Abstract
Introduction: Alcohol withdraw syndrome (AWS) and delirium tremens (DTs) are part of the clinical picture of alcohol dependence (AD). The mechanisms by which these conditions develop are not entirely understood, but there are data suggesting that several factors are largely responsible for the emergence of the AWS and DTs. According to some authors, with the prolongation of the AD and increasing tolerance, the AWS aggravates, somatic and neurovegetative manifestations worsen. Russian authors accept that the degree of clinical manifestations depends on the individual characteristics of the response to ethanol. Materials and methods: The present work is a systematic study of 28 alcohol-dependent patients that developed clinical signs and symptoms of pre-delirium and delirium during alcohol detoxification treatment. Results: The relationship between sex, age, type and duration for abuse, type of alcoholic beverage, and the clinical picture has been analyzed and discussed as well as the criteria for their diagnosis and follow-up. Conclusion: Our analysis of the development and the manifestations of pre-delirium and DTs, during detoxification of AD patients, underlines the protracted course of the illness and the need for multidisciplinary intensive care. Dynamic monitoring of the patients and timely correction of the therapeutic program guarantee the success of the detoxification.
Public Interest Statement
Alcohol withdraw is a serious clinical syndrome and also a part of the clinical picture of Alcohol use disorder. As alcohol use is widely spread and socially acceptable in most countries around the world, it is important for health care professionals to recognize its consequences and be able to provide adequate management of such patients based on reliable assessments of its severity. In our article, the relationship between sex, age, type and duration of use, type of alcoholic beverage, and the clinical picture has been analyzed and discussed.
Competing Interests
The authors have no competing interests.
1. Introduction
Alcohol withdraw syndrome (AWS) and delirium tremens (DTs) are part of the clinical picture of alcohol dependence (AD)—condition due to a number of pathological processes in the central nervous system (CNS) determined by the abuse of ethanol and its specific metabolism. The fast metabolization of ethanol determines the build-up of acetaldehyde in the organism (Gerevich, Citation2007). In addition to the direct toxic effects on neurons, which is functionally metabolic, active metabolites from its degradation lead to morphologically destructive changes, turning the process into exo- and endo-intoxication. The mechanisms by which these conditions develop are not entirely understood, but there are data suggesting that several factors are largely responsible for the emergence of the AWS and DTs (Gerevich, Citation2007).
1.1. Impact on the receptors in the central nervous system
The ethanol attacks the lipid component built mainly from phospholipids and covering the main protein ingredient of GABA-receptors. A disharmony occurs in the permeability of the cell membrane, the stimulation of secondary messengers and impairments in central brain neurotransmission and the hormonal secretion as well (Tatebayashi, Motomura, & Narahashi, Citation1998).
The blocking of vasopresin leads to general dehydratation effect as well as in the cells of the brain and on the cells of the stroma (Mander et al., Citation1985).
Acetaldehyde (a metabolite that is much more aggressive than ethanol) plays a very important role in the emergence of the AWS and DTs, mainly through its propensity to condensation with neurotransmitters in the CNS (Koob & Le Moal, Citation2006; Licata & Renshaw, Citation2010).
In Table are shown the neurotransmitter systems (NM) which are affected by the acute and chronic (systemic) action of ethanol (Koob & Le Moal, Citation2006; Licata & Renshaw, Citation2010).
Table 1. Change in the activity of the affected neurotransmitter systems by ethanol.
The oxidation of ethanol, apart from the synthesis of acetaldehyde, is a process leading to the intracellular accumulation of NADH 2 and NADPH 2 and is a prerequisite for a number of pathological syntheses which start intra-cellulary (Thurman, Glassman, Handler, & Forman, Citation1989).
Energy source of these processes (syntheses) comes from the high caloricity of alcoholic beverages—the separation of large quantity of hydrogen as well as from the opportunity to be used in the cycle of the Krebs as products of ketogenesis (Khanna & Israel, Citation1980).
1.2. Interaction of acetaldehyde with neurotransmitters in the CNS
To begin with, acetaldehyde condenses with dopamine (DA) and a condensate is formed—salsalinol, which has hallucinogenic properties. Apart from that during his amine oxidation, tetra-hydro-isoquinolines (THIQ) are formed. The latter are extremely stable compounds, practically they do not degrade, but accumulate in the CNS and play an important role in the occurrence of withdraw reactions (Naoi, Maruyama, & Nagy, Citation2004).
The harmaline is degraded extremely slow, it stimulates the release of DA in the CNS; it is a cholinesterase inhibitor and also blocks MAO-inhibitors. Decreased synthesis of melatonin and is a powerful indole stimulator in the CNS. It determines the agitation and hallucinatory symptoms (Collins, Citation1988).
Of course, other contributing factors for the development of the AWS and DTs cannot be ignored, such as amyl alcohol—the result of the degradation of the carbon skeleton of the branched chain amino acids. It can be found in almost all alcoholic beverages (Westerhoff, Groen, & Wanders, Citation1984).
There are also many other factors, coming mainly from impairments of the hepatocyte metabolism: reduced synthesis of aldehyde-dehydrogenase; high homocysteine levels; change in acid–base balance, leaning toward acidosis; dehydration of body; general enzyme deficiency, as well as hypovitaminosis of water soluble vitamins and the insufficiency of zinc (Zn) (Seeto, Fenn, & Rockey, Citation2000).
There are two phases in the course of the AWS—in the beginning, after an interruption of the alcoholic use, symptoms (not at the same time) amplify and reach a maximum after which they gradually (not at the same time) decrease. The tremor, asteno-adynamic syndrome, dyspeptic nad cardio-vascular symptoms develop during the first 6–12 h after the last alcohol consumption, hallucinations and seizures—after 24–28 h and DTs—3–5 days after the last drink (Bayard, McIntyre, Hill, & Woodside, Citation2004).
According to some authors, with the prolongation of the alcohol dependence and the increasing of the tolerance, the AWS aggravates, and the somatic and neurovegetative manifestations worsen (Entin, Citation1990). Russian authors accept that the degree of clinical manifestations depends on the individual characteristics of the response to ethanol (Ivanetz & Igonin, Citation1987).
There are authors who claim that the severity of AWS is not age-dependent (Wetterling, Citation2001). Delirium tremens is the worst expression of AWS and the most frequent type of alcohol- induced disorder of cognition. It develops in 5–15% of alcoholic dependent patients. It is observed when people abusing mainly with concentrated drinks, more often after 30 years of age (Lofwall, Schuster, & Strain, Citation2008).
Delirium can be observed during a long intoxication and in the course of withdraw (American Psychiatric Association, Citation2000; Esel, Citation2006). People with alcohol dependence rarely stop drinking abruptly. Frequently in the presence of the level of alcohol in the blood, the first symptoms of AWS emerge along with symptoms of alcohol intoxication (Elholm, Larsen, Hornnes, Zierau, & Becker, Citation2010). Clinical practice indicates that the objective assessment of the existence and the severity of the abstinence are not uncommonly hampered by a number of nonmedical factors. Patient anxiety, low insight, craving for alcohol, social stigma , pressure from relatives for aggressive treatment or no treatment at all are common factors that distort the course of gathering accurate information about the condition and the real alcohol consumption of the patients. For this reason, the standardized assessment with specially developed scales has a number of advantages.
Few studies have validated scales for the evaluation of the AWS (Elholm, Larsen, Hornnes, Zierau, & Becker, Citation2011). At this stage, there is no consensus on the standardized monitoring of AWS and DTs as well (Seeto et al., Citation2000). The most widely used and the most well studied in scale for the evaluation of the AWS is Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar) (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, Citation1989). It contains 10 items with a weight of 0–7 by which a score is calculated, that gives the clinician an idea of the severity of the AWS. One of its advantages is that there are different protocols for guidance of the treatment plan (especially the dosage of benzodiazepines) based on the score of the scale even in multiple patient assessments (Ng, Dahri, Chow, & Legal, Citation2011).
When a disorder of consciousness is suspected there are a number of standardized clinical tools for its evaluation and for the verification of the presence of a delirium (irrespective of the etiology). Such are the Richmond Agitation-Sedation Scale; Delirium Triage Screen (DTS); brief Confusion Assessment Method; Confusion Assessment Method is (CAM); Confusion Assessment Method is for the ICU (CAM-ICU).
2. Aim
The current study analyses of the various factors—sex, type and duration of alcohol use, type of drink (high concentration and/or low concentration of alcohol), in order to predict the dynamics and severity of the alcoholic delirium in the period of detoxification.
3. Materials and methods
Target group are patients in pre-delirium or with the clinical picture of DTs. All have gone through detoxification in clinic “Emergency toxicology” of the MHAT-Sofia, MMA between the years 2009 and 2012. The mean age is 43, 50 ± 13, 74 years, in the range between 19 and 69 years.
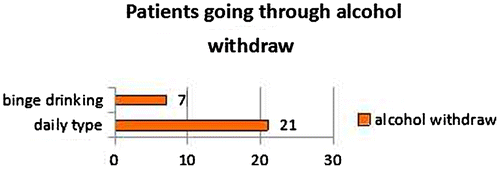
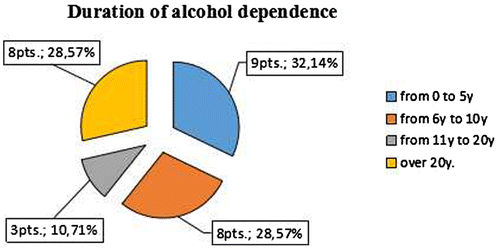
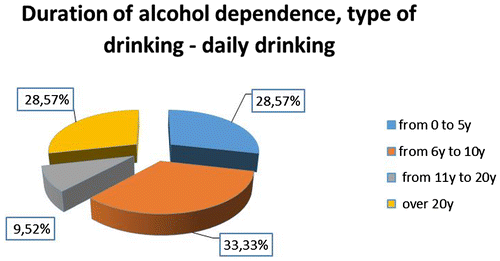
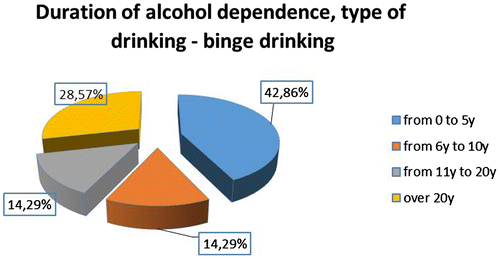
The distribution by sex is—3 women and 25 men and according to the type of alcohol use. Seven of all patients had binge type of drinking and 21—daily type, as described in Figure . The duration of alcohol dependence among patients and the distribution according to the type of drinking is described in Figures –.
The methods used are: documentary, clinical, statistical—all analyzes have been carried out with the program IBM SPSS Statistics 22.0. In cases in which subgroups with a smaller number of persons (<8) have been analyzed, non-parametrical statistical tests were applied.
4. Results
The main criterion for the selection of patients is the presence of alcohol withdraw—pre-delirium or delirium (DTs). Their development usually is preceded by a long-term alcohol consumption—“daily” or “binge drinking” type. The diagnosis is made by the consultant—psychiatrist.
In the target group of patients with AD, we have observed withdraw symptoms more or less pronounced, of pre-delirium or delirium tremens, after the termination or reduction of alcohol consumption. With respect to differential diagnosis hypoglycemia was ruled-out as possible etiology.
Predominantly affected were patients in the age range of 30–39 years (32%), which raises the hypothesis that alcohol use has started at a young age.
The long-term information for the systematic use of alcohol is the attribute “duration”. According to it predilection (33%) have patients with abuse of between 6 and 10 years of “Daily” beer drinkers and the most small is of these between 11 and 20 years old (around 10%). In the subgroup with “binge drinking” type—most of the patients have duration of drinking between 0 and 5 years (almost 43%).
The distribution by sex is 8:1 men toward women. The three female patients have exhibited pre-delirious symptoms.
5. Discussion
The syndrome of alcoholic withdraw with pre-delirium in AD patients is characterized by polymorphic clinical picture. It occurs most frequently between the 10th and 30th hour, but may also start after 40–50 h after the last alcoholic drink. The clinical manifestations observed by our team were: Hyperactivity, fear affect or depressivity, slightly increased temperature, tachycardia, increased diaphoresis, nausea with or without vomiting (in 25 of the patients—89.28%). A remarkable feature of neurological symptoms in patients in pre- delirious state is the absence or very low intensity of the tremor of the upper extremities. Three patients (10.72%) were with a heavy expressed form of AWS—delirium tremens. In their case, in addition to the above stated symptoms combativeness, desorientation, visual and auditory hallucinations were also observed. The psychosis in patients with DTs passed with formally preserved autopsychic (for own personality), but with impaired alopsychichic (for time and place) orientation. The average duration was 15 days.
The structure of the psychopathologic disorders during detoxification varied in relation to the level of anxiety and depressive disorders.
At the time of the study, the patients had laboratory tests done for the assessment of liver pathology. In 75% of them there was an increase in the serum aminotransferase levels, with the predominance of the ratio ASAT > ALAT—indicator of impaired liver function. The levels of gamma glutamyl-transpeptidase (GGT–cholestatic enzyme)—are with increased average value several times above the upper limit of normal. Increase has been noted also in the average values of total cholesterol, TG and LDL-cholesterol.
Patients often complain of withdraw symptoms, but there are no objective evidence of this. This is why close monitoring (hourly in severe cases) for signs of pre-delirium or DTs through observation, BP and pulse, and by applying scales such as CIWA-Ar, DTS and bCAM is of great importance.
This gives the following advantages: individualized medical intervention in accordance with changes in the severity of the AWS (symptom-dependent); facilitates the selection of proper medication regimen and reduces the risk of somatic and neurological complications; shortens the period of detoxification; economic effect—reduces unnecessary treatment costs.
6. Implications
| (1) | All patients with AD develop withdraw symptoms. AWC is often short lasting, but quickly transforms in pre-delirious or delirious state. In the pre-crisis period somatic manifestations are discreet—a slight tremor, tendency toward tachycardia, small increase in BP, sweating. Careful monitoring and working in liaison with the psychiatric department unit are essential for recognizing those early signs. Beginning treatment in a timely manner often prevents the occurrence of delirium. | ||||||||||||||||||||||||||||
| (2) | Number of factors are important for the development of alcohol delirium:
| ||||||||||||||||||||||||||||
| (3) | According to various literature sources pre-delirium or DTs occur in individuals abusing mainly concentrated alcoholic drinks and more frequently after age 30. Our research shows that most often delirium occurs in patients abusing concentrated alcoholic drinks or concentrated along with “light” alcoholic beverages (96.42%). | ||||||||||||||||||||||||||||
| (4) | The intensity of autonomic disorders correlates with the severity of anxiety and depressive disorders. Patients with psychotic symptoms during the AWS, rarely develop depressive symptoms. The severity of psychopathologic signs is determined by a number of factors (including the characteristics of the AD; severity of AWS). | ||||||||||||||||||||||||||||
| (5) | Of great importance for the duration of delirious states is the degree of damage to the liver functioning. | ||||||||||||||||||||||||||||
| (6) | Quick and competent correction of the dysfunction of the liver often significantly reduces the duration and intensity of delirious events. | ||||||||||||||||||||||||||||
| (7) | Standardized assessments i.e. the use of approbated clinical scales for the diagnosis and monitoring of patients going through alcohol withdraw and delirium could be beneficial for the guidance of early interventions in the course of the detoxification and a symptom triggered approach of treatment. | ||||||||||||||||||||||||||||
7. Conclusion
Our analysis of the development and the manifestations of pre-delirium and DTs, during detoxification of AD patients, underlines the protracted course of the illness and the need for multidisciplinary intensive care. Dynamic monitoring of the patients and timely correction of therapeutic program are a guarantee for the success of the detoxification.
Additional information
Funding
Notes on contributors
Krasimir Kostadinov
The Military Medical Academy (MMA)—Sofia is a large complex for medical treatment, as well as education, located in Sofia, Bulgaria. It has several branches and smaller clinics in other cities in the country. The current investigation was carried out by the Department of Emergency Toxicology of The MMA—Sofia, led by Lyudmila Neykova-Vasileva and in close collaboration with Tony Donchev and Krasimir Kostadinov of the Department of Psychiatry, MMA-Sofia. Our team, as members of the both clinics, is dedicated to providing quality care for patients with substance abuse disorders both in acute and chronic settings.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision (DSM-IV-TR). doi:10.1176/appi.books.9780890423349
- Bayard, M., McIntyre, J., Hill, K. R., & Woodside, Jr., J. (2004). Alcohol withdrawal syndrome. American Family Physician, 69, 1443–1450.
- Collins, M. A. (1988). Acetaldehyde and its condensation products as markers in alcoholism. Recent Developments in Alcoholism, 6, 387–403.
- Elholm, B., Larsen, K., Hornnes, N., Zierau, F., & Becker, U. (2010). A psychometric validation of the short alcohol withdrawal scale (SAWS). Alcohol and Alcoholism, 45, 361–365. doi:10.1093/alcalc/agq033
- Elholm, B., Larsen, K., Hornnes, N., Zierau, F., & Becker, U. (2011). Alcohol withdrawal syndrome: symptom-triggered versus fixed-schedule treatment in an outpatient setting. Alcohol And Alcoholism, 46, 318–323. doi:10.1093/alcalc/agr020
- Entin, M. (1990). Treamtent of acloholism (pp. 28–31). Moscow: Moscow Medicine.
- Esel, E. (2006). Neurobiology of alcohol withdrawal inhibitory and excitatory neurotransmitters. Turk Psikiyatri Derg, 17, 129–137.
- Gerevich, J. (2007). Drug and alcohol abuse: A clinical guide to diagnosis and treatment. Journal of Epidemiology and Community Health, 61, 173–174. Retrieved from http://doi.org/10.1136/jech.2006.04770410.1136/jech.2006.047704
- Ivanetz, N. N., & Igonin, A. L. (1987). Withdraw syndrome in alcoholism (a physician manual) (pp. 89–97). Moscow: Moscow Medicine.
- Khanna, J. M., & Israel, Y. (1980). Ethanol metabolism. International Review of Physiology, 21, 275–315.
- Koob, G. F., & Le Moal, M. (2006). Neurobiology of Addiction. London: Academic Press.
- Licata, S. C., & Renshaw, P. F. (2010). Neurochemistry of drug action. Annals of the New York Academy of Sciences, 1187, 148–171.10.1111/j.1749-6632.2009.05143.x
- Lofwall, M. R., Schuster, A., & Strain, E. C. (2008). Changing profile of abused substances by older persons entering treatment. The Journal of Nervous and Mental Disease, 196, 898–905. doi:10.1097/NMD.0b013e31818ec7ee
- Mander, A. J., Smith, M. A., Kean, D. M., Chick, J., Douglas, R. H. B., Rehman, A. U., Best, J. J. K. (1985). Brain water measured in volunteers after alcohol and vasopressin. The Lancet, 326, 1075. Retrieved from http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(85)90950-X/abstract10.1016/S0140-6736(85)90950-X
- Naoi, M., Maruyama, W., & Nagy, G. M. (2004, Janauary). Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology, 25, 193–204.
- Ng, K., Dahri, K., Chow, I., & Legal, M. (2011). Evaluation of an alcohol withdrawal protocol and a preprinted order set at a tertiary care hospital. The Canadian Journal of Hospital Pharmacy, 64, 436–445.
- Seeto, R. K., Fenn, B., & Rockey, D. C. (2000). Ischemic hepatitis: clinical presentation and pathogenesis. The American Journal of Medicine, 109, 109–113.10.1016/S0002-9343(00)00461-7
- Sullivan, J., Sykora, K., Schneiderman, J., Naranjo, C., & Sellers, E. (1989). Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Addiction, 84, 1353–1357.10.1111/add.1989.84.issue-11
- Tatebayashi, H., Motomura, H., & Narahashi, T. (1998). Alcohol modulation of single GABAA receptor-channel kinetics. NeuroReport, 9, 1769–1775.10.1097/00001756-199806010-00018
- Thurman, R. G., Glassman, E. B., Handler, J. A., & Forman, D. T. (1989). The swift increase in alcohol metabolism (SIAM): A commentary on the regulation of alcohol metabolism in mammals. In K. E. Crow & R. D. Batt (Eds.), Human metabolism of alcohol (Vol. 2, pp. 17–30). Boca Raton, FL: CRC Press.
- Westerhoff, H., Groen, A., & Wanders, R. (1984). Modern theories of metabolic control and their applications. Bioscience Reports, 4, 1–22.10.1007/BF01120819
- Wetterling, T. (2001). The severity of alcohol withdrawal is not age dependent. Alcohol And Alcoholism, 36, 75–78. doi:10.1093/alcalc/36.1.75