Abstract
Background: A combination of genetic fine-mapping and complementation testing was used previously to assign the juvenile alopecia mutation (abbreviated jal) to the GATA binding protein 3 (Gata3) gene on Chromosome 2 in mice. However, sequence analysis of Gata3 exons (including coding and noncoding regions) revealed no differences between wild type C3H/HeJ and co-isogenic C3H/HeJ-jal/J mutant mice. Results: Using a PCR-based scanning method, here we have tested the hypothesis that jal might result from insertion of a transposable element in or near the Gata3 gene. We show that the jal mutation is specifically associated with an intracisternal A particle (IAP) element of the I∆1 subtype that has transposed to Intron 3–4 in the Gata3 gene, and use the same panel of recombinants used previously to fine-map jal to show that this IAP element and jal are located within the same small genetic interval. Conclusion: Transposition of an IAP element of the I∆1 subtype into Intron 3–4 of the mutant Gata3jal allele is the likely cause of the juvenile alopecia phenotype in mutant mice.
Public Interest Statement
In previous work, a recessive form of progressive hair loss known as juvenile alopecia was assigned to the Gata3 gene on mouse Chromosome 2, but no DNA defect was discovered. In this report, we identify a retrovirus-like element that is integrated within the Gata3jal allele and is the likely cause of the mutant phenotype. This mutational mechanism would explain the variable expressivity of the hair loss phenotype in juvenile alopecia mice, and suggests that these animals may serve as a living resource for the further study of epigenetic gene regulation.
Competing interests
The authors declare no competing interests.
1. Introduction
A significant amount of the mouse genome (8–10%) is composed of endogenous retroviruses (ERVs) and other DNA elements with long terminal repeats (LTRs) (Mouse Genome Sequencing Consortium (MGSC), Citation2002), and many of these remain “active,” in that their RNA can be reverse-transcribed and integrated into new genomic sites by a retrovirus-like transposition mechanism. Indeed, it has been estimated that some 10–15% of new mutations in mice are due to transposition of an ERV element into or near the altered gene (Maksakova et al., Citation2006). One family of ERV elements, the intracisternal A particle (IAP), is present in about 1,000 full-length or partially deleted copies in the haploid mouse genome (Kuff & Lueders, Citation1988), and one subclass of deleted IAP elements, the I∆1 subclass (which has a 1.9 kb deletion in gag-pol), is responsible for nearly all of the known, ERV-induced, de novo germline mutations in mice (Maksakova et al., Citation2006). Most of these I∆1 insertional mutations have occurred in the C3H/HeJ standard inbred mouse strain, suggesting that one or a small number of I∆1, IAP elements in this strain must persist in evading host suppression mechanisms (Maksakova et al., Citation2006).
Our group has previously assigned (by a positional-candidate approach) the juvenile alopecia mutation (abbreviated jal)—which arose spontaneously on the C3H/HeJ genetic background—to the Gata3 gene in mice (Ramirez et al., Citation2013). While complementation testing between jal and the engineered Gata3tm1Gsv null allele (van Doorninck et al., Citation1999) verified allelism, we could find no DNA defect in the exonic portions of Gata3 in jal mutants (Ramirez et al., Citation2013). The jal mutation’s C3H/HeJ strain-of-origin, the lack of an obvious coding defect in Gata3jal, and the markedly variable expressivity of the mutant phenotype (vibrissae defects and focal alopecia that may range from global to undetectable), combine to suggest that an IAP integration near or within an intron of Gata3 might be the basis of this natural variant. Here we test that hypothesis, and report the detection, orientation, and precise location of an I∆1, IAP element in Exon 3–4 of Gata3 that appears to be specific to the C3H/HeJ-jal/J strain and maps to the same small region as the jal mutation itself.
2. Methods
Animals were housed and fed according to Federal guidelines, and the Institutional Animal Care and Use Committee at CCSU approved of all procedures involving mice (Animal Protocol Application numbers 101, 119, and 122). Standard inbred mice (from strains C57BL/6 J, A/J, C3H/HeJ) and C3H/HeJ-jal/J mutant mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). We maintained our coisogenic C3H/HeJ-jal/J line by crossing heterozygous females with homozygous males, to produce segregating litters. Mutant jal/jal mice were mostly detected by vibrissae defects that were observable shortly after birth, and those homozygotes displayed distinct patches of hair loss by two weeks of age that persisted throughout life (Figure ). The mutant phenotype was not fully penetrant, and was highly expressive such that the amount of body surface affected by baldness varied from less than 5% to greater than 95% (see additional photographs shown in Figure and Additional file 1 in Ramirez et al., Citation2013).
Genomic DNA was isolated from 3 mm tail tip biopsies taken from two-week-old mice using Nucleospin kits from BD Biosciences (Palo Alto, CA, USA). The polymerase chain reaction (PCR) was performed using the Titanium PCR kit from Clontech (Mountain View, CA, USA), and oligonucleotide primers for PCR were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA). Gata3-specific primers were based on sequence information available online (CitationEnsembl Mouse Genome Server (EMGS), XXXX) and IAP-element-specific primers were based on the IAP LTR sequence (GenBank D63767) published by Ishihara, Tanaka, Wan, Nojima, and Yoshida (Citation2004). Specifically, the primer designated ISP-R1 or ISP-F4 herein was 5’ GAGCTGACGTTCACGGGAAAAAC 3’; the primer designated ISP-F2 or ISP-R3 was 5’ ACGACCACTTGTACTCTGTTTTTC 3’. Primers designed to overlap or flank the gag-pol deletion that is characteristic of the I∆1 subtype were based on the widely-studied, full-length IAP element MIA14 (GenBank M17551), published by Miets, Grossman, Lueders, and Kuff (Citation1987). The specific primers designated ISP-Fa, ISP-Fb and ISP-Fc herein are shown in Supplementary Figure S1. PCR products were visualized by electrophoresis through 3.5% NuSieve 3:1 agarose gels (Lonza, Rockland, ME, USA) for products under 1,000 bp and through 1.5% SeaKem agarose gels (Lonza) for products over 1,000 bp in length. Gels were stained with ethidium bromide (0.5 μg/mL) and photographed under ultraviolet light. For sequence analysis, 1.5 μg of individual PCR amplimers were concentrated into 30 μl using QIAquick PCR Purification kits (Qiagen, Valencia, CA, USA). Purified amplimers were shipped to the Keck DNA Sequencing Lab at Yale University (New Haven, CT, USA) for primer-extension analysis. All datasets supporting the results of this article are included here in the five formal Figures and single Supplementary Figure. The nucleotide sequences described in this study are also accessioned in GenBank, NCBI (accession numbers KX555562 and KX555563).
The creation of a 374-member [(A/J x C3H/HeJ-jal/J)F1-jal/+ x C3H/HeJ-jal/J-jal/jal] backcross (N2) “mapping panel” that was used previously to locate jal on proximal Chr 2 was described in Ramirez et al. (Citation2013). DNA samples from recombinant panel members, which had been previously characterized for numerous microsatellite and single-nucleotide polymorphisms (SNPs) on proximal Chr 2 (see Ramirez et al., Citation2013) and stored at −80° C for retrospective analysis, were used here to locate the IAP element integrated with Gata3 with respect to jal and to these other marker sites.
3. Results
To detect an IAP element associated with the Gata3jal allele, gene-specific primer (GSP) pairs were designed that lay about 1,000 bp apart in the noncoding regions of Gata3. A GSP pair might fail to generate amplimers of the expected (wild type) length in PCRs using jal/jal template DNAs if a large IAP element (which range in size from 5.4 to 7.3 kb) were integrated between their annealing sites (Figure (a)). One such GSP pair (which directed amplification of wild type but not jal templates) was found (see Figure (b)) and these primers were tested individually with primers designed to anneal within the LTR region of the IAP element (based on the sequence published by Ishihara et al., Citation2004; GenBank D63767), designated here as ISP-R1, -F2, -R3, and -R4 (see Figure (c)). For example, the “failed” GSP in the forward orientation (GSP-F) might generate an amplimer with ISP-R1 if an IAP in the sense orientation was integrated downstream of the GSP, or with ISP-R3 if an IAP element in the antisense orientation was integrated downstream of the GSP. The “failed” GSP in the reverse orientation (GSP-R) might generate an amplimer with ISP-F2 if an IAP in the sense orientation was integrated upstream of that GSP, or with ISP-F4 if an IAP element in the antisense orientation was integrated upstream of the GSP. Figure (d) shows the results of PCRs that reveal the integration of an IAP element in the antisense orientation at a position about 700 bp downstream of GSP-F and about 250 bp upstream of GSP-R. The amplimers produced in these positive reactions were sequenced by primer-extension analysis, and the precise integration site of an IAP element in Intron 3–4 of the Gata3jal allele, designated Gata3IAP, was determined, as shown in Figure .
Figure 2. Scanning the Gata3jal allele for an integrated intracisternal A particle (IAP) element. (a) Annealing sites for a gene-specific primer (GSP) pair, one in the forward and one in the reverse orientation, are shown within an intron of the Gata3 gene (represented by a green line with the sense orientation indicated). In the top diagram, the GSP are close enough together to yield a PCR product of the wild-type length. In the bottom diagram, an IAP (represented by a thick blue line) has integrated between the primer-annealing sites, moving the GSP pair so far apart that no product is generated in the PCR. (b) One GSP pair, designated GSP-F and GSP-R (sequences given in Figure ), produced a 1,016 bp amplimer from wild-type DNA templates, but no products from jal/jal mutant DNA templates, as expected if an IAP integration occurred between the primer-annealing sites. (c) Here an intron with an IAP insertion is depicted in each of the two possible orientations, sense (top diagram) or antisense (bottom diagram), with respect to the Gata3 gene. If the IAP is integrated in the sense orientation, GSP-F should yield a PCR product with IAP-specific primer (ISP) -R1, and GSP-R should yield a product with ISP-F2. If the IAP is integrated in the antisense orientation, GSP-F should yield a PCR product with ISP-R3, and GSP-R should yield a product with ISP-F4. (d) GSP-F yielded a 959 bp product from jal templates with ISP-R3 but none with ISP-R1. GSP-R yielded a 486 bp product from jal templates with ISP-F4 but none with ISP-F2. Therefore, the IAP integrated in Intron 3–4 in the Gata3jal allele is in the antisense orientation with respect to the gene.
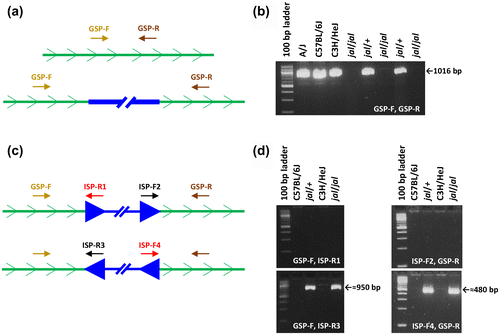
Figure 3. Nucleotide sequence at the site of IAP integration in Intron 3–4 of the mutant Gata3jal allele. Sequences in lower case are located within Gata3, Intron 3–4 (beginning with nucleotide number 2:9869836); nucleotides from Exon 4 are shown in blue, upper case letters (ending with nucleotide number 2:9868821). Sequences that correspond to the primers described in the text and in Figure as GSP-F and GSP-R are highlighted in yellow. The six-nucleotide direct repeat created in mouse genomic DNA by IAP element insertion (the target site duplication, TSD) is shown in red (and corresponds to nucleotides 2:9869167–9869162). The IAP element’s 5’ and 3’ LTRs, in antisense orientation, are shown in green; IAP sequences located between the LTRs are abbreviated by blue stars. Sequences that correspond to IAP-specific primers described in the text and in Figure as ISP-R3 and ISP-F4 are highlighted in blue and pink, respectively. Base-pair positions on mouse Chromosome 2 are from NCBI Build 37.2 (Ensembl Mouse Genome Server, Citation2016; Mouse Genome Database, Citation2016).

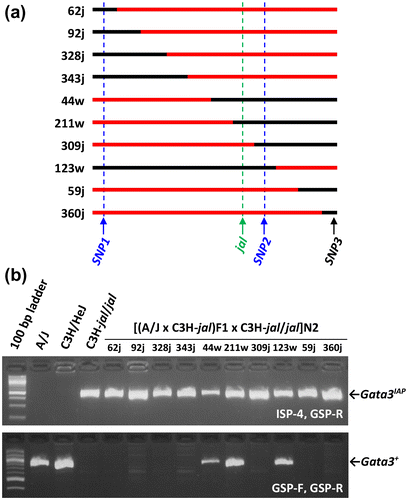
The characterization of a panel of 374 [(A/J × C3H/HeJ-jal/J)F1 × C3H/H3 J-jal/J] backcross offspring for the inheritance of jal and various molecular markers on proximal mouse Chromosome 2 established a location for the jal mutation between positions designated as SNP1 and SNP2 by Ramirez et al. (Citation2013), SNPs officially known as rs27112885 and rs27131573, respectively (CitationEnsembl Mouse Genome Server, XXXX). Specifically, 6 meiotic crossovers were identified in this 374-member panel that fell between SNP1 and jal, and 1 crossover was identified that fell between jal and SNP2 (summarized in Figure (a)). If the IAP element inserted in Intron 3–4 of Gata3jal is the mutational basis of the jal mutant phenotype, then this IAP element should map to the same site as jal, that is, Gata3IAP should co-segregate with jal in the 374-member backcross panel. As shown in Figure (b), the Gata3IAP genotype of each rare, recombinant panel member matches its jal genotype, suggesting that the Gata3IAP and the jal mutation are at least very closely linked (mapping less than 0.3 cM apart), if not identical.
Figure 4. Gata3IAP and jal are meiotically inseparable among a panel of 374 backcross mice. (a) Ramirez et al. (Citation2013) described the production of a panel of 374 backcross mice from a cross of (A/J x C3H/HeJ-jal/J)F1 females with C3H/HeJ-jal/J mutant males. These panel members segregated for jal and for various other markers on proximal Chromosome 2. Here, the most salient 10 members of that panel (i.e., those with crossovers nearest the jal mutation) are each represented by a line that depicts the region of Chr 2 between markers SNP1 (officially designated rs27112885) and SNP3 (a.k.a. rs27100936) that was inherited from their F1 parent. A/J-derived sequences are drawn in black, and C3H-derived sequences are drawn in red. Each recombinant is identified by its pedigree number, followed by a letter to indicate its phenotype as wild type (w) or mutant (j). Six crossovers (found in recombinants 62, 92, 328, 343, 44, and 211) lie telomeric to SNP1 but centromeric to jal; one crossover (found in recombinant 309) lies telomeric to jal but centromeric to SNP2 (a.k.a. rs27131571). Thus, jal must lie between SNP1 and SNP2, an interval that includes only one gene known to be expressed in skin, Gata3. Official SNP designations are from dbSNP Build 142 (Ensembl Mouse Genome Server, Citation2016; Mouse Genome Database, Citation2016). (b) Recombinant DNA samples from the panel described by Ramirez et al. (Citation2013) and controls were characterized for the presence of none, one, or two copies of Gata3IAP. All recombinants that were phenotypically mutant were homozygous for Gata3IAP, and the wild-type recombinants, heterozygous jal/+, were heterozygous for Gata3IAP/Gata3+. Co-segregation of Gata3IAP and jal in this backcross suggests that they are very close or identical.
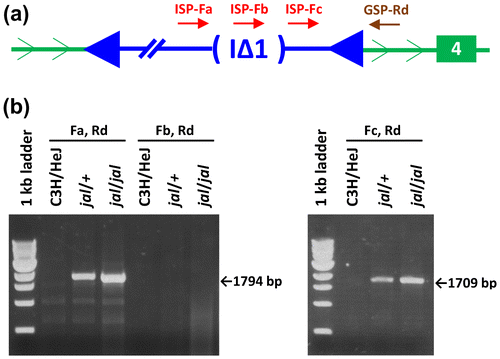
The I∆1 subtype of IAP elements is known to have a 1.9 kb deletion that extends from position 1708 in gag to position 3608 in pol (Kuff & Lueders, Citation1988). To determine if the IAP element integrated in the Gata3 gene in C3H/HeJ-jal/J mice might be of the I∆1 subtype (which is known to be most frequently associated with de novo insertional mutations in mice, Ishihara et al., Citation2004; Maksakova et al., Citation2006), a set of forward primers for PCR were designed that would anneal just before (ISP-Fa), within (ISP-Fb), and just after (ISP-Fc) this characteristic deletion in the IAP sequence. These primers were tested individually with a gene-specific reverse primer (GSP-Rd) that would anneal just downstream of the integration site of the IAP element in Intron 3–4 of Gata3jal (see Figure (a)). The presence and length of the PCR products obtained (see Figure (b)) were consistent with the Gata3IAP being of the I∆1 subtype, and primer-extension sequence analysis of the entire interval defined by the annealing sites for ISP-Fa and GSP-Rd in the Gata3jal allele (see Supplementary Figure S1) confirmed this classification.
Figure 5. Characterization of the IAP subtype associated with the Gata3jal insertion. (a) The relative location of the ISP-Fa, -Fb, -Fc and GSP-Rd annealing sites are shown. Note that each of these primer sequences is displayed in Supplementary Figure S1. (b) The ISP-Fa, GSP-Rd primer pair should produce a jal-specific amplimer only if the 1.9 kb gag-pol deletion characteristic of IAP elements of the I∆1 subtype should position primer-annealing sites for -Fa and -Rd near one another, similar to the ISP-Fc to GSP-Rd distance. The I∆1 deletion should remove the annealing site for ISP-Fb, so a deleted IAP template is not expected to produce an amplimer with the -Fb, -Rd pair. The -Fa and -Rd pair directed the amplification of a 1,794 bp product from jal templates, only 85 bp longer than the 1,709 bp jal-specific product yielded by the -Fc, -Rd primer pair, suggesting that the Gata3jal-associated IAP is of the I∆1 subtype. The full DNA sequence of the jal-specific, -Fa to -Rd amplimer (which includes the I∆1 deletion breakpoints in the Gata3jal-associated IAP insertion) is shown in Supplementary Figure S1.
4. Discussion
Although mice homozygous for germline null alleles of Gata3 die around embryonic day 11 (van Doorninck et al., Citation1999; Lim et al., Citation2000; Pandolfi et al., Citation1995), conditional ablation in the epidermis has revealed a role for Gata3 in hair follicle development and skin cell lineage determination (Kaufman et al., Citation2003; Kurek, Garinis, van Doorninck, van der Wees, & Grosveld, Citation2007). These null mutants, however, show a complete absence of hair, while mice homozygous for the spontaneous juvenile alopecia allele of Gata3, which show patchy hair loss, typically display at least some normally furred sectors. On this basis, we have previously suggested that Gata3jal likely encodes a normal primary protein sequence that is, by some stochastic mechanism, improperly regulated (Bisaillon et al., Citation2014; Ramirez et al., Citation2013).
While we have not attempted here to directly elucidate the mutagenic mechanism of the Gata3IAP insertion that we now propose to be the basis of the juvenile alopecia phenotype, several well-characterized examples of antisense integration of I∆1, IAP elements into the introns of other mouse genes (reviewed by Maksakova et al., Citation2006), suggest a few likely possibilities. For example, IAP insertions within introns can cause aberrant splicing or premature termination of the disrupted gene’s mRNA (Druker, Bruxner, Lehrbach, & Whitelaw, Citation2004; Gunn et al., Citation2001; Vasicek et al., Citation1997; Ware et al., Citation1997), but our previous investigation of Gata3 cDNA did not reveal any anomalous, jal-specific transcripts (Ramirez et al., Citation2013). Most commonly, antisense integrations of I∆1, IAP elements have been seen to cause ectopic gene expression from a promoter located in the 5’ LTR (Duhl, Vrieling, Miller, Wolff, & Barsh, Citation1994; Michaud et al., Citation1994; Vasicek et al., Citation1997). Many of these mutant alleles show variable expressivity among genetically identical mice (Druker et al., Citation2004; Michaud et al., Citation1994; Rakyan et al., Citation2003), as we have documented for Gata3jal (see Additional file 1 in Ramirez et al., Citation2013). Such variable expressivity has been shown to correlate with the methylation state of the 5’ LTR, which seems to be established somewhat stochastically (Druker et al., Citation2004; Michaud et al., Citation1994; Rakyan et al., Citation2003). Perhaps if the 5’LTR is mostly methylated, its antisense promoter will be inactive, and little effect on Gata3 expression in that skin patch leads to normal hair development and maintenance in that sector. However, if the LTR is unmethylated, its promoter may drive aberrant gene expression that results in follicular dysgenesis. For example, an active LTR promoter may cause ectopic expression of the functional domains of Gata3 protein that are encoded by Exons 4–6 in hair-follicle stem cells, leading to their terminal differentiation and loss in that skin patch. Alternatively, host silencing mechanisms (such as hypermethylation of DNA; Walsh, Chaillet, & Bestor, Citation1998) directed at the intron-integrated IAP element may interfere with the production of a normal Gata3 transcript, leading to alopecia in those skin sectors. In any case, the Gata3jal mutation appears to offer a valuable and convenient model for the study of a “metastable epiallele” (Rakyan, Blewitt, Druker, Preis, & Whitelaw, Citation2002), the expression of which appears to be regulated by a stochastic, epigenetic mechanism that can vary between genetically matched individuals or even between different skin patches on the same individual.
As in mice, a large (8%) portion of the human genome is of retroviral origin (International Human Genome Mapping Consortium (IHGMC), Citation2001). While the majority of human ERV’s represent ancient integrations and lack function due to accumulated mutations and deletions, recent work has identified a few nearly intact ERV integrations in humans (including at least one HERV-K provirus that may retain the potential for infectivity), and expression of such proviruses in tissues associated with cancer and autoimmune disease suggests at least the potential for pathogenic effects (Wildschutte et al., Citation2016). Although ERV insertions in humans are not an important source of new germline mutations (as they are known to be in mice), study of ERV-mediated biological effects in animal models like the juvenile alopecia mouse are still needed to better understand the regulatory effects of existing ERVs and LTRs on the many human genes they are integrated into or near (Jern & Coffin, Citation2008; van de Lagemaat, Landry, Mager, & Medstrand, Citation2003; Medstrand, van de Lagemaat, & Mager, Citation2002), to anticipate and control the potential side effects of retroviral vectors that might be used therapeutically (Dahl et al., Citation2015; Kagiava et al., Citation2016; Yi, Jong Noh, & Hee Lee, Citation2011), and to further explore epigenetic silencing of proviruses (mediated by RNA or drug action, for example)(Herrera-Carrillo & Berkhout, Citation2015; Lee et al., Citation2002; Tyagi & Karn, Citation2007) as an approach to finding a functional cure for retroviral infection.
5. Conclusions
We describe an intracisternal A-type particle element that has integrated into Intron 3–4 of the mouse Gata3 gene and is specifically associated with the Gata3jal mutation, which arose spontaneously on the standard inbred C3H/HeJ genetic background and was first described in 1999. This IAP element is in the antisense orientation with respect to the gene, is flanked by a six-base direct repeat, and is shown by sequence analysis to be a deleted element of the I∆1 subtype. The Gata3IAP element was mapped to the same small genetic interval as the coisogenic jal mutation itself, suggesting that this IAP insertion is the molecular basis of the juvenile alopecia mutant phenotype.
| Abbreviations | ||
| ERV | = | endogenous retrovirus |
| IAP | = | intracisternal A particle |
| PCR | = | polymerase chain reaction |
| LTR | = | long terminal repeat |
| SNP | = | single nucleotide polymorphism |
| GSP | = | gene-specific primer |
| ISP | = | IAP-specific primer |
| TSD | = | target site duplication |
Author’s contributions
MEC led all aspects of this study, including experimental design, data acquisition and interpretation. TRK conceived of the study, carried out all procedures involving mice, and drafted the manuscript. Both authors read, edited, and approved the final manuscript.
Supplementary Material
Supplementary material for this article can be accessed here at http://dx.doi.org/10.1080/23312025.2016.1264691.
Supplementary_material.pdf
Download PDF (402.8 KB)Acknowledgments
The authors thank Mary Mantzaris for excellent animal care.
Additional information
Funding
Notes on contributors
Thomas R. King
The research group led by Thomas R. King at Central Connecticut State University (New Britain, CT) aims to identify the genetic basis of spontaneous hair variants in mice and rats. Identification of such causative gene mutations can make probes available for the molecular analysis of both normal and disrupted development of the mammalian integument, and can help to identify human conditions these animal resources might model. Malcolm E. Connor is a graduate student in the Department of Biomolecular Sciences.
References
- Bisaillon, J. J., Radden II, L. A., Szabo, E. T., Hughes, S. R., Feliciano, A. M., Nesta, A. V., & ... King, T. R. (2014). The retarded hair growth (rhg) mutation in mice is an allele of ornithine aminotransferase (Oat). Molecular Genetics and Metabolism Reports, 1, 378–390.10.1016/j.ymgmr.2014.08.002
- Dahl, M., Doyle, A., Olsson, K., Månsson, J. E., Marques, A. R., Mirzaian, M., & ... Karlsson, S. (2015). Lentiviral gene therapy using cellular promoters cures Type 1 gaucher disease in mice. Molecular Therapy, 23, 835–844.10.1038/mt.2015.16
- Druker, R., Bruxner, T. J., Lehrbach, N. J., & Whitelaw, E. (2004). Complex patterns of transcription at the insertion site of a retrotransposon in the mouse. Nucleic Acids Research, 32, 5800–5808.10.1093/nar/gkh914
- Duhl, D. M., Vrieling, H., Miller, K. A., Wolff, G. L., & Barsh, G. S. (1994). Neomorphic agouti mutations in obese yellow mice. Nature Genetics, 8, 59–65.10.1038/ng0994-59
- Ensembl Mouse Genome Server. (2016). Mouse genome sequencing consortium: The European Bioinformatics Institute (EBI) and Welcome Trust Sanger Institute (WTSI). Release 84. Retrieved 2016, from http://www.ensembl.org
- Gunn, T. M., Inui, T., Kitada, K., Ito, S., Wakamatsu, K., He, L., … Barsh, G.S. (2001). Molecular and phenotypic analysis of Attractin mutant mice. Genetics, 158, 1683–1695.
- Herrera-Carrillo, E., & Berkhout, B. (2015). The impact of HIV-1 genetic diversity on the efficacy of a combinatorial RNAi-based gene therapy. Gene Therapy, 22, 485–495.10.1038/gt.2015.11
- International Human Genome Mapping Consortium. (2001). A physical map of the human genome. Nature, 409, 934–941.
- Ishihara, H., Tanaka, I., Wan, H., Nojima, K., & Yoshida, K. (2004). Retrotransposition of limited deletion type intracisternal A-particle elements in the myeloid leukemia cells of C3H/He mice. Journal of Radiation Research, 45, 25–32.10.1269/jrr.45.25
- Jern, P., & Coffin, J. M. (2008). Effects of retroviruses on host genome function. Annual Review of Genetics, 42, 709–732.10.1146/annurev.genet.42.110807.091501
- Kagiava, A., Sargiannidou, I., Theophilidis, G., Karaiskos, C., Richter, J., Bashiardes, S., … Kleopa, K. A. (2016). Intrathecal gene therapy rescues a model of demyelinating peripheral neuropathy. Proceedings of the National Academy of Sciences, 113, E2421–E2429.10.1073/pnas.1522202113
- Kaufman, C. K., Zhou, P., Pasolli, H. A., Rendl, M., Bolotin, D., Lim, K.-C., … Fuchs, E. (2003). GATA-3: An unexpected regulator of cell lineage determination in skin. Genes & Development, 17, 2108–2122.10.1101/gad.1115203
- Kuff, E. L., & Lueders, K. K. (1988). The intracisternal A-particle gene family: Structure and functional aspects. Advances in Cancer Research, 51, 183–276.10.1016/S0065-230X(08)60223-7
- Kurek, D., Garinis, G. A., van Doorninck, J. H., van der Wees, J., & Grosveld, F. G. (2007). Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development, 134, 261–272.10.1242/dev.02721
- Lee, N. S., Dohjima, T., Bauer, G., Li, H., Li, M.-J., Ehsani, A., … Rossi, J. (2002). Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nature Biochemistry, 19, 500–505.
- Lim, K. C., Lakshmanan, G., Crawford, S. E., Gu, Y., Grosveld, F., & Engel, J. D. (2000). Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature Genetics, 25, 209–212.
- Maksakova, I. A., Romanish, M. T., Gagnier, L., Dunn, C. A., van de Lagemaat, L. N., & Mager, D. L. (2006). Retroviral elements and their hosts: Insertional mutagenesis in the mouse germ line. PLoS Genetics, 2, e2.10.1371/journal.pgen.0020002
- Medstrand, P., van de Lagemaat, L. N., & Mager, D. L. (2002). Retroelement distributions in the human genome: Variations associated with age and proximity to genes. Genome Research, 12, 1483–1495.10.1101/gr.388902
- Michaud, E. J., van Vugt, M. J., Bultman, S. J., Sweet, H. O., Davisson, M. T., & Woychik, R. P. (1994). Differential expression of a new dominant agouti allele (Aiapy) is correlated with methylation state and is influenced by parental lineage. Genes & Development, 8, 1463–1472.10.1101/gad.8.12.1463
- Miets, J. A., Grossman, Z., Lueders, K. K., & Kuff, E. L. (1987). Nucleotide sequence of a complete mouse intracisternal A-particle genome: Relationship to known aspects of particle assembly and function. Journal of Virology, 61, 3020–3029.
- Mouse Genome Database. (2016). Mouse genome database group: The mouse genome Informatics website. The Jackson Laboratory, Bar Harbor, ME. Retrieved July 2016, from http://www.informatics.jax.org
- Mouse Genome Sequencing Consortium (2002). Initial sequencing and comparative analysis of the mouse genome. Nature, 420, 520–562.
- Pandolfi, P. P., Roth, M. E., Karis, A., Leonard, M. W., Dzierzak, E., Grosveld, F. G., … Lindenbaum, M. H. (1995). Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nature Genetics, 11, 40–44.10.1038/ng0995-40
- Rakyan, V. K., Blewitt, M. E., Druker, R., Preis, J. I., & Whitelaw, E. (2002). Metastable epialleles in mammals. Trends in Genetics, 18, 348–351.10.1016/S0168-9525(02)02709-9
- Rakyan, V. K., Chong, S., Champ, M. E., Cuthbert, P. C., Morgan, H. D., Luu, K. V., & Whitelaw, E. (2003). Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proceedings of the National Academy of Sciences, 100, 2538–2543.10.1073/pnas.0436776100
- Ramirez, F., Feliciano, A. M., Adkins, E. B., Child, K. M., Radden II, L. A., Salas, A., … King, T. R. (2013). The juvenile alopecia mutation (jal) maps to mouse Chromosome 2, and is an allele of GATA binding protein 3 (Gata3). BMC Genetics, 14, 40.10.1186/1471-2156-14-40
- Tyagi, M., & Karn, J. (2007). CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. The EMBO Journal, 26, 4985–4995.10.1038/sj.emboj.7601928
- van de Lagemaat, L. N., Landry, J. R., Mager, D. L., & Medstrand, P. (2003). Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends in Genetics, 19, 530–536.10.1016/j.tig.2003.08.004
- van Doorninck, J.H., van der Wees, J., Karis, A., Goedknegt, E., Engel, J.D., Coesmans, M., … De Zeeuw, CI. (1999). GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. Journal of Neuroscience, 19, 1–8.
- Vasicek, T. J., Zeng, L., Guan, X.-J., Zhang, T., Costantini, F., & Tilghman, S. M. (1997). Two dominant mutations in the mouse Fused gene are the result of transposon insertions. Genetics, 147, 777–786.
- Walsh, C. P., Chaillet, J. R., & Bestor, T. H. (1998). Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nature Genetics, 20, 116–117.10.1038/2413
- Ware, M. L., Fox, J. W., González, J. L., Davis, N. M., Lambert de Rouvroit, C. L., Russo, C. J., … Walsh, C. A. (1997). Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron, 19, 239–249.10.1016/S0896-6273(00)80936-8
- Wildschutte, J. H., Williams, Z. H., Montesion, M., Subramanian, R. P., Kidd, J. M., & Coffin, J. M. (2016). Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proceedings of the National Academy of Sciences, 113, E2326–E2334.10.1073/pnas.1602336113
- Yi, Y., Jong Noh, M., & Hee Lee, K. (2011). Current advances in retroviral gene therapy. Current Gene Therapy, 11, 218–228.10.2174/156652311795684740

