citation2012). The present study also shows similar effects in sublethal, lower sublethal, and adaptive split dose radiation groups. The deleterious effects of radiation include formation of free radicals and development of oxidative stress. Radiation has been shown to induce oxidative stress by increasing membrane peroxidation and reducing antioxidants in the blood. There is an overall reduction in the blood antioxidants, reduced glutathione, and the antioxidant enzymes like SOD, catalase, glutathione S transferase, and glutathione peroxidase (Verma et al., Citation2011). The current study reveals a similar response with sublethal, lower sublethal, and adaptive split dose irradiation. Radiation also inflicts damage to the DNA and leads to loss of cell viability and increased apoptosis (Madhu, Suchetha Kumari, Naveen, & Sanjeev, Citation2012). From the present study, we report an increased DNA damage in sublethal and lower irradiation groups and a reduced DNA damage in adaptive split dose groups.
Sulfhydryl compounds like amifostine (Kouvaris, Kouloulias, & Vlahos, Citation2007), cysteine, cysteamine, amino ethyl thiourea, glutathione (Nair, Parida, & Nomura, Citation2001) have been shown to reduce the radiation induced tissue damage by scavenging the free radicals or by repairing the radioactivated polymers or activating the repair enzymes (Suzen, Citation1999). The naturally occurring organosulfur compounds diallyl sulfide (DAS), diallyl disulfide (DADS), allyl methyl sulfide (AMS), allyl isothio cyanate (AITC), and phenyl isothio cyanate (PITC) have also been shown for their radioprotective activity by enhancing the glutathione content (Chittezhath & Kuttan, Citation2006). The studies have also revealed that these compounds must be present in the system before radiation (Montoro et al., Citation2011). Hence, sulfhydryl compounds have only prophylactic effect and no therapeutic effect. Intra cellular non-protein sulfydryl groups are mainly involved in enhancing the membrane stability and antioxidant capacity and DADS in the present study has shown enhanced GSH levels, total antioxidant capacity, and reduced the malondialdehyde levels in sublethal and lower sublethal dose radiation groups. DADS has shown reduced DNA damage in adaptive split dose groups and also has contributed to the radio-adaptive response.
Recent studies have concluded that C. papaya (L.) leaf extract significantly increased the platelet and RBC counts in murine models (Dharmarathna, Wickramasinghe, Waduge, Rajapakse, & Kularatne, Citation2013). Studies done by Joseph (Citation1997) have demonstrated the hematological potential of C. papaya (Linn.) leaf extracts. Our present study also indicates a thrombopoietic and erythropoietic potential of C. papaya (L.) leaf extract in radiation induced-hematopoietic suppression. According to a study done by Patil et al. (Citation2013) C. papaya (L.) leaves contain various phytoconstituents like saponins, tannins, cardiac glycosides, and alkaloids. The alkaloids present include carpaine, pseudocarpaine, and dehydrocarpaine I and II. These constituents may act on the bone marrow, prevent its destruction and enhance its ability to produce platelets. Moreover, it can also prevent platelet destruction in the blood and thereby increase the life of the platelet in circulation. In the present study, papaya leaf extract has shown moderate increase in antioxidant levels and reduced lipid peroxidation at sublethal and lower sublethal doses. At adaptive split doses, it reduced the DNA damage levels thus contributing to the radio-adaptive response in mice.
The overall survival of mice against a lethal dose of radiation is dependent on the resistance acquired by mice or the antioxidant load given to it (Jagetia, Citation2007). This demonstrates the amount of radiation dose the animal can withstand with minimal damages. The median lethal dose with and without the compound and extract was calculated. Without any intervention the LD50 was found to be 8 Gy and with intervention the LD50 was found to be 9 Gy and 10 Gy for C. papaya (L.) and DADS, respectively. Enhancement of the median lethal dose for the normal cells will increase the efficacy of the radiotherapy. A similar DRF of 1.12 and 1.25 for C. papaya (L.) and DADS, respectively, obtained have increased the percentage survival of pretreated mice by approximately 0.1 Gy.
5. Conclusions
The results indicate a potential hematoprotective property of C. papaya (L.) and DADS against radiation-induced hematological and biochemical alterations in Swiss albino mice. An adaptive response was also seen in C. papaya (L.) and DADS pretreatment. Further studies may be carried out to understand the dose and time dependant modifications that take place post irradiation and development of specific target molecules to counter the radiation-induced hematological and biochemical damages.
Acknowledgements
The authors are grateful to the board of research in nuclear sciences (BRNS) sanction No. 2012/34/26/BRNS for funding the study and Nitte University for providing the laboratory facility. The authors acknowledge the support of Dr Ganesh Sanjeev, Professor, Microtron center, Mangalore University, Dr Damodhara Gowda, Department of Physiology and Dr Chandrika Rao, Department of Pathology, K.S. Hegde Medical Academy, Nitte University. The authors also thank Dr Sanal T.S., Associate professor, Department of Biostatistics, K.S. Hegde Medical Academy, Nitte University.
Additional information
Funding
Notes on contributors
Suchetha Kumari N.
The authors of Central Research Laboratory, K S Hegde Medical Academy, Nitte University are majorly involved in studies related to radiation protectors, mitigators, and their mode of action in different systems of Invitro and Invivo. Most of the resources that are being studied are of plant origin as they are non-toxic and inexpensive. The present study is an attempt to understand the radioprotective role and radio-adaptive response of papaya leaf extract and diallyl disulfide using electron beam as a source of ionizing radiation.
References
- Aebi, H. (1974). Catalase estimation. In H. V. Berg Meyer (Ed.), Methods of enzymatic analysis (pp. 673–684). New York, NY: Verlag Chemie.10.1016/B978-0-12-091302-2.50032-3
- Amagase, H. (2006). Clarifying the real bioactive constituents of garlic. Journal of Nutrition, 136, 716S–725S.
- Begum, N., Prasad, N. R., & Thayalan, K. (2012). Apigenin protects gamma-radiation induced oxidative stress, hematological changes and animal survival in whole body irradiated Swiss albino mice. International Journal of Nutrition, Pharmacology, Neurological Diseases, 2, 45–52.
- Block, E. (1985). The chemistry of garlic and onions. Scientific American, 252, 94–99.
- Chittezhath, M., & Kuttan, G. (2006). Radioprotective effects of naturally occurring organosulfur compounds. Tumori, 92, 162–169.
- Chung, L. Y. (2006). The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin and allyl disulphide. Journal of Medicinal Food, 9, 205–213.
- Citrin, D., Cotrim, A. P., Hyodo, F., Baum, B. J., Krishna, M. C., & Mitchell, J. B. (2010). Radioprotectors and mitigators of radiation-induced normal tissue injury. The Oncologist, 15, 360–371.10.1634/theoncologist.2009-S104
- Dharmarathna, S. L. C. A., Wickramasinghe, S., Waduge, R. N., Rajapakse, R. P. V. J., & Kularatne, S. A. M. (2013). Does Carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian Pacific Journal of Tropical Biomedicine, 3, 720–724.10.1016/S2221-1691(13)60145-8
- Hafeman, D. G., Sundae, R. A., & Houestra, W. G. (1974). Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. Journal of Nutrition, 104, 580–587.
- Ikpeme, E. V., Ekaluo, U. B., Kooffreh, M. E., & Udensi, O. (2011). Phytochemistry and heamatological potential of ethanol seed leaf and pulp extracts of carica papaya (Linn.). Pakistan Journal of Biological Sciences, 14, 408–411. doi:10.3923/pjbs.2011.408.411
- Jagetia, G. C. (2007). Recent advances in Indian herbal drug research guest editor: Thomas Paul Asir Devasagayam radioprotective potential of plants and herbs against the effects of ionizing radiation. Journal of Clinical Biochemistry and Nutrition, 40, 74–81.10.3164/jcbn.40.74
- Jagetia, G. C. R., & Tiyyagura, K. (2002). The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: A micronucleus study. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 519, 37–48.10.1016/S1383-5718(02)00111-0
- Joseph, W. F. (1997). Pharmacologic approaches to protection against radiation- induced lethality and other damage. Environmental Health Perspectives, 105, 1473–1478.
- Kala, C. P. (2012). Leaf juice of Carica papaya (L) A remedy of dengue fever. Medicinal Aromatic Plants, 1, 1–2.
- Kouvaris, J. R., Kouloulias, V. E., & Vlahos, L. J. (2007). Amifostine: The first selective-target and broad-spectrum radioprotector. The Oncologist, 12, 738–747. doi:10.1634/theoncologist.12-6-73810.1634/theoncologist.12-6-738
- Kumar, M., Samarth, R., Kumar, M., Senthamil, S. R., Saharan, B., Kumar, A.. (2006). Protective effect of Adhatoda vascia nees against radiation-induced damage at cellular, biochemical and chromosomal levels in Swiss albino mice. eCAM, 1–8.
- Lawson, L. D., Wang, Z. J., & Hughes, B. G. (1991). Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Medica, 57, 363–370.10.1055/s-2006-960119
- Madhu, L. N., Suchetha Kumari, N., Naveen, P., & Sanjeev, G. (2012). Protective effect of Nardostachys jatamansi against radiation-induced damage at biochemical and chromosomal levels in swiss albino mice. Indian Journal of Pharmaceutical Sciences, 74, 460–465.
- Mc Cord, J. M., & Fridovich, I. (1969). Superoxide dismutase enzyme function for erythrocaprein. Journal of Biochemistry, 244, 6049–6056.
- Montoro, A., Barquinero, J. F., Almonacid, M., Montoro, A., Sebastià, N., Verdù, G., … Soriano, J. M. (2011). Concentration-dependent protection by ethanol extract of propolis against γ-ray-induced chromosome damage in human blood lymphocytes. Evidence-Based Complementary and Alternative Medicine, Article ID 174853, 7 doi:10.1155/2011/174853
- Moron, M. A., Depierre, J. W., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta (BBA)—General Subjects, 582, 67–78.10.1016/0304-4165(79)90289-7
- Nageshwar Rao, B., Satish Rao, B. S., Kiran Aithal, B., & Sunil Kumar, M. R. (2009). Radiomodifying and anticlastogenic effect of Zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutation Research, 677, 33–41.
- Nair, C. K. K., Parida, D. K., & Nomura, T. (2001). Radioprotectors in radiotherapy. Journal of Radiation Research, 42, 21–37.10.1269/jrr.42.21
- Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.10.1016/0003-2697(79)90738-3
- Patil, S., Shetty, S., Bhide, R., & Narayanan, S. (2013). Evaluation of platelet augmentation activity of carica papaya leaf aqueous extract in rats. Journal of Pharmacognosy and Phytochemistry, 1, 58–61.
- Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Analytical Biochemistry, 269, 337–341.10.1006/abio.1999.4019
- Singh, N. P., McCoy, M. T., Tice, R. R., & Schneider, E. L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191.10.1016/0014-4827(88)90265-0
- Suzen, S. (1999). Radioprotective agents in the treatment of cancer and the role of amifostine. Journal of the Faculty of Pharmacy of Ankara University, 28, 117–128.
- Tice, R. R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., … Sasaki, Y. F. (2000). Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis, 35, 206–221.10.1002/(SICI)1098-2280(2000)35:3<>1.0.CO;2-I
- Upadhyay, S. N., Dwarakanath, B. S., Ravindranath, T., & Mathew, T. L. (2005). Chemical radioprotectors. Defence Science Journal, 55, 403–425.10.14429/dsj.55.2003
- Vennila, S., Mohana, S., Bupesh, G., Mathiyazhagan, K., Dhanagaran, D., Baskar, M., … Leeba, B. (2012). Qualitative phytochemical screening and invitro antioxidant activity of Helicteres isora L. Herbal Tech Industry, 14–18.
- Verma, P., Sharma, P., Parmar, J., Sharma, P., Agrawal, A., & Goyal, P. K. (2011). Amelioration of radiation-induced hematological and biochemical alterations in Swiss albino mice by panax ginseng extract. Integrative Cancer Therapies, 10, 77–84.10.1177/1534735410375098
- Yan, X., Wang, Z., & Barlow, P. (1992). Quantitative estimation of garlic oil content in garlic oil based health products. Food Chemistry, 45, 135–139.10.1016/0308-8146(92)90024-V
- Yuhas, J. M. (1980). Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3- aminopropylamino)-ethylphosphorothioic acid. Cancer Research, 40, 1519–1524.
- Zohra, S. F., Meriem, B., Samira, S., & Alsayadi Muneer, M. S. (2012). Phytochemical screening and identification of some compounds from Mallow. Journal of Natural Product and Plant Resources, 2, 512–516.