Abstract
This study aims to investigate the hepatoprotective activity of Ganoderma lucidium mushroom growing on the dead ironwood tree in the Central Highlands of Vietnam. The total extract was orally administrated to experimental mice with hepatotoxicity induced by cyclophosphamide at the dose of 150 mg/kg (intraperitoneal injection). The liver malondialdehyde (MDA) content and the level of endogenous antioxidant glutathione (GSH) were measured. The results revealed that the total extract at the oral doses of 330, 230, and 120 mg/kg body weight which were equivalent to 5, 10, and 15 g/kg of dry material alleviated the increase of heaptic MDA content and restored the decrease of GSH level significantly which almost have the same potent as reference drug (silymarin). Biochemical observations were also supported with histopathological examination of liver. This finding demonstrated that G. lucidium in Vietnam could represent a promising approach for effective liver protective agents.
Public Interest Statement
Medicinal mushrooms have a long history of ultilization in traditional therapy; and fungal metabolite are increasingly operated not to be considered as food, some of them could have biological properties including antioxidant, antitumor, and hepatoprotective activity. In Vietnam, Ganoderma lucidium has been found growing on the dead ironwood tree in the Central of Highlands. It is boardly presented to a commercial scale or harvested in nature and commonly purchased for its medicinal values. This study has provided the scientific evidence to validate the usage of this mushroom in folk medicine as the treatment of liver disorders and the prevention of hepatic injury.
Competing Interests
The authors declare no competing interest
1. Introduction
Asian countries have a long convention of handling mushrooms as medicine, whereas in Western this has been used since last decade (Lindequist, Niedermeyer, & Julich, Citation2005). Mushrooms have been known to be a potential source of antioxidants as well as hepatoprotective agents and capable of strong inhibition of lipid peroxidation (Acharya, Chatterjee, & Ghosh, Citation2011; Acharya, Yonzone, Rai, & Acharya, Citation2005; Pal, Ganguly, Tahsin, & Acharya, Citation2000). Ganoderma lucidium (reishi mushroom, Ling Zhi) has been an economically crucial species, particularly in the Far East countries. It is broadly grown on a commercial scale or harvested in nature and commonly purchased for its medicinal and spiritual properties. In a broad review about the hepatoprotective property of G. lucidium, Gao et al. (Citation2003) collected evidence to suggest possible molecular mechanisms to explain its hepatoprotective action.
This annual mushroom grows on a great variety of dead or dying tree, e.g. deciduous trees, especially oak, maple, elm, willow, sweet gum, magnolia, and locust. G. lucidium grows originally on plum trees and found on stumps near the soil surface (Wasser, Citation2005). It had been found growing in the dead ironwood tree in the Central of Highlands of Vietnam. Due to the growth of this mushroom in the green ironwood tree, it was a so-called “Green Ironwood mushroom.” Local people harvested the fruiting body of G. lucidium and then dried under shade. It has been often sold to patients suffering cancer or even disorders such as cirrhosis. G. lucidium is also believed that the regular consumption of this mushroom may preserve human vitality and to promote the longevity. In general, the mushroom has been traditionally used for the prevention or treatment of numerous diseases related to liver although whether it was effective or not is presumably unknown. The aim of this study is to validate the use of this mushroom in folk medicine by evaluating the hepatoprotective activity of G. lucidium which grows on the dead ironwood tree against the cyclophosphamide (CP)-induced liver toxicity. The lipid peroxidation and antioxidant parameters MDA and GSH were observed in liver homogenates and histopathological examination of liver sections was conducted to confirm the activity. The potent hepatoprotective effect of natural material exhibit an appealing advance for liver protective agents, especially at the moment there is an urgent need concerning on new innovative drugs.
2. Materials and methods
2.1. Materials
The fruiting body of G. lucidium was harvested from Central Highlands of Vietnam in August 2014. The mushroom was identified by Associate Prof. Dr Tran Van Minh-Institute of Tropical Biology, Vietnam. A voucher specimen was deposited in the herbarium of Applied Biochemistry Laboratory, Department of Applied Chemistry, School of Biotechnology, International University, Vietnam National University Ho Chi Minh City, Vietnam with voucher No. HB-B10–08-09–14.
2.2. Chemicals
Methanol, KCl, EDTA–phosphate, and thiobarnituric acid were purchased from Chemsol Co., Ltd. (Viet Nam). CP, malondialdehyde, glutathione, ellman or 5,5′-dithiobis-(2-nitrobenzoic acid), and silymarin were supplied from Sigma Co., Ltd. (USA).
2.3. Animals
Adult (5–6 weeks) Swiss albino male mice (23 ± 2 g) were obtained from Pasteur Institute of Ho Chi Minh city and kept in animals’ house of the university animal facility. All mice were acclimated for a week prior to commencement of experiments. They were provided standard pellet diet, water ad libitum and maintained a controlled 12 light–dark cycles at room temperature. All the ethical protocols and guideline for experimental animal handling and treatment were strictly followed the guidelines enunciated in the “Guideline for the Treatment of Animals in Behavioral Research and Teaching” and complied with ethical measures for animal research.
2.4. Preparation of mushroom extract
The fruiting body of G. lucidium was first rinsed thoroughly with tap water, and subjected to dry heat treatment before grinding it into powdery form using an electric grinder. The extraction was done by cold maceration of 2 kg in 96% of ethanol and kept aside for 3 days with frequent agitation. The filtrate obtained was concentrated using rotary evaporator. The residue subsequently referred to as the extract, was stored in a refrigerator until required for further use.
2.5. Cyclophosphamide-induced hepatotoxicity and treatment
The animal treatment was designed to study the hepatoprotective effect detailed in Table
Table 1. Experimental treatment design
After 8-day experimental period, the animals were sacrificed and liver tissues were immediately excised and rinsed in ice-cold saline then homogenized for the subsequent assays
2.6. Assessment of liver function
Livers were resected and then homogenized in KCl (1, 15%) in 1 min at 13,000 rpm. 2 mL of tissue homogenized was added to 1 mL of Tris (pH 7.4). The mixture was incubated at 37°C for 60 min, then stopped the reaction by 1 mL of 10% trichloroacetic acid. The next step was the centrifugation of the mixture at 5°C with 10,000 rpm/min for 10 min.
2.7. Estimation of malondialdehyde (MDA) level
Two milliliters of supernatant reacted with 1 mL of 0.8% thiobarbituric acid (TBA) at 100°C for 15 min and the absorbent were measured at λ = 532 nm. 2-Thiobarbituric acid reactive substance (TBARS) assay values are usually reported in MDA equivalents, a compound that results from the decomposition of polyunsaturated fatty acid lipid peroxide. The amount of MDA formed was quantified by reaction with TBA. It was formed according to the methods of Stroev and Makarova (Citation1989) and Abraham and Sugumar (Citation2008) with few modifications (Ngo & Nguyen, Citation2011; Nonanka et al., Citation2005).
2.8. Estimation of glutathione (GSH) level
About 1 mL of supernatant reacted with 0, 2 mL of Ellman 5,5′-dithiobis (2-nitrobenzoic acid) and 0, 8 mL of phosphate–EDTA. After 3 min, the absorbent was determined at λ = 412 nm. The method is based on the reduction of 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) with reduced GSH to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 412 nm. Noeman, Hamooda, and Baalash (Citation2011) with some modifications (Ngo & Nguyen, Citation2011).
2.9. Histopathological examination
The livers were excised and soaked by optimal cutting temperature O.C.T compound. The samples were then freezed and fit into Tissue-Tek Cryo 3 (Sakura, USA). They were cut into slides by MX35 Premier and Microtome Blade (Thermo Scientific, Japan) before being melt by block heater (Stuart, United Kingdom). Slides were preserved in 4% paraformaldehyde, stained with hematoxylin and eosin. The samples were then observed under Leica microscope for histopathological study.
2.10. Statistical analysis
All data were presented as the mean ± Standard Error of Mean (SEM). Mean values were assessed for significance by Student’s t-test at p < 0.05. One-way ANOVA and Dunnett’s method were used for multiple comparisions of data. Statistical analysis was processed using the SigmaStat, version 3.5. Correlation between variables was evaluated using Pearson’s correlation coefficient with level of significance p < 0.05.
3. Results
3.1. Liver parameters
The hepatoprotective effects of G. lucidium on CP-induced hepatic injury and normal mice are shown in Table .
Table 2. The effect of G. lucidium total extract on MDA content in cyclophosphamide-untreated and -treated mice
As can be seen in Table , the MDA content in hepatic tissues of CP (+) mice in negative control group was found to be significantly (p < 0.05) increased (110.91 ± 4.46 nM/g protein) as compared with CP (−) normal control (58.82 ± 4.06 nM/g protein), reflecting the hepatic damage induced by CP. The MDA level in hepatic tissues in mice of CP (+) and G. lucidium co-treated group at the dose of 330, 230, and 120 mg/kg b.wt were figured out almost the same (61.17 ± 7.71, 59.46 ± 5.22, 61.52 ± 5.31 nM/g protein, respectively) and showed a statistically significant decrease of MDA content as compared with the CP (+) negative control, (p < 0.05). Meanwhile, silymarin served as positive control also produced a decrease of MDA in liver tissue significantly (63.54 ± 5.09 nM/g protein).
As can be observed from Table , GSH level in liver of total extract treatment were significantly recorded high (p < 0.05) with respect to CP (−) normal group, proving the effect of G. lucidium extract in enhancing the GSH content. However, GSH after a single dose of CP 150 mg/kg b.wt decreased significantly (p < 0.05) when compared with CP (−) mice normal control group. Statistically, 50% depletion of hepatic GSH in CP-induced mice was observed.
Table 3. The effect of treatment with the G.lucidium total extract on GSH content in cyclophosphamide-treated and normal mice for 8 days
Data in Table also showed a significant increase of GSH level in the mice with CY (+) and G. lucidium total extract co-treated group after 8-day treatment. However, mice treated with the dose of 230 mg/kg b.wt made the highest GSH level in liver (7,063.59 ± 402.09 nM/g protein) among the tested samples, followed by the dose of 120 mg/kg b.wt (6,114.42 ± 443.58 nM/g protein), and 330 mg/kg b.wt (5,803.09 ± 416.35 nM/g protein). Meanwhile, silymarin at the dose of 100 mg/kg b.wt used as a reference drug also produced a significant increase of GSH (5,935.06 ± 354.05 (nM/g protein).
3.2. Histopathological examiation
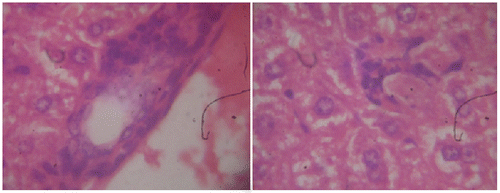
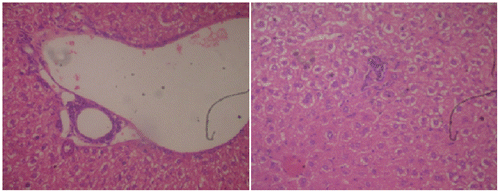
Histopathological observations of normal mice group showed normal hepatocyte with polygonal shape and no lobular inflammation. The central vein did not contain lymphocytes and none of necrosis of liver cells (Figure ). Liver sections of CP (150 mg/kg b.wt) treated group expressed the portal tracts with moderate to marked inflammation. Hepatocellular necrosis appeared lobular inflammation with lyphocytic infiltration. Liver of mice in this group showed a severe active hepatitis (Figure (a) and (b)). Meanwhile, liver of mice from CP and G. lucidium total extract co-treated group (120 mg/kg b.wt) showed a mild active hepatitis. Portal space presented slight to moderate inflammation, no necrotic liver cells and small focal lobular inflammation (Figure (a) and (b)). Liver sections of silymarin treatment group were manifested in Figure . The central vein contained an adhesion of the lymphocytes on the endothelial cell, moderate portal, and lobular inflammation. No necrotic liver cell occurred, the liver were exposed to be a moderate active hepatitis.
Figure 1. Normal group, section of mouse liver showing no lobular inflammation, none of necrosis and fibrosis. No abnormality of central vein and hepatocytes.
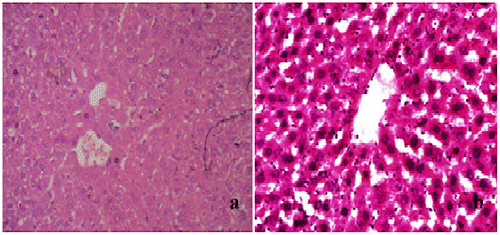
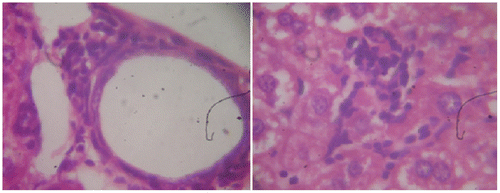
Figure 2a. Cyclophosphamide (150 mg/kg)-treated group, a section of mouse liver showing portal inflammation, hepatocellular necrosis, and lymphocytic inflammatory infiltrations (Hematoxylin and eosin-stained paraffin section; H&E 200).
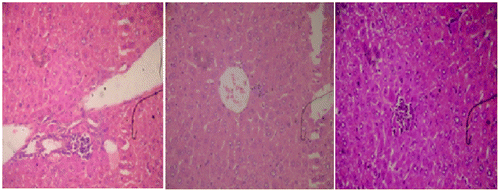
Figure 2b. Cyclophosphamide (150 mg/kg)-treated group, a section of mouse liver showing portal inflammation, hepatocellular necrosis, and lymphocytic inflammatory infiltrations (Hematoxylin and eosin-stained paraffin section; H&E 400).
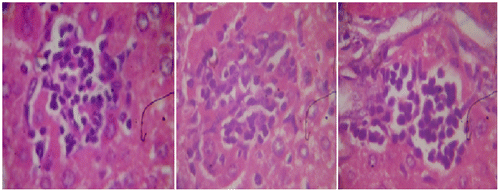
Figure 3a. Section of mouse liver treated with 120 mg/kg of G. lucidium total extract showing a mild active hepatitis with no inflammatory at central vein and slight portal inflammation. (Hematoxylin and eosin-stained paraffin section; H&E 200).
4. Discussion
CP is one of the commonly used anticancer drugs for its therapeutic efficacy against various cancers. Earlier studies have proved that ameliorative dose of CP could cause liver toxicity (Mahsa & Shivanandappa, Citation2013). CP goes through a metabolic activation by hepatic microsomal cytochrome P450 mixed functional oxidase system to produce the two metabolites, phosphoramide mustard and acrolein. Phosphoramide mustard is believed to have an antineoplastic activity, while acrolein highly reactive metabolite with a short biological half-life, may be responsible for CP-induced liver injury (Ludeman, Citation1999; Selvakumar, Prahalathan, Mythili, & Varalakshmi, Citation2005). Experimental evidence recommends that oxidative stress is answerable for CP hepatotoxicity. It could generate reactive oxygen species (ROS) like superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2) during its oxidative metabolism and discourages the antioxidant defense mechanism in liver (Bhattacharya et al., Citation2003). A number of studies have shown that natural products with antioxidant activity protect against CP hepatotoxicity (Haque et al., Citation2003; Kumar & Kuttan, Citation2005; Sharma, Trikha, Athar, & Raisuddin, Citation2000).
G. lucidium has been of research interest because it is commonly use as traditional folk medicine to treat liver disorders. There have been various studies on the efficacy of hepatoprotective property including in vitro and animal assays using the extracts with varying solvents. A queous and alcoholic extracts of G. lucidium utilized the protective action against acute hepatitis. These achievements were attributed to the inhibitory activities of the G. lucidium extract on the membrane lipid peroxidation and the formation of free radical (Lakshmi, Ajith, Jose, & Janardhanan, Citation2006; Shieh et al., Citation2001; Wang, Liu, Che, & Lin, Citation2002). On the other hand, these components contained in the mushroom had been subjected to investigate the key role in liver protection. In a report by Soares et al. (Citation2013), polysaccharides and triterpenoids might possess protective activity against liver injury induced by toxic agents. Recent studies suggest CP generates ROS like superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2) which cause lipid peroxidation of cellular membrane. During their oxidative metabolism, they depress the antioxidant defense mechanisms in liver (Lakhanpal & Rana, Citation2005; Mishra & Singh, Citation2010).These achievements were attributed to the inhibitory activities of the G. lucidium extract on the membrane lipid peroxidation and the formation of free radical (Lakshmi et al., Citation2006; Shieh et al., Citation2001; Wang et al., Citation2002).
G. lucidium in Vietnam is naturally harvested in the forest and the fruiting body is developed on the dead ironwood tree. We evaluated the hepatoprotective activity of G. lucidium against the hepatic damage induced by CP. Administration of G. lucidium for eight consecutive days after CP injection showed a significant inhibition in the liver injury compared to CP-treated mice without G. lucidium supplement. At the dose of 230 mg/kg b.wt, the hepatoprotective activity was determined by the depletion of MDA and restored the decrease of endogenous hepatic GSH antioxidant content in the liver. When the polyunsaturated lipids were degenerated by ROS, an ascent of cellular MDA occurs. The production of this aldehyde, typically quantified as thiobarbituric acid reactive substances (TBARS). It is normally considered as a biomarker to evaluate lipid peroxidation which could be indicated to the free radical scavenging activity as well as suppressing oxidative stress in an organism (Devasagayam, Boloor, & Ramasarma, Citation2003). The results of this study could support the previous findings of Shi, Sun, He, Guo, and Zhang (Citation2008), Nonanka et al. (Citation2005) and Truong and Nguyen (Citation2010) that oral supplement of G. lucidium could prevent the CP-induced lipid peroxidation. The current study also revealed a significant reduction of GSH following CP administration which might result from the direct conjugation of CP metabolites with glutathione as part of mechanism of hepatoprotective action.
5. Conclusion
The results of present study suggest that administration of G. lucidium developing on the dead ironwood tree harvested in the Central Highlands of Vietnam might have potential in protecting liver against cyclophosphamide-induced hepatotoxicity. Further study is necessary to elucidate target compounds from G. lucidium in Vietnam responsible for liver protection
Acknowledgments
I would like to thank MSc Huynh Ngoc Linh for thought-provoking comments and discussions. Special thanks to Anatomical Department, Pham Ngoc Thach University of Medicine, for providing necessary facilities to carry out the histological examination.
Additional information
Funding
Notes on contributors
Hong Ngoc Pham
Our research group is interested in exploring the bioactive and novel compounds from Vietnamese medicinal materials. Recent interest studies have concerned about hepatoprotective activity of some valuable mushrooms in Vietnam including Tramates versicolor, and Ganoderma colossum collected in nature. This study had focused on Lim Xanh (Ganoderma lucidium) as a potential source for elucidating the target hepatoprotective agents.
References
- Abraham, P., & Sugumar, E. (2008). Increased glutathione levels and activity of PON1 (phenyl acetate esterase) in the liver of rats after a single dose of cyclophosphamide: A defense mechanism? Experimental and Toxicologic Pathology, 59, 301–306.10.1016/j.etp.2007.06.006
- Acharya, K., Chatterjee, S., & Ghosh, S. (2011). Comparative evaluation on the free radical scavenging activity of eleven Idian cultivated strains of Pleurotus ostreatus. Pharmacologyonline, 1, 440–450.
- Acharya, K., Yonzone, P., Rai, M., & Acharya, R. (2005). Antioxidant and nitric oxide synthase activation properties of Ganoderma applanatum. Indian Journal of Experimental Biology, 43, 926–929.
- Bhattacharya, A., Lawrence, R. A., Krishnan, A., Zaman, K., Sun, D., & Fernandes, G. (2003). Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. Journal of the American College of Nutrition, 22, 388–399.10.1080/07315724.2003.10719322
- Devasagayam, T. P. A., Boloor, K. K., & Ramasarma, T. (2003). Methods for estimating lipid peroxidation: An analysis of metrits and demertis. Indian Journal of Biochemistry & Biophysics, 40, 300–308.
- Gao Y. H., Huang M., Lin Z. B., & Zhou, S. F. (2003). Hepatoprotective activity and the mechanisms of action of Ganoderma lucidium (Curt: Fr) P. Karst. (Ling Zhi, Reishi Mushroom) (Aphyllophormomycetidae). International Journal of Medicinal Mushrooms, 5, 111–131.
- Haque, R., Bin-Hafeez, B., Parvez, S., Pandey, S., Sayeed, I., Raisuddin, S., & Raisuddin, S. (2003). Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamideinduced biochemical toxicity. Human & Experimental Toxicology, 22, 473–480.10.1191/0960327103ht388oa
- Kumar, K. B. H., & Kuttan, R. (2005). Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomedicine, 12, 494–500.10.1016/j.phymed.2004.03.009
- Lakhanpal, T. N., & Rana, M. (2005). Medicinal and nutraceutical genetic resources of mushrooms. Plant Genetic Resources: Characterization and Utilization, 3, 288–303.10.1079/PGR200581
- Lakshmi, B., Ajith, T. A., Jose, N., & Janardhanan, K. K. (2006). Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. Journal of Ethnopharmacology, 107, 297–303.10.1016/j.jep.2006.03.027
- Lindequist, U., Niedermeyer, T. H. J., & Julich, W. D. (2005). The pharmacological potential of mushrooms. eCAM, 2, 285–299.
- Ludeman, S. M. (1999). The chemistry of metabolites of cyclophosphamide. Current Pharmaceutical Design, 5, 627–643.
- Mahsa, Z., & Shivanandappa, T. (2013). Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food and Chemical Toxicology, 57, 179–184.
- Mishra, S., & Singh, R. B. (2010). Effect of mushroom on the lipid profile, lipid peroxidation and liver functions of aging Swiss Albino rats. The Open Nutraceuticals Journal, 3, 248–253.10.2174/1876396001003010248
- Ngo, Q. H., & Nguyen, T. T. H. (2011). Antioxidant effect of polysaccharide extracted from Ganoderma colossum on cyclophosphamide-induced hepatotoxicity. Journal of HCMC Medicine, 15, 50–55.
- Noeman, S. A., Hamooda, H. E., & Baalash, A. A. (2011). Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetology & Metabolic Syndrome, 3, 17.10.1186/1758-5996-3-17
- Nonanka, Y., Ishibashi, H., Nakai, M., Shibata, H., Kiso, Y., & Abe, S. (2005). Soothing effect of Ganoderma lucidium antlered form on cyclophosphamide-induced adverse reaction. Gan To Kagaku Ryoho, 32, 1586–1588.
- Pal, J., Ganguly, S., Tahsin, K. S., & Acharya, K. (2000). In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus [Mont.] Singer. Indian Journal of Experimental Biology, 47, 1210–1218.
- Selvakumar, E., Prahalathan, C., Mythili, Y., & Varalakshmi, P. (2005). Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-α-lipoic acid. Molecular and Cellular Biochemistry, 272, 179–185.10.1007/s11010-005-7322-4
- Sharma, N., Trikha, P., Athar, M., & Raisuddin, S. (2000). Inhibitory effect of Emblica officinalis on the in vivo clastogenicity of benzo[a]pyrene and cyclophosphamide in mice. Human & Experimental Toxicology, 19, 377–384.10.1191/096032700678815945
- Shieh, Y. H., Liu, C. F., Huang, Y. K., Yang, J. Y., Wu, I. L., Lin, C. H., & Lin, S. C. (2001). Evaluation of the hepatic and renal-protective effects of Ganoderma lucidum in Mice. The American Journal of Chinese Medicine, 29, 501–507.10.1142/S0192415X01000526
- Shi, Y., Sun, J., He, H., Guo, H., & Zhang, S. (2008). Hepatoprotective effects of Ganoderma lucidum peptides against d-galactosamine-induced liver injury in mice. Journal of Ethnopharmacology, 117, 415–419.10.1016/j.jep.2008.02.023
- Soares, A. A., de Sá-Nakanishi, A. B., Bracht, A., da Costa, S. M. G., Koehnlein, E. A., de Souza, C. G. M., & Peralta, R. M. (2013). Hepatoprotective effects of mushrooms. Molecules, 18, 7609–7630.10.3390/molecules18077609
- Stroev, E. A., & Makarova, V. G. (1989). Determination of lipid peroxidation rate in tissue homogenate laboratory. In Laboratory manual in biochemistry (pp. 243–256). Moscow: Mir.
- Truong, T. M. C. & Nguyen, T. T. H. (2010). In vitro and in vivo study on antioxidant activity of Ganoderma lucidium (Lingzhi) and Trametes versicolor (Yunzhi). Journal of HCMC Medicine, 14, 135–141.
- Wang, M. Y., Liu, Q., Che, Q. M., & Lin, Z. B. (2002). Effects of total triterpenoids extract from Ganoderma lucidium (Curt: Fr) P. Karst. (Reishi mushroom) on experimental liver injury models induced by carbon tetrachloride or D-galactosamine in mice. International Journal of Medicinal Mushrooms, 4, 337–342.
- Wasser, S. P. (2005). Reishi or Ling Zhi (Ganoderma lucidium). Encyclopedia of Dietary Supplements, 2005, 603–623.