Abstract
The disease-free (control) and blast infected leaf samples of 11 rice genotypes were evaluated for activity profile of defense-related and antioxidative enzymes. The amplification genomic DNA with two SSR markers RM124 and RM224 were also performed for identification of blast resistance and susceptible genotypes. The activity of chitinase, PAL and β-glucosidase of post pathogen-infected leaf samples increased significantly in all rice genotypes, thought the increase was comparable less in to blast susceptible genotypes Chimansal and EK-70. The activity of antioxidative enzymes was comparatively higher in the infected leaf of blast resistant genotypes recording highest increase in NLR-20104 and KJT-5. The activity of defense-related and antioxidative enzymes in the disease-free leaf samples differed among the genotypes and was even higher in the two blast susceptible genotypes. RM144 and RM224 SSR primers clearly amplified in blast resistant KJT-5, NLR-20104, KJT-2, Tetep genotypes whereas RM144 missing in susceptible Chimansal but prominently present in susceptible genotype EK-70. This study revealed that higher level of induction of defense-related and antioxidative enzymes and presence of specific amplified fragments with RM144 and RM224 could be useful for screening the resistant and susceptible rice genotypes against Magnaporthe oryzae.
Public Interest Statement
Rice blast disease is a serious fungal disease caused by Magnaporthe oryzae. The use of chemical is expensive and not environment-friendly; hence utilization of host resistance has been the best way to manage the disease. To identify biochemical and molecular markers selectively differentiating the germplasm which can be environmentally sustainable plan for developing rice resistant genotypes. In this report, disease-free and infected leaf samples of 11 rice genotypes were screened against fungal blast disease for biochemical analysis of some defense-related and antioxidative enzymes. Amplification genomic DNA with two SSR markers RM124 and RM224 were also performed for identification of blast resistance and susceptible genotypes. This study revealed that higher level of induction of defense-related and antioxidative enzymes and presence of specific amplified fragments with RM144 and RM224 could be useful for screening the resistant and susceptible rice genotypes against M. oryzae.
1. Introduction
Rice blast disease is caused by the filamentous ascomycete fungus Magnaporthe oryzae, is the most devastating fungal disease in the rice growing world thus resulting in huge yield losses (Samalova, Meyer, Gurr, & Fricker, Citation2014). The disease symptoms appear on the aerial parts of the plant. Most infections occur on the leaves during vegetative phase and on panicle and neck during reproductive phase of the crop. Plant diseases are often severe during periods of warm temperatures and high moisture. Generally, rice blast is favored by moderate temperatures 24°C and periods of high moisture that is 12 h or longer, conditions readily attainable in flooded rice fields.
In India, management of rice blast diseases is highly dependent on chemical fungicides, and due to low levels of host plant resistance in many of the cultivated rice varieties. The expensive use of fungicide is not an environment-friendly for disease control and hence utilization of host resistance has been the best way to manage the disease, for which identification of sources of resistance are necessary (Bonman, Khush, & Nelson, Citation1992). A better understanding of the mechanisms involved in defense to M. oryzae infection and responsible for damage to the host plant may provide new methods to control this disease. The need for a better understanding of this disease becomes clear if we consider the poor durability of many blast resistant cultivars of rice, which have a typical field life of only 2–3 growing seasons before disease resistance is overcome. Rice blast control strategies that can be deployed as part of an environmentally sustainable plan for increasing the efficiency are therefore urgently required.
Plants defend themselves against pathogen challenge by the activation of defense response pathways (Staskawicz, Ausubel, Baker, Ellis, & Jones, Citation1997). The systemic resistance induction process increases enzymatic activity of peroxidase (POX) and polyphenol oxidase (PPO) which are responsible for catalyzing lignin formation, and phenylalanine ammonia lyase (PAL) which is involved in the biosynthesis of phytoalexins and phenols. The pathogenesis-related proteins (PRPs) β-1,3-glucanase and chitinase, enzymes that belong to PR-2 and PR-3 families, respectively (van Loon, Rep, & Pieterse, Citation2006) have been related more often to Systemic acquired resistance (SAR) and sometimes to Induced systemic resistance (ISR). All these enzymes have been shown to be involved in plant defense against pathogens in several pathosystem (Kini, Vasanthi, & Shetty, Citation2000). The activation and the expression levels of defense genes vary in different plant-pathogen interactions. Plants have also developed complex antioxidant defense systems that respond to biotic and abiotic stresses and mitigate the deleterious effects of reactive oxygen species (Panda, Citation2007). The levels of ROS and the extent of oxidative damage depend largely upon the level of coordination among ROS-scavenging enzymes (Liang, Chen, Liu, Zhang, & Ding, Citation2003). In transgenic rice plant, phenolic compounds and activity profile of some enzymes such as SOD, POX, APX and hydrolytic enzyme such as chitinase, β-glucosidase have shown to play active role in resistant mechanism of plant disease.
A combination of major resistance genes and defensive response genes form the basis for durable resistance. It has been reported that DNA markers that co-segregate with the resistant gene are a powerful method to accelerate development of a resistant cultivar (Fjellstrom et al., Citation2004a, Citation2004b). The availability of different molecular markers allows characterization of genes of interest. Single sequence repeat (SSR) can be applied to identify markers tightly linked to blast resistance genes and to detect genes and QTLs on rice chromosomes (Fjellstrom et al., Citation2004a; Liu, Wang, Chen, Lin, & Pan, Citation2005; Zhu, Wang, & Pan, Citation2004) which also extensively used in diversity analyses (Baraket et al., Citation2011; Swapna, Sivaraju, Sharma, Singh, & Mohapatra, Citation2010), marker-assisted selection (Zhu et al., Citation2009) and inheritance studies (Campoy et al., Citation2011). During the last few years, genetics of blast resistance in rice has been extensively studied and many dominant R genes conferring complete resistance to M. oryzae have been identified. In this study, the genomic DNA of four blast resistant and two blasts susceptible (Chimansal and EK-70) genotypes were amplified using these two flanking SSR markers. RM 224/RM 144 are flanking SSR markers for blast resistance located on chromosome 11 of rice crop which were used to distinguish resistance and susceptible cultivars of rice in present study.
Biochemical studies on defense-related and antioxidative enzymes can also be applied to identify markers tightly linked to blast resistance genes and QTLs on rice chromosomes. This information also helped in understanding the nature and mechanisms of resistance and aid in screening for disease resistant genotypes. The constitutive and induced biochemical defense of rice genotypes with amplification of identified two SSR markers RM-124 and RM-224 against rice blast was therefore undertaken at Lonavala region of Pune district in India which is a hot spot of rice blast disease (Krishnaveni et al., Citation2012).
2. Materials and methods
2.1. M. oryzae inoculation and disease rating
Five week old M. oryzae infected and disease-free leaf samples (Control) of rice seedlings of same plant from different blast resistant (KJT-2, TeTep, NLR 20104, KJT-5, Rp-Biopatho-3, Swarnadhan, RAU-631-9-10, CN-1447-9-4-2 and CB-06-555) and susceptible (EK-70 and Chimansal) genotypes were collected from Agricultural Research Station, Lonavala during rainy season of 2014. For screening of rice genotypes against leaf blast, a pot culture experiment was carried out in green house condition (20°C) at ARS, Lonavala, The inoculum load of the pathogen used was 106 spores ml−1 by spraying the leaves until run off. After 10 days of inoculum, spraying samples was collected immediately frozen in liquid nitrogen for biochemical analysis. The rating scale used for different lesion types on the basis of disease severity symptomatic leaf blades was measured according to the severity of the lesions described in Table (Anonymous, Citation2002).
Table 1. Grade description for screening against M. oryzae
The chitinase (EC.3.2.1.14) activity was assayed by the method of Giri et al. (Citation1998). For enzyme extraction 0.5 g of infected and control leaf samples were weighed separately and macerated with 2 ml of 0.1 M sodium citrate buffer in precooled mortar and pestle. The homogenate was centrifuged at 10,000 rpm for 10 min at 10°C and the supernatant was used as crude source of chitinase. 0.5 ml of supernatant was added to 2 ml of chitin suspension containing 7.5 mg of BSA and was incubated in water bath at 37°C for 3 h. From that an aliquot of 0.1 ml was taken for the estimation of N-acetyl glucosamine as per the method of Nelson-Smogyi. The chitinase activity was expressed in terms of μg N-acetyl glucosamine released per min per mg of protein. The PAL activity (E.C.4.1.3.5) was assayed by the method of Campos, Nonogaki, Suslow, and Saltveit (Citation2004). Infected and control seedlings were separately weighed and 0.5 g were macerated with 2 ml of 50 mM borate buffer (pH 8.5) containing 5 mM of 2-mercaptoethanol and 0.4 g polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 20,000 rpm at 4°C for 20 min. The collected supernatant was used as an enzyme source. The assay mixture containing 1 ml aliquots of supernatant and 110 μl of 100 mM L-phenylalanine were incubated at 40°C for 30 min. 1 ml of 4% tri-chloro acetic acid (TCA) was added in it to terminate the reaction. Similarly, the TCA was added in one of the test tubes at zero min to serve as blank. The assay mixture was incubated with TCA for 5 min at room temperature and the absorbance was read at 290 nm. PAL activity was calculated as μ moles of trans-cinnamic acid released per min per mg proteins under the specific condition.
The β-glucosidase activity was assayed by the modified method of Agrawal and Bahl (Citation1969). Enzyme extraction was done using 0.5 g of infected and control seedlings and samples were macerated with 0.05 M of sodium acetate buffer (pH 4.6). The homogenate was centrifuged at 23,000 rpm at 41°C for 20 min. The supernatant was used as an enzyme source. A reaction mixture was prepared by adding 100 μl of a solution of p-nitro phenyl-β-D glucopyranoside to 350 μl of 0.05 m sodium buffer (pH 4.6), followed by initial incubation at 30°C for 5 min. After the addition of 50 μl of enzyme extract, the mixture was further incubated at 30°C for 15 min. The reaction was stopped by adding 700 μl of 0.2 M sodium carbonate. The yellow color formed was measured at 420 nm by spectrophotometer. Enzyme activity was calculated as μ moles of p-nitro phenol released per min per mg of protein. The activity was calculated based on molar extinction coefficient (U) = 1.12 × 104 M−1 cm−1.
Ascorbate peroxidase (APX) (EC.1.11.1.11) activity was assayed as per the method described by Nakona and Asada (Citation1981). Enzyme extract for APX was prepared by grinding 0.5 g of controlled and infected leaf samples separately with 2 ml of 100 mM Potassium phosphate buffer (pH = 7.5). The homogenate was centrifuged at 15,000 rpm for 15 min at 4°C and the supernatant used as the enzyme source. The reaction mixture contained 2.3 ml phosphate buffer, 0.2 ml ascorbic acid, 0.2 ml (EDTA, 50 μl enzyme extract, 50 μl H2O2 and 0.3 ml distilled water. The reaction was started with addition of 0.2 ml of hydrogen peroxide. Decrease in absorbance after 30 s. was measured at 290 nm in UV–visible spectrophotometer. The activity was determined using molar extinction coefficient U = 2.8 mM−1 cm−1
Superoxide dismutase (SOD) (EC.1.15.1.1) activity was measured immediately in fresh extract as described by Dhindsa, Plumb-Dhindsa, and Thorpe (Citation1981). Enzyme extract for SOD was prepared by grinding 0.5 g of controlled and infected leaf samples separately with 2 ml of 100 mM Potassium phosphate buffer (pH 7.5). The homogenate was centrifuged at 15,000 rpm for 15 min at 4°C and the supernatant was used as the enzyme source. The reaction mixture contained, 1.5 ml phosphate buffer, 0.2 ml methionine, 0.1 ml Ethylene-diaminetetraacetic acid (EDTA), 0.1 ml sodium carbonate, 0.1 ml enzyme extract, 0.1 ml NBT, 0.9 ml distilled water, and 0.1 ml riboflavin. The reaction was started by adding 0.1 ml of riboflavin and placing the tubes under two 15 W fluorescent lamps for 15 min. A complete reaction mixture without enzyme, which gives the maximal color, served as control. Switching off the lights and putting the tubes into dark stopped the reaction. The non-irradiated complete reaction mixture served as blank.
The assay of peroxidase activity (EC.1.11.1.7) was performed as described by Sadasivam and Manickam (Citation1996). Enzyme extract for peroxidase was prepared by grinding 0.5 g of controlled and inoculated leaf samples separately with 2 ml of 100 mM potassium phosphate buffer (pH 7.5). The homogenate was centrifuged at 15,000 rpm for 15 min at 4°C and the supernatant was used as the enzyme source. For the peroxidase assay, the reaction mixture was prepared by adding 2.85 ml 0.1 M phosphate buffer (pH 7.0), 50 μl of 20 mM guaiacol solution, 50 μl of 12.3 mM hydrogen peroxide and 50 μl of enzyme extract. The reaction was allowed to proceed for 3 min. Absorbance at 470 nm were measured 30 s after adding the enzyme extract to the substrate, and change in the absorbance was recorded up to 3 min. The peroxidase activity was determined using molar absorption coefficient U = 26.6 mM−1 cm−1. The protein content in the crude enzyme extract was estimated according to the method of Lowry, Rosebrough, Farr, and Randall (Citation1951).
2.2. DNA Isolation, purification and amplification of PCR product
Isolation of genomic DNA from young seedlings was carried out by modified cetyltrimethyl ammonium bromide (CTAB) method described by Keim, Olson, and Shoemaker (Citation1988). Fresh young leaves of rice, about 0.5 g were taken and cut into small pieces (about 10 mm²) with blade, powdered in liquid nitrogen with mortar and pestle. The powder was homogenized in prechilled 1.5 ml CTAB buffer with mortar and pestle and transferred in 2 ml eppendorf tubes. The tubes were incubated for 60 min at 65°C in thermostatic water bath. The contents in the tubes were mixed after every 15 min by inversion during incubation and the tubes were allowed to cool at room temperature. An equal volume of CI (24:1) was added into the contents of fresh tube. The tubes were then centrifuged at 10,000 rpm for 10 min at 4°C in a high speed refrigerated centrifuge (Kubota 6,500, Japan). Then aqueous phase was carefully recovered and transferred to a fresh tube for precipitation of DNA. The tubes were again centrifuged at 12,000 rpm for 10 min and collect upper layer in 1.5 ml tube. About 500 μl, ice cold 70% (v/v) ethanol was added into the tubes and 50 μl of 7.5 M ammonium acetate was added to it and kept it for precipitation for 1 h at −20°C. Then the pellets were washed with 70% ethanol. The DNA pellet was air dried till the last traces of ethanol was evaporated. The pellets were resuspended in suitable volume of TE (10/1) buffer for PCR use.
For purification, 500 μl of DNA sample was taken in a fresh eppendorf tube with 10 μl of RNase A (10 mg/ml) and incubated at 37°C for 1 h with occasional gentle shaking. After incubation, 20 μl of Proteinase K and 100 μl of 3 M sodium acetate (pH 4.8) was added followed by incubation for 60°C for 1 h. Further steps was followed as per above procedure. DNA amplification was carried out in a 0.2 ml PCR tubes having 25 μl reaction volume as described by Mahatma, Bhatnagar, Solanki, Mittal, and Shah (Citation2011) with some modification. The reaction mixture containing 17 μl of sterile distilled water with 10x reaction buffer 2 μl with 15 mM Mgcl2, 1 μl of dNTPs (10 mM), 0.5 μl(3U) of Tag DNA polymerase, 2 μl (1 μl forward and 1 μl reverse primer form 10 pmol stock and 2 μl (20 ng/μl) template DNA (Table ).
Table 2. Chromosome location (CL), size range (SR), primers sequence and annealing temperature (AT) for two SSR markers used in the study
The 25 μl reaction mixture was gently vortexed and spinned down. The DNA amplification was carried out on a thermal cycler (Eppendorf Master Cycle gradient, Germany). PCR reaction performed as follows: The first step consist of 1 cycle for initial denaturation at 94°C for 5.0 min, annealing as per the TM values of primers for 45 s and primer extention at 72°C for 1.0 min and 30 s. A final extention at 72°C for 7 min was given at the end of the cycles and the samples were held at 4°C till retrieval. The amplification of PCR product was subjected to 3% agarose gel electrophoresis. For this 3 g of metaphore agarose gel was added to 100 ml of 1x TBE buffer and melted by heating the solution in a microwave oven. The solution was cooled to about 50°C and 5 μl ethidium bromide was added in it. In prepared gel 25 μl PCR product were analyzed by mixing 2 μl of tracking dye and loaded carefully in the wells of gel and amplification was performed. The amplified PCR products were observed under UV transilluminater in gel imaging and spot picking work station documentation system and image was captured.
All biochemical parameters were analyzed in three replication. The data obtained by biochemical constituents and enzymes determination were subjected to factorial completely randomized design (CRD) for the significance of various data analyzed and means were compared by Tukey test (p = 0.05) using SAS, Institute (Citation2002).
3. Results
3.1. Disease severity
Five-week old disease-free rice seedlings when challenge with pathogen (106 spores ml−1) by spraying and after 10 significant differences was observed for disease severity. From the Table , it is observed that out of 11 genotypes, NLR 20104, KJT-5-1-10-22-38-13, CB-06-555, TeTep, Swarnadhan, RAU-631-9-10 and RP-Biopatho-3 were categorized as a resistant genotypes to leaf blast, whereas CN-1447-9-4-7 and KJT-2 were found to be moderately resistant. On the contrary, Ek70 and Chimansal, which are the susceptible check, recorded highly susceptible reaction to leaf blast. There was no any infection on any genotypes/entry before inoculation.
Table 3. Leaf blast severity were recorded by following 0–9 SES scale as per IRRI, Philippines
3.2. Activity of antioxidative enzymes
The activity of SOD recorded in range from 3.91 to 14.15 U mg−1 protein in the control leaf samples of 11 rice genotypes and in infected it was ranged between 8.06 and 27.12 U mg−1protein (Figure ). The activity increased significantly highest in NLR-20104 followed by KJT-5. The induction levels of SOD in term of fold increase after inoculum spraying was higher (2.96 fold) in KJT-5 followed 2.63 fold in RAU-631-9-10 resistant and 1.64 fold in CN-1447-9-4-2 moderately blast resistant and least in EK-70 (0.33 fold) and Chimansal (0.41 fold) the two blast susceptible genotypes of rice.
Figure 1. Activity profile of superoxide dismutase (SOD).
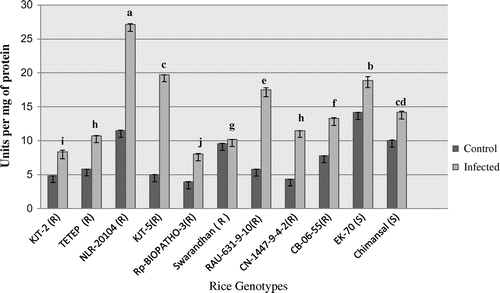
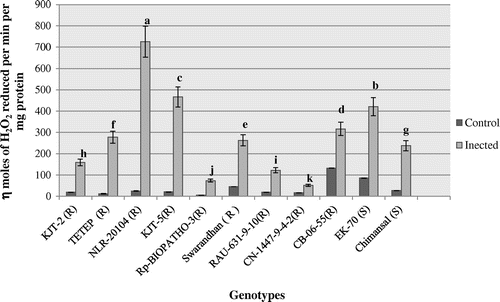
The peroxidase activity profile ranged between 5.67 and 132.61 n moles H2O2 oxidized min−1 mg−1 protein in the infected leaf samples of all 11 rice genotypes (Figure ). The activity profile of peroxidase (POX) in the infected leaf samples ranged between 51.16 and 725 n moles H2O2 oxidized min−1 mg−1 protein. After 10 days of inoculum spraying, the POX activity significantly induced in blast resistant genotypes by 27.96 fold followed by 21.82 fold in NLR-20104 and KJT-5 followed by TETEP (21.61 fold) (p < 0.05). The least level of induction was recorded in Chimansal (3.92 fold), a blast susceptible rice. It thus appears that the level of induction higher than 20 fold over the uninfected control (constitutive) may be considered as a criterion for selection.
Figure 2. Activity profile of peroxidase (POX).
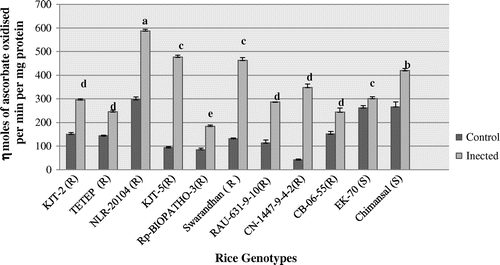
APX activity profile ranged between 42.48 and 296.59 n moles ascorbate oxidized/min/mg protein in the uninfected leaf samples of 11 rice genotypes (Figure ). Whereas in the infected leaf samples, it was ranged between 184.43 and 587.67 n moles ascorbate oxidized min1 mg−1 protein. Significantly (p < 0.05) highest APX activity was recorded after inoculum sprayed leaf samples of NLR-20104 (587.67 n moles ascorbate oxidized min−1 mg−1 protein) followed by KJT-5 (476.67 n moles ascorbate oxidized min−1 mg−1 protein) followed by Swarandhan (463.23 n moles ascorbate oxidized min−1 mg−1 protein), respectively. EK-70 but these blast susceptible genotypes recorded constitutive higher levels of APX as against other resistant genotypes but level of induction was nonsignificant. After 10 days of inoculum spraying the APX activity significantly induced in blast resistant genotypes by 8.20 fold in CN-1447-9-4-2(R) followed by 5.09 fold in KJT-5 and least in EK-70 (1.14 fold).
Figure 3. Activity profile of ascorbate peroxidase (APX).
3.3. Activity profile of defense-related enzymes
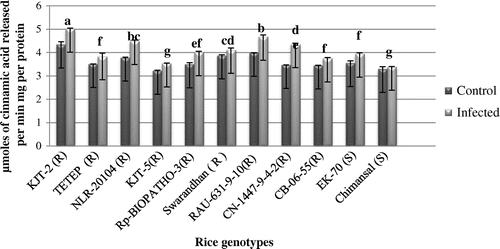
The PAL activity profile 11 rice genotypes ranged between 3.21 and 4.34 μ moles cinnamic acid released/min/mg protein in the disease-free leaf samples whereas it was ranged between 3.39 and 5.01 μ moles cinnamic acid released/min/mg protein in the infected leaf samples (Figure ). Highest increase of PAL activity recorded in KJT-2 (R) (4.67 μ moles cinnamic acid released min1 mg−1 protein). The order of induction of PAL activity on infection with the pathogen was in the order KJT-2(R) followed by NLR-20104 and RAU-631-9-10 and the least level of induction was recorded in Chimansal a blast susceptible rice genotype. From Figure , it was also observed that the PAL activity constitutively recorded higher in both group of genotypes.
Figure 4. Activity of phenylalanine ammonia lyase (PAL).
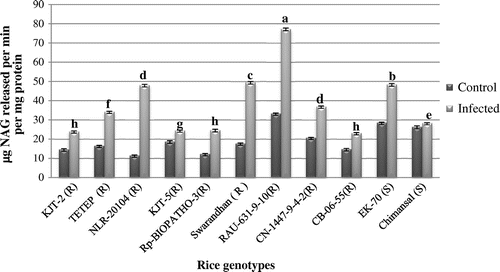
The leaf chitinase activity profile ranged between 11.53 and 33.35 μg NAG released min1 mg−1 protein in the disease-free leaf samples of 11 rice genotypes. As compared to control samples chitinase activity significantly increased after infection of M. oryzae from 23.21 to 77.15 μg NAG released/min/mg protein (Figure ). The leaf chitinase activity was significantly higher both in the uninfected (constitutive) and infected (induced) recorded in RAU-631-9-10 that is 33.35 to 77.15 μg NAG released min−1 mg−1 protein followed by Swarandhan and in susceptible EK-70. Chimansal recorded constitutively higher activity after infection but it was low as compared to other resistant rice genotypes. The order of induction level of chitinase activity significantly (p < 0.05) higher in NLR-20104 and CN-1447-9-4-2 (R) (4.17) and was at par in Swarandhan and RAU-631-9-10 and least level of induction was recorded in Rp-Biopath-3 (R).
Figure 5. Activity of Chitinase.
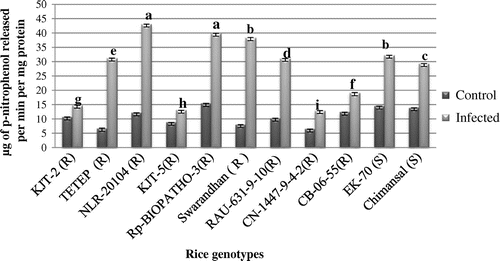
In case of β-glucosidase, as compared to control samples (6.45 to 15.42 n moles p-nitro phenol released min−1 mg−1 protein) the activity profile significantly induced in all genotypes and it was ranged from 12.93 to 43.08 n moles p-nitro phenol released min−1 mg−1 protein in infected leaf samples of all 11 rice genotypes (Figure ). Highest β-glucosidase activity in the infected leaf samples was recorded in NLR-20104 and Rp-BIOPATHO-3R of 43.08 and 39.87 n moles p-nitro phenol released/min/mg protein respectively followed by Swarandhan (R) 38.34 n moles p-nitro phenol released min−1 mg−1 protein and EK-70(S) (n moles p-nitro phenol released min−1 mg−1 protein) and Swarandhan with. Constitutive higher β-glucosidase activity recorded in both group of rice genotypes.
Figure 6. Activity of β-glycosidase.
3.4. Genomic DNA amplification using SSR primers
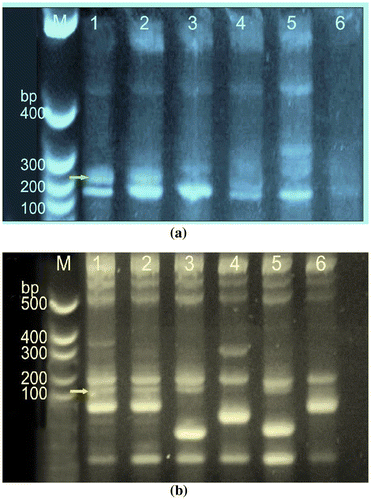
The genomic DNA of four blast resistant (KJT-2, KJT-5, Tetep and NLR-20104) and two blast susceptible (Chimansal and EK-70) genotypes were amplified using these two SSR markers, RM 224 with an expected allele size fragment of 122 bp, corresponding to PiL locus and RM 144 with an expected allele size of 254 bp corresponding to Pik locus. It is observed that a band corresponding ~122 bp with RM 224 is clearly visible in all the four blast resistant genotypes, while the same is not very prominent in the two blast susceptible genotypes (Figure (a)). As regard to the amplification pattern using RM144, a band corresponding to a allelic size of ~254 bp was distinctly visible in line 1, 2 and 5 corresponding to KJT-2, KJT-5 and NLR-20104 a three blast resistant genotypes, which was missing in the susceptible genotype Chimansal, however, the same was prominently present in another susceptible genotype EK-70 (Figure (b)).
Figure 7. The purified genomic DNA was quantified and equal amount of DNA was used for PCR amplification. (a) Amplified genomic DNA of blast resistant and susceptible genotypes with SSR primer RM 224 and (b) with SSR RM144. The Lane M:100 bp, lane 1:1-KJT-2 (R),Lane 2:KJT-5 (R), Lane 3-TETEP (R), Lane 4:Chimansal(S), Lane 5:NLR-20104 (R),Lane 6:-EK-70 (S).
4. Discussion
In plant-pathogen interactions formation of new proteins that have direct or indirect effect on plant resistance to pathogen include a heterogeneous group of proteins collectively called as pathogenesis-related (PR) proteins (Jones & Dangl, Citation2006). PR protein such as PAL, β-glucosidase and chitinase has been suggested to be involved in plant resistance against fungal pathogens (Kini et al., Citation2000). The ROS can be second messengers in resistance mechanisms leading to activation of defense-related genes and interferes with other important signaling molecules (Chen, Kidd, Carvalhais, & Schenk, Citation2014). Uncontrolled accumulation of ROS results in spreading cell death which in some cases can enhance plant susceptibility (Torres, Jones, & Dang, Citation2006). Thus, ROS production and elimination are tightly controlled during plant-pathogen interactions. Enzymatic antioxidants such as SOD and peroxidase (POX) are participating in scavenging various types of ROS (Barna, Fodor, Harrach, Pogány, & Király, Citation2012). The enzyme SOD constitutes the first line of defense against ROS by catalyzing the dismutation of O2− to O2 and H2O2 (Alscher, Erturk, & Heath, Citation2002). In our study, SOD activity was significantly induced in infected leaf of both resistant and susceptible rice genotypes (Figure ). POX is one of the most important enzymes active in elimination of ROS and catalyzes the oxidoreduction of various substrates using hydrogen peroxide. Depending upon physiological condition peroxidase may acts as either H2O2 scavanger or generator (Almagro et al., Citation2009). Many reports have suggested that POX plays a role in resistance to pathogens (Kawaoka et al., Citation2003). In our study, SOD and POX activities induced significantly over controls suggesting their activation might be due to generation of ROS there by activating other defense cascade (Rahman, Uddin, & Wenner, Citation2014) or display direct toxicity toward invading pathogen (Torres, Jones, & Dang, Citation2006), thus this two enzymes might be more efficiently control ROS (Figures and ).
APX is an enzymatic antioxidant present in practically all sub cellular compartments. In rice, Agrawal, Jwa, Han, Agrawal, and Rakwal (Citation2003) reported cytosolic APX genes are up regulated upon wounding suggesting that the cytosolic APX isozymes play a protective role against stressful conditions. In our study, constitutive higher APX activity has been recorded in both group of rice genotypes and it was induced on post infection (Figure ). This indicates their participation in removal of H2O2 produced by induced SOD activity. Similar increase of APX activity with glutathione reductase activity is reported in pearl millets Sclerospora graminicola by Kumar, Naik, Satbhai, and Patil (Citation2015). Overall, this study revealed that priming in ROS production and the activity of antioxidant enzymes such as SOD, POX and APX occurred during interaction with the pathogen more in resistant rice cultivar compared to susceptible cultivar. Furthermore, the greater resistance of NLR-20104 (R), KJT-5 (R), Swarnadhan (R) genotypes might be associated with greater lignin contents in this genotypes. Together, these findings suggest the critical role of early O2− and H2O2 accumulation, also SOD and POX and APX dependent lignifications as a defense mechanism involved in basal resistance in our pathosystem. The induced levels of antioxidants after post infection in rice plants might be due to pathogen-associated molecular pattern triggered immunity and effectors triggered immunity that accompanied by ROS generation (Filippi et al., Citation2011; Taheri, Irannejad, Goldani, & Tarighi, Citation2014) and that also strengthening of plant cell wall to limit proliferation of pathogen under control condition as a basal defense and during the pathogen attack of M. oryzae (Chisholm, Coaker, Day, & Staskawicz, Citation2006; Jones & Dangl, Citation2006). In addition to the enzymatic H2O2 scavenging system, phenolics are strong non-enzymatic antioxidants due to availability of their phenolic hydrogen. Some phenolics are constituents of lignin and these phenolics are oxidized by POX using H2O2 (Nikraftar, Taheri, Falahati Rastegar, & Tarighi, Citation2013; Sharma, Jha, Dubey, & Pessarakli, Citation2012).
PAL catalyses the first committed step for biosynthesis of the phenyl propanoid pathway in higher plants and involved in the synthesis of both phytoalexin and lignin. These PR protein prevent cell wall penetration by the pathogen (Dixon, Citation2001). In this study PAL also recorded higher constitutive level in both group of rice genotypes with similar trend of increment in infected samples (Figure ). Earlier positive correlation of PAL in six rice cultivars differing in resistance to M. oryzae with the degree of resistance has been reported (Zhang, Duan, & Yu, Citation1987). PAL activity in highly resistant cultivars was 63.5% higher than in susceptible cultivars which cause hardening of infection sites, thus preventing pathogen entry into the host plant reported by Hsieh, Ma, Yang, and Lee (Citation2010).
In this study, the chitinase activity constitutively higher at control in all 11 rice genotypes that indicate their importance and involvement in basal defense response in rice. Early research showed low constitutive expression of chitinases in healthy plants and induced to much higher levels upon infection or wounding (Boller, Citation1988). Shimizu et al. (Citation2010) reported that Slp1, a novel effector secreted by M. oryzae competes for chitin binding with the rice pattern recognition receptor (PRR) chitin elicitor binding protein (CEBiP), which is required for chitin triggered immunity in rice, acting in cooperation with the LysM receptor-like kinase Os-CERK1. Transgenic over expression lines, using family 19 chitinases from bean, tobacco, and rice confirmed that higher constitutive expression of some chitinases indeed does contribute to increased fungal resistance (Datta et al., Citation2001).
β-glucosidase a enzymes with a broad substrate specificity is a microbial cell wall degrading enzymes and have relationship to pathogenicity (Takeda et al., Citation2010). In our study, KJT(5), CN-1447-9-4-2 and CB-06-55 a resistant rice genotypes recorded lowest activity than susceptible EK-70 and Chimansal both at control and post infection indicates these enzymes showed more activation to avoid infection but their action might insufficient to limit the spread of M. oryzae than the resistant (Figure ). Yang, Jiang, Yan, and Zhu (Citation2008) reported microbial and fungal β-glucosidase (EC 3.2.1.21) are produced extra cellularly and intra cellularly, and are thought to play a significant role in saccharifying cellulosic materials and acquiring nutrients by producing glucose. Earlier Whetten, MacKay, and Sederoff (Citation1998) reported that the plant β-glucosidase may be involved in the processing and release of fungal glucan elicitors, triggering a chain of reactions in the host, including phytoalexin formation and the biosynthesis of phenylpropanoids and lignin-like phenol aglucones by hydrolyzing B-phenyl glucosides. These aglucones are basically fungi toxic and fungi static in action and may limit the spread of M. oryzae in resistant plants. A similar result in accumulation of β-1-3 glucanase, PAL and chitinase enzymes in incompatible interactions of pearl millets has been reported recently by Kumar et al. (Citation2015). Suppression of blast infection M. oryzae has been reported with induction of this enzymes by soil drenching of rhizobacteria in rice field by Filippi et al.(Citation2011). This induced resistance (IR) may control the pathogens or damaging factors, completely or partially (Chen et al., Citation2014; Kuc, Citation1982). Several studies have shown that genes expressed during IR responses produce proteins with chitinase, glucanase and other enzymatic activities that are involved in defense reactions to a wide spectrum of pathogens (van Loon, Rep, & Pieterse, Citation2006). In resistant rice genotypes, the activity of defense-related and antioxidative enzymes increased might associated with hypersensitive response (HR) and or M. oryzae has robust the defense system. Our results are in agreement with the results of above quotation.
PCR-based microsatellite markers have been widely used to screen, characterize and evaluate genetic diversity in cereal species. In particular, microsatellite based methods offer an high through put and non labour intensive way to tag resistance genes in breeding programs. RM 224/RM 144 are flanking SSR markers for blast resistance loci located on chromosome 11 of rice crop which were used to detect allelic differences in resistance and susceptible cultivars of rice. In present study all the four resistant genotypes KJT-2, KJT-5, Tetep and NLR-20104 could amplify the fragment 139 and 254 bp using RM244 and RM144 primers, respectively (Figure (a) and (b)). These genotypes also recorded lower score of blast infection. Suh et al. (Citation2009) reported the presence or absence of the eight major blast-resistance genes (Pib, Pia, Piz, Piz-t, Pi9, Pi5, Pita, and Pi40) was validated using foreground selection with gene specific DNA markers in the parents. According to report by Liu, Pi-l is located 6.8 cM and Pi-k is located 1.2 cM away from the RM-144 microsatellite. More recently, there has been a report concerning the identification of rice blast resistance using RM 144. Markers RM 144 and RM224 co-segregated with both Pi-kh and Pi-ks resistance factors found at this locus (Fjellstrom et al., Citation2004a, Citation2004b). The presence of the Pi-kh and Pi-ks alleles can be differentiated by RM-224 and RM-144. The Pi-kh gene is associated with the RM-224 = 139 nucleotide (nt) and RM-144 = 255 nt alleles, whereas the Pi-ks allele is associated with the RM-224 = 120 nt and RM-144 = 255 nt alleles. It is reported that RM-144 an allelic fragment size of 254 is expected in resistant genotypes corresponding to Pik locus. However, in the present finding, a band corresponding to 200 bp was observed both in susceptible and resistant genotype. These markers are ideally suited for marker assisted selection for blast resistance in rice because of their tight linkage with resistance genes and ease of use through analysis of amplification products (Fjellstrom et al., Citation2004a, Citation2004b). Fjellstrom, McClung, and Shank (Citation2006) reported, SSR markers linked to the Pi gene were useful for selection of resistance genes at the Pi gene locus in rice germplasm. Eizenga, Agrama, Lee, Yan, and Jia (Citation2006) reported marker RM225 to be close to the blast resistant genes Pi22 and Pi27, where as Temnykh et al. (Citation2001) mapped the marker RM225 on short arm of chromosome 6 at 1.1 cM away from the marker RM204. It suggests that marker RM224 identified in present study is in close vicinity to the gene.
5. Conclusions and future prospects
The activity of defense-related enzymes i.e. pathogenesis related protein was induced differentially in different genotypes upon pathogen infection and appreciable differences was observed in both resistant and susceptible rice genotypes. However, the activity profile of these enzymes in the disease-free leaf samples of some of the resistant genotypes was less than the activity recorded in blast susceptible genotypes (Chimansal). The resistant genotypes NLR-20104, KJT-5 (R), Swaranadhan, KJT-2 recorded higher levels of defense-related and antioxidative enzymes both at constitutive and post infection be considered as most promising blast resistant genotypes. Whereas significant higher induction levels of chitinase and with higher disease severity in susceptible EK-70 than the other resistant genotypes needs further validation and needs to be tested at different stages of post inoculation. In addition to two SSR markers RM-224, RM-144 some already reported SSR markers on different linkage group needs to be attempted for further study. This study revealed that, the post infection induction in activity level of defense-related and antioxidant enzymes with least disease severity and validation of genomic DNA with RM144 and RM224 could be effectively exploited to screen the rice genotypes for blast resistance.
Table_4_supplimentary_information_table.docx
Download MS Word (25 KB)Additional information
Funding
Notes on contributors
P.U. Anushree
We at the department of Biochemistry are undertaking research work to identify biochemical and molecular markers to screen the available germplasm of crop plants for major abiotic and biotic stresses particularly in pigeon pea, chickpea for wilt and sterility mosaic, rice for blast and blight, sorghum for shoot fly. The work is also being undertaken for abiotic stresses particularly drought and salinity and also for combinational stress. The present work is similar attempt which was carried out in collaboration with rice pathologist working at ARS, Lonavala, India which is a hot spot for blast disease of rice. Disease-free and infected leaf samples of 11 rice genotypes were collected and biochemical analysis of some defense-related and antioxidative enzymes was carried out. Flanking markers RM144 and RM224 also amplified for validation of blast resistant and susceptible rice genotypes in the present study. These efforts are helping the plant breeder to understand breeding program and developing mapping population is being utilized for marker trait association.
References
- Agrawal, G. K., Jwa, N. S., Han, K. S., Agrawal, V. P., & Rakwal, R. (2003). Isolation of a novel rice PR4 type gene whose mRNA expression is modulated by blast pathogen attack and signaling components. Plant Physiology and Biochemistry, 41, 81–90.10.1016/S0981-9428(02)00012-8
- Agrawal, K. M. L., & Bahl, O. P. (1969). Glycosidases of Phaseolus vulgaris. The Journal of Biological Chemistry, 243, 103–111.
- Almagro, L., Gomez Ros, L. V., Belchi-Navarro, S., Bru, R., Ros Barcelo, A., & Pedreno, M. A. (2009). Class III peroxidases in plant defence reactions. Journal of Experimental Botany, 60, 377–390.10.1093/jxb/ern277
- Alscher, R. G., Erturk, N., & Heath, L. S. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53, 1331–1341.10.1093/jexbot/53.372.1331
- Anonymous. (2002). Find out how the qualities of rice are evaluated and scored in this authoritative sourcebook. Standard evaluation system for rice (p. 1518). Laguna: IRRI.
- Baraket, G., Chatti, K., Saddoud, O., Abdelkarim, A. B., Mars, M., & Trifi, M. (2011). Comparative assessment of SSR and AFLP markers for evaluation of genetic diversity and conservation of fig, Ficus carica L., genetic resources in Tunisia. Plant Molecular Biology Reporter, 29, 171–184.10.1007/s11105-010-0217-x
- Barna, B., Fodor, J., Harrach, B. D., Pogány, M., & Király, Z. (2012). The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiology and Biochemistry, 59, 37–43.10.1016/j.plaphy.2012.01.014
- Boller, T. (1988). Ethylene and the regulation of antifungal hydrolases in plants. Oxford Surveys of Plant Molecular and Cell Biology, 5, 145–174.
- Bonman, J. M., Khush, G. S., & Nelson, R. J. (1992). Breeding rice for resistance to pests. Annual Review of Phytopathology, 30, 507–528.10.1146/annurev.py.30.090192.002451
- Campos, R., Nonogaki, H., Suslow, T., & Saltveit, M. S. (2004). Isolation and characterization of a wound inducible phenylalanine ammonia-lyase gene (LsPAL1) from Romaine lettuce leaves. Physiologia Plantarum, 121, 429–438.10.1111/ppl.2004.121.issue-3
- Campoy, J. A., Ruiz, D., Egea, J., Rees, D. J. G., Celton, J. M., & Martínez-Gómez, P. (2011). Inheritance of flowering time in apricot (Prunus armeniaca L.) and analysis of linked quantitative trait loci (QTLs) using simple sequence repeat (SSR) markers. Plant Molecular Biology Reporter, 29, 404–410.10.1007/s11105-010-0242-9
- Chen, Y. C., Kidd, B. N., Carvalhais, L. C., & Schenk, P. M. (2014). Molecular defense responses in roots and the rhizosphere against Fusarium oxysporum. Plant Signaling & Behavior, 9(12), e977710. doi:10.4161/15592324.2014.977710
- Chisholm, S. T., Coaker, G., Day, B., & Staskawicz, B. J. (2006). Host-microbe interactions: Shaping the evolution of the plant immune response. Cell, 124, 803–814.10.1016/j.cell.2006.02.008
- Datta, K., Tu, J., Oliva, N., Ona, I. I., Velazhahan, R., Mew, T. W., Muthukrishnan, S., & Datta, S. K. (2001). Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Science, 160, 405–414.10.1016/S0168-9452(00)00413-1
- Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.10.1093/jxb/32.1.93
- Dixon, R. A. (2001). Natural products and plant disease resistance. Nature, 411, 843–847.10.1038/35081178
- Eizenga, G. C., Agrama, H. A., Lee, F. N., Yan, W., & Jia, Y. (2006). Identifying novel resistance genes in newly introduced blast resistant rice germplasm. Crop Science, 46, 1870–1878.10.2135/cropsci2006.0143
- Filippi, M. C. C., da Silva, G. B., Silva-Lobo, V. L., Côrtes, M. V. C. B., Moraes, A. J. G., & Prabhu, A. S. (2011). Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biological Control, 58, 160–166.10.1016/j.biocontrol.2011.04.016
- Fjellstrom, R., Conaway-Bormans, C. A., McClung, A., Marchetti, M. A., Shank, A. R., & Park, W. D. (2004a). Development of DNA markers suitable for marker assisted selection of three genes conferring resistance to multiple pathotypes. Crop Science, 44, 1790–1791.10.2135/cropsci2004.1790
- Fjellstrom, R., Conaway-Bormans, C. A., McClung, A. M., Marchetti, M. A., Shank, A. R., & Park, W. D. (2004b). Development of DNA markers suitable for marker assisted selection of three Pi. Genetics, 138, 1251–1274.
- Fjellstrom, R., McClung, A. M., & Shank, A. R. (2006). SSR markers closely linked to the Pi-z locus are useful for selection of blast resistance in a broad array of rice germplasm. Molecular Breeding, 17, 149–157.10.1007/s11032-005-4735-4
- Giri, A. P., Harsulkar, A. M., Patankar, A. G., Gupta, V. S., Sainani, M. N., Deshpande, V. V., & Ranjekar, P. K. (1998). Association of induction of protease and chitinase in chickpea roots with resistance to Fusarium oxysporum f.sp. ciceri. Plant Pathology, 47, 693–699.10.1046/j.1365-3059.1998.00299.x
- Hsieh, L. S., Ma, G. T., Yang, C. C., & Lee, P. D. (2010). Cloning, expression, site-directed mutagenesis and immunolocalization of phenylalanine ammonia-lyase in Bambusa oldhamii. Phytochemistry, 71, 1999–2009.10.1016/j.phytochem.2010.09.019
- Jia, Y., & Moldenhauer, K. (2010). Development of monogenic and digenic rice lines for blast resistance genes. Journal of Plant Registrations, 4, 163–166.10.3198/jpr2009.04.0223crmp
- Jones, J. D. G., & Dangl, J. (2006). The plant immune system. Nature, 444, 323–329.10.1038/nature05286
- Kawaoka, A., Matsunaga, E., Endo, S., Kondo, S., Yoshida, K., Shinmyo, A., & Ebinuma, H. (2003). Ectopic expression of a horseradish peroxidase enhances growth rate and increases oxidative stress resistance in hybrid aspen. Plant Physiology, 132, 1177–1185.10.1104/pp.102.019794
- Keim, P., Olson, T. C., & Shoemaker, R. C. (1988). A rapid protocol for isolating soybean DNA. Soybean Genetics Newsletter, 15, 150–152.
- Kini, K. R., Vasanthi, N. S., & Shetty, H. S. (2000). Induction of β-1,3-glucanase in seedlings of pearl millet in response to infection by Sclerospora graminicola. European Journal of Plant Pathology, 106, 267–274.10.1023/A:1008771124782
- Krishnaveni, D., Laha, G. S., Prasad, M. S., Ladha Lakshmi, D., Mangrauthia, S. K., Prakasam, V., & Viraktamath, B. C. (2012). Sources of resistance in “Disease resistance in rice” (p. 35). Hyderabad: Directorate of Rice Research.
- Kuc, J. (1982). Induced immunity to plant diseases. BioScience, 32, 854–860.
- Kumar, S., Naik, R., Satbhai, R., & Patil, H. (2015). Activity profile of defense related enzymes in pearl millet against downy mildew (Sclerospora graminicola). Journal of Pure and Applied Microbiology, 9, 1465–1474.
- Liang, Y. C., Chen, Q., Liu, Q., Zhang, W. H., & Ding, R. X. (2003). Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). Journal of Plant Physiology, 160, 1157–1164.10.1078/0176-1617-01065
- Liu, X. Q., Wang, L., Chen, S., Lin, F., & Pan, Q. H. (2005). Genetic and physical mapping of Pi36(t), a novel rice blast resistance gene located on rice chromosome 8. Molecular Genetics and Genomics, 274, 394–401.10.1007/s00438-005-0032-5
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
- Mahatma, M. K., Bhatnagar, R., Solanki, R. K., Mittal, G. K., & Shah, R. R. (2011). Characterisation of downy mildew resistant and susceptible pearl millet (Pennisetum glaucum (L.) R.Br.) genotypes using isozyme, protein, randomly amplified polymorphic DNA and inter-simple sequence repeat markers. Archives of Phytopathology and Plant Protection, 44, 1985–1998.10.1080/03235408.2011.559038
- Nakona, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbic specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 868–880.
- Nikraftar, F., Taheri, P., Falahati Rastegar, M., & Tarighi, S. (2013). Tomato partial resistance to Rhizoctonia solani involves antioxidative defense mechanisms. Physiological and Molecular Plant Pathology, 81, 74–83.10.1016/j.pmpp.2012.11.004
- Panda, S. K. (2007). Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. Journal of Plant Physiology, 164, 1419–1428.10.1016/j.jplph.2007.01.012
- Rahman, A., Uddin, W., & Wenner, N. G. (2014). Induced systemic resistance responses in perennial ryegrass against Magnaporthe oryzae elicited by semi-purified surfactin lipopeptides and live cells of Bacillus amyloliquefaciens. Mole Plant Pathology. doi:10.1111/mpp.12209
- Sadasivam, S., & Manickam, A. (1996). Biochemical methods (2nd ed.) New Delhi: New Age International.
- Samalova, M., Meyer, A. J., Gurr, S. J., & Fricker, M. D. (2014). Robust anti-oxidant defences in the rice blast fungus Magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytologist, 201, 556–573.10.1111/nph.12530
- SAS, Institute. (2002). Version 9.2. Cary, NC: Author.
- Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1–26.
- Shimizu, T., Nakano, T., Takamizawa, D., Desaki, Y., Ishii-Minami, N., Nishizawa, Y., … Shibuya, N. (2010). Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. The Plant Journal, 64, 204–214.10.1111/tpj.2010.64.issue-2
- Staskawicz, B. J., Ausubel, F. M., Baker, B. J., Ellis, J. G., & Jones, J. D. C. (1997). Molecular genetics of plant disease resistance. Science, 268, 661–667.
- Suh, J. P., Roh, J. H., Cho, Y. C., Han, S. S., Kim, Y. G., & Jena, K. K. (2009). The Pi40 gene for durable resistance to rice blast and molecular analysis of Pi40 - Advanced backcross breeding lines. Phytopathology, 99, 243–250.10.1094/PHYTO-99-3-0243
- Swapna, M., Sivaraju, K., Sharma, R. K., Singh, N. K., & Mohapatra, T. (2010). Single-strand conformational polymorphism of EST-SSRs: A potential tool for diversity analysis and varietal identification in sugarcane. Plant Molecular Biology Reporter. 11105-010-0254.
- Taheri, P., Irannejad, A., Goldani, M., & Tarighi, S. (2014). Oxidative burst and enzymatic antioxidant systems in rice plants during interaction with Alternaria alternata. European Journal of Plant Pathology, 140, 829–839.10.1007/s10658-014-0512-8
- Takeda, T., Takahashi, M., Nakanishi-Masuno, T., Nakano, Y., Saitoh, H., Hirabuchi, A., Fujisawa, S., & Terauchi, R. (2010). Characterization of endo-1,3–1,4-β-glucanases in GH family 12 from Magnaporthe oryzae. Applied Microbiology and Biotechnology, 88, 1113–1123.10.1007/s00253-010-2781-2
- Temnykh, S., DeClerck, G., Lukashova, A., Lipovich, L., Cartinhour, S., & McCouch, S. (2001). Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Research, 11, 1441–1452.10.1101/gr.184001
- Torres, M. A., Jones, J. D. G., & Dang, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiology, 141, 373–378.10.1104/pp.106.079467
- van Loon, L. C., Rep, M., & Pieterse, C. M. (2006). Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology, 44, 135–162.10.1146/annurev.phyto.44.070505.143425
- Whetten, R. W., MacKay, J. J., & Sederoff, R. R. (1998). Recent advances in understanding lignin biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 585–609.10.1146/annurev.arplant.49.1.585
- Yang, S., Jiang, Z., Yan, Q., & Zhu, H. (2008). Characterization of a thermostable extracellular β-glucosidase with activities of exoglucanase and transglycosylation from Paecilomyces thermophila. Journal of Agricultural and Food Chemistry, 56, 602–608.10.1021/jf072279+
- Zhang, J. T., Duan, G. M., & Yu, Z. Y. (1987). Relationship between Phenylalanine ammonia lyase (PAL) activity and resistance to rice blast. Plant Physiology Communications, 6, 34–37.
- Zhu, M., Wang, L., & Pan, Q. H. (2004). Identification and characterization of a new blast resistance gene located on rice chromosome 1 through linkage and differential analyses. Phytopathology, 94, 515–519.10.1094/PHYTO.2004.94.5.515
- Zhu, W., Lin, J., Yang, D., Zhao, L., Zhang, Y., Zhu, Z., Chen, T., & Wang, C. (2009). Development of chromosome segment substitution lines derived from backcross between two sequenced rice cultivars, indica recipient 93-11 and japonica donor nipponbare. Plant Molecular Biology Reporter, 27, 126–131.10.1007/s11105-008-0054-3
