 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Senna alata has attracted the attention of many researchers due to its numerous medicinal properties. This study aims to test the acute and sub-acute toxicity of its leaf extracts in Swiss albino mice. Studies were carried out with a fixed dose of 1,000, 2,000, and 3,000 mg/kg body weight through oral administration daily. Signs of toxicity in terms of behavior and mortality were noted after every two hours till 24 h of administration for acute toxicity and further administration of extracts till 15 days to analyze the physical, biochemical, hematological parameters, and histopathological studies in liver, kidney, and spleen for sub-acute study. The highest dose administered did not produce mortality or changes in the general behavior of the test animals. All parameters were unaltered throughout the study. The present study revealed no obvious toxicity in mice treated with S. alata. These results indicate the safety of the oral administration of leaf extract.
Keywords:
Public Interest Statement
Helminthiasis is a neglected disease and has shown resistance to some available marketed drugs. Scientists continue the search for new anthelmintic agents especially from natural sources and plants have proved to be a potential source for this purpose. There are few species of Senna plant that showed to have medicinal properties, three species viz. S. alexandrina, S. alata and S. occidentalis leaf extracts have been reported for the first time from our laboratory to have cestocidal property. Amongst the three plants, S. alata leaf extracts showed to have more anthelmintic efficacy and is apparently believed to be nontoxic, but detail pre-clinical toxicological evaluation in animals have not been evaluated. Moreover, not all medicinal plants are safe for consumption in the crude form. Some level of toxicity arises from the potent toxic compounds present in it and nontoxic compounds can also behave like a toxic compound even at a lower dose, and can produce an adverse effect in human or animal cells. Thus, it is required to examine the toxicity profile of S. alata leaf extract, given its widespread consumption by human.
Competing Interests
The authors declare no competing interest
1. Introduction
According to World Health Organization (Citation2003), more than 80% of the world’s population rely on traditional medicine for their primary health care and more than 30% of the plant species have been used for this purpose. A major population of the world are attracted to this type of traditional medicine due to scarcity and high costs of available drugs (Hudaib et al., Citation2008) and an easy access to these plants in some regions of the world (Humber, Citation2002). Besides, a large amount of evidences has shown immense potential of medicinal plants for prevention, diagnosis, and treatment of various diseases (Abera, Citation2014; Ghosh, Sahoo, Das, Duley, & Palhy, Citation2014). In India, over 3,000 plants were officially recognized for their medicinal value, but it is generally estimated that over 6,000 plants are being used in traditional, folklore, and herbal medicines. Although the scientific study of some medicinal plants clearly validates the effectiveness and reliability of ethno-medical knowledge and traditional use in managing diseases, however herbal medicines are complex mixtures of many bioactive phytochemicals which may differ in different mechanisms (Sengupta, Sharma, & Chakraborty, Citation2011). Some level of toxicity arises from the potent toxic compounds present in it, and nontoxic compounds can also behave like a toxic compound even at a lower dose, and can produce an adverse effect by interacting with human or animal cells. Therefore, not all medicinal plants are safe for consumption in the crude form. Thus, such plants should be investigated to better understand their properties, safety and efficiency.
Senna alata Linn. (Family Fabaceae) has been recognized in traditional medicine for its medicinal activities (Lim, Citation2013; Karthika, Manivannan, & Mohamed, Citation2016) and was also recently reported to have anthelmintic activity (Kundu, Roy, & Lyndem, Citation2012, Citation2014). Though this plant is apparently believed to be nontoxic, but detail pre-clinical toxicological evaluation in animals have not been evaluated. Thus, it is required to examine the toxicity profile of S. alata leaf extract given its widespread consumption by human.
2. Results and discussion
During the 15-day period of toxicity study, mice showed no signs of behavioral distress or change in skin color, no changes in the eyelids, sleep, food, and water intake, and no observable toxicity symptoms or death. The experimented mice survived till the completion of the experimental duration at all levels of treatment (Table ). Thus, this indicates there was no disturbance in carbohydrate, protein, or fat metabolism (Klaassen, Citation2001).
Table 1. Acute toxicity study of ethanol extract of Senna alata in mice
Though the body weight gradually increased in control and treated groups, there was no significant difference in mean body weight amongst the different treated groups and the control (Table ) which indicated that the extract has negligible levels of toxicity on the growth of the animals as also observed by Mir, Sexena, and Malla (Citation2013) and Rajalakshmi, Jayachitra, Gopal, and Krithiga (Citation2014). There were no significant changes in organ weight and relative organ weight of liver, kidney, and spleen with respect to the body weight as well (Table ). Kluwe (Citation1981), documented that the increase in organ weight had been observed to be a relative sensitive indicator of nephrotoxicity. Thus, S. alata did not induce any toxic effect on the kidneys and the other organs going by this indicator.
Table 2. Body weight of mice during sub-acute toxicity study after administration of S. alata extract
Table 3. Absolute organ weight (g) and relative organ weight (g) during sub-acute toxicity study of S. alata extract
Hematological tests showed no significant differences in hemoglobin, RBC, WBC (total and differential), and platelet count in all doses as compared to control. In WBC differential count, lymphocytes showed slight variation in the dose of 2,000 and 3,000 mg/kg body weight compared to 1,000 mg/kg body weight and also to control, while eosinophil count showed no significant differences at all dose levels compared to control (Table ). According to Onyeyilli, Iwuoha, and Akinniyi (Citation1998), administration of an agent can result in loss of blood cells and/or inhibition of blood cell synthesis and decrease in such hematological parameters in experimental animals has been associated with anemia. The above results suggest the nontoxicity of S. alata in mice. A similar observation was reported by Ping, Darah, Chen, Sreeramanan, and Sasidharan (Citation2013), after oral administration of Euphorbia hirta, Carica papaya, Petroselinum crispum, and Lygodium flexuosum.
Table 4. Hematological parameters of sub-acute toxicity study of S. alata extract in mice
The serum analyses showed no significant difference in calcium and chloride level between the control and experimental group. However, there is a less significant level of difference in phosphorus at 1,000 and 2,000 mg/kg body weight compared to control group (Table ). Similarly, levels of creatinine and uric acid were not significantly different between the control and the experimental group of mice (Table ). This observation was also made by Ping et al. (Citation2013). Moreover, levels of total protein, albumin, SGPT, total bilirubin, direct bilirubin, cholesterol, triglyceride, alkaline phosphatase (ALP), and aspartate transaminase (AST) also showed no significant difference, however slight variation in the latter two was observed at 1,000 mg/kg body weight of the control group (Table ). Liver injury is characterized as hepatocellular when there is predominant elevation of the ALT, while AST is a mitochondria enzyme whose increased activity reflects severe tissue injuries (Martin, Citation2006). Hypo-proteinaemia, a common finding in liver damage (Larrey, Citation2002), was also not observed in the present study. This indicates that the extract did not cause any overt liver damage at the dose levels studied. Further, there was a low level of glucose in the treated group as compared to the control group (Table ) which may be due to inadequate insulin secretion that indicates normal functioning of the liver. Similar observations were made by Rajalakshmi et al. (Citation2014), Nabukenya et al. (Citation2014), Priyadarshini, Mazumder, and Choudhury (Citation2014) and Bello et al. (Citation2016). Cholesterol and triglyceride levels have no significant difference in treated animals which concurs that this plant extract does not present any risk of hypercholesterolemia or artherosclerosis at a high level of doses (Bello et al., Citation2016).
Table 5. Sub-acute toxicity study on the electrolytes of mice after treatment with S. alata alcoholic leaf extracts
Table 6. Sub-acute toxicity study on biomarkers of kidney malfunction in mice treated with S. alata
Table 7. Sub-acute toxicity study of biomarkers of liver malfunction in mice treated with S. alata
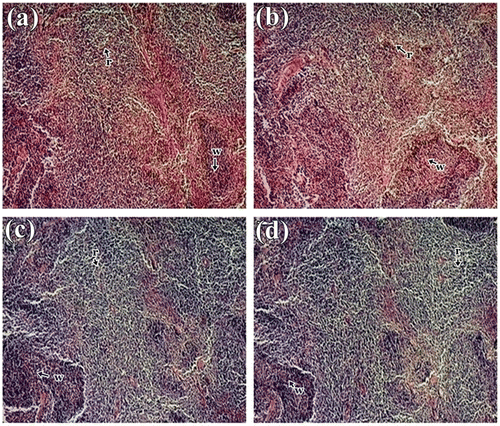
Histological studies revealed no abnormalities in liver, kidney and spleen tissue in treated mice. The liver tissue displayed normal hepatocytes without any enlargement in sinusoidal vein, central vein, and portal triad in all treated groups compared to control (Figure ). Similar type of observation was also seen by Bello et al. (Citation2016) in rat liver. Kidney micrograph revealed normal architecture of glomerulus and Bowman’s capsules with no degeneration, necrosis, or inflammation (Figure ), which are comparable with the study made by Ping et al. (Citation2013) and Nabukenya et al. (Citation2014). Histological features of spleen showed normal splenocytes with prominent nucleus in both treated and control groups (Figure ). These observations agreed with that of Ping et al. (Citation2013) in rat model that have been treated with Euphorbia hirta. Thus, histopathological evaluation indicated that the extract did not have any adverse effect on morphology of the tissues and these observations supported the biochemical results mentioned above. Therefore, it is concluded that S. alata did not produce any toxic effect in male albino mice.
Figure 1. Histology study of liver from of mice: (a) control group; (b) 1,000 mg/kg; (c) 2,000 mg/kg and (d) 3,000 of S. alata leaf extract in a 15-day sub-acute toxicity.
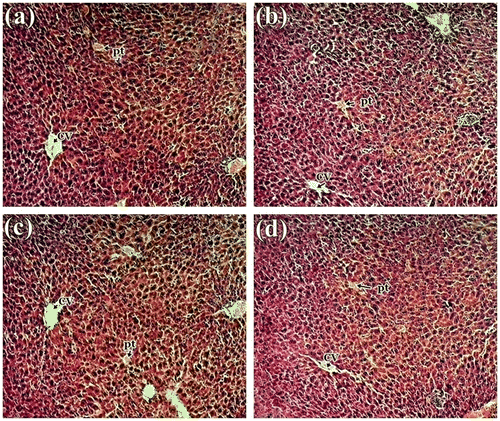
Figure 2. Histology study of kidney of mice: (a) control group; (b) 1,000 mg/kg; (c) 2,000 mg/kg and (d) 3,000 of S. alata leaf extract in a 15-day sub-acute toxicity.
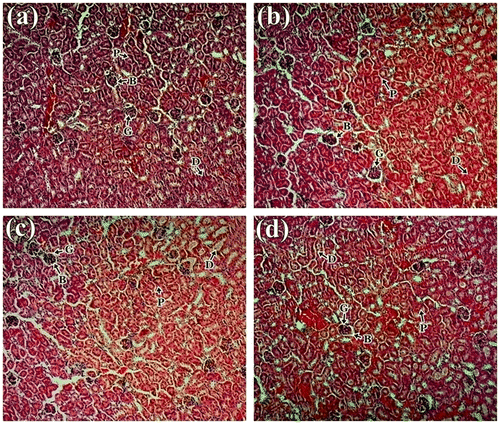
Figure 3. Histology study of spleen of mice showing normal splenocytes with defined red pulp and white pulp in control and treated group: (a) Control; (b) 1,000 mg/kg; (c) 2,000 mg/kg and (d) 3,000 mg/kg body weight of S.alata leaf extract.
3. Materials and methods
3.1. Preparation of plant extract
S. alata leaves were collected from in and around the University campus of Visva-Bharati, Santiniketan. Young leaves were washed with distilled water, allowed to dry in an oven at 50°C, and crushed to powder. About 250 g of the powdered form was extracted with 1 L of ethanol (90%) in a Soxhlet apparatus for 7–8 h, and the final crude extract was recovered using rotary evaporator and stored at 4°C until further use.
3.2. Expremental designs
Twenty-four Swiss albino male mice weighed 24–28 g were divided into four groups of six animals each (Group 1–4). Group 1 is control group, fed daily with only normal laboratory diet and water. Group 2–4 were treated with a dose of 1,000, 2,000, and 3,000 mg/kg body weight, respectively, for 15 days through an oral needle following a period of 10-h fasting. All animals were maintained on standard laboratory diets with water ad libitum. The experimental protocol and procedures used in this study were approved by the Institutional Animal Ethical Committee (IAEC), Visva-Bharati, Santiniketan.
3.3. Acute oral toxicity study
After administration of the extract, animals were monitored continuously for every two hours for a day to detect acute changes in morphological and behavioral responses, spontaneous activity, irritability, corneal reflex, tremors, convulsion, salivation, diarrhea, lethargy if any, and also monitored for any mortality during the course of toxicity study.
3.4. Sub- acute oral toxicity study
Body weight of each animal was recorded every five days interval till the last day of experiment. After the 15th day, all animals were sacrificed after light chloroform inhalation of anesthesia and different hematological and biochemical studies were performed.
3.4.1. Hematological assay
About 1.5–2 ml of blood was drawn directly with a hypodermic syringe to minimize the damage of serum contamination through a cardiac puncture (Jochems, Valk, Stafleu, & Baumans, Citation2002). About 100 μl of the collected blood sample was used for the determination of hematological parameters like hemoglobin concentration, total RBC count, total WBC count, WBC differential count, and total platelet count following the methods of Smith (Citation1995) and Kjeldsberg (Citation1998).
3.4.2. Analysis of serum biochemical parameters
The rest of the collected blood sample was prepared for serum isolation according to the method of Singh and Rana (Citation2007). In brief, blood was kept for 20 min at room temperature of 30°C and then centrifuged at 2,500 rpm for 5 min at 4°C. The serum obtained as supernatant, was collected in an eppendorf tube and kept at 4°C till use. Determination of glucose, calcium, phosphorus, chloride, total protein, albumin, ALP, AST, SGPT, total bilirubin, direct bilirubin, creatinine, cholesterol, triglyceride, and uric acid were analyzed by different assay kits following the manufacturer protocol.
3.4.3. Measurement of relative organ weight
Liver, kidneys, and spleen were carefully dissected out and weighed separately. The relative organ weight of each animal was then calculated as follows:
3.4.4. Histological examination
Each selected organs were cut into small pieces and kept in Bouin’s fixative for 24 h, and processed for histological study following methods of Mayer (Citation1896) with slight modification and later observed under a light microscope.
3.5. Chemicals
All the chemicals used were of analytical grade. Ethanol was supplied by Bengal Chemicals, Kolkata. Stains and fixatives were purchased from Sigma–Aldrich. All biochemical assay kits were purchased from Coral clinical system company, Goa, India, and all other reagents were obtained from Merck Life Science Pvt. Ltd., Merck India.
3.6. Statistical analysis
Data are expressed as a mean ± SD. Total variations present in a set of data were estimated by one way Analysis of Variance (ANOVA) comparisons were made between the treated groups. All data were analyzed using Duncan’s Multiple Range Test (DMRT). p < 0.05 was considered as the level statistical significance.
4. Conclusions
The absence of gross and histopathological lesions in the organs as well as no significant differences in hematological and biochemical test in the treated groups from the control could suggest the level of safety of the leaf alcoholic extract on the animals. In conclusion, to our knowledge, this is the first investigation of the various parameters of toxicity studies made on the S. alata at higher dose. This study has shown that sub-acute administration of the alcoholic leaf extract of S.alata may be safe and thereby provide a support to the use of S.alata leaves as an alternative system of medicine.
Cover image
Source: Author.
Acknowledgments
We also wish to thank the Department of Zoology, Centre for Advanced Studies, Visva-Bharati for providing infrastructural support.
Additional information
Funding
Notes on contributors
S. Roy
Larisha Mawkhlieng Lyndem PhD is an associate professor in the Department of Zoology, Visva-Bharati University, West Bengal, India. She teaches Parasitology and related courses for graduate and postgraduate students. Among other areas, her research interest includes the development of anthelmintic agents from natural sources mainly plant source. Together with her research team members Lyndem has extensive publications on the anthelmintic efficacy of medicinal plants widely used in traditional medicine. Saptarshi Roy and Bidisha Ukil are active members of Lyndem’s team of research.
References
- Abera, B. (2014). Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. Journal of Ethnobiology and Ethnomedicine, 10, 40.10.1186/1746-4269-10-40
- Bello, I., Bakkouri, A. S., Tabana, Y. M., Hindi, B. A., Mansoub, M. A. A., Mahmud, R., & Asmawi, M. Z. (2016). Acute and sub-acute toxicity evaluation of the methanolic extract of Alstonia scholaris stem bark. Medical Sciences, 4, 4.
- Ghosh, G., Sahoo, S., Das, D., Duley, D., & Palhy, R. N. (2014). Antibacterial and antioxidant activities of methanol extract and fractions of Clerodendrum viscosum vent. leaves. Indian Journal of Natural Products and Resources, 5, 134–142.
- Hudaib, M., Mohammad, M., Bustanji, Y., Tayyem, R., Yousef, M., Aburjaie, M., & Aburjai, T. (2008). Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. Journal of Ethnopharmacology, 120, 63–71.10.1016/j.jep.2008.07.031
- Humber, J. M. (2002). The role of complementary and alternative medicine: Accommodating pluralism. JAMA: The Journal of the American Medical Association, 288, 1655–1656.
- Jochems, C. E. A., Valk, J. B. F., Stafleu, F. R., & Baumans, V. (2002). Ethical or scientific problem? ATIA, 30, 219–227.
- Karthika, C., Manivannan, S., & Mohamed, R. K. (2016). Phytochemical analysis of Ruellia patula using gas chromatography-mass spectrometry. Asian Journal of Pharmaceutical and Clinical Research, 9, 253–257.
- Kjeldsberg, C. R. (1998). Principios de examehematologico. In G. R. Lee, T. C. Bithell, J. Forester, J. W. Athens, & J. N. Lukens (Eds.), Wintrobehematol.clinica. (pp. 7–42). São Paulo: Manole.
- Klaassen, C. D. (2001). Principles of toxicology. In C. D. Klaassen (Ed.), Casarett and Doull’s toxicology: The basic science of poisons (5th ed., p. 13). New York, NY: McGraw-Hill.
- Kluwe, W. M. (1981). Renal function tests as indicators of kidney injury in subacute toxicity studies. Toxicology and Applied Pharmacology, 57, 414–424.10.1016/0041-008X(81)90239-8
- Kundu, S., Roy, S., & Lyndem, L. M. (2012). Cassia alata L.: Potential role as anthelmintic agent against Hymenolepis diminuta. Parasitology Research, 111, 1187–1192.10.1007/s00436-012-2950-6
- Kundu, S., Roy, S., & Lyndem, L. M. (2014). Broad spectrum anthelmintic potential of Cassia plants. Asian Pacific Journal of Tropical Biomedicine, 4, S436–S441.10.12980/APJTB.4.2014C1252
- Larrey, D. (2002). Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Seminars in Liver Disease, 22, 145–156.10.1055/s-2002-30101
- Lim, T. K. (2013). Edible medicinal and non-medicinal plants (pp. 841–859). Dordrecht: Springer Science Business Media.
- Martin, A. C. (2006). Clinical chemistry and metabolic medicine (7th ed., pp. 7–15). London: Edward Arnold.
- Mayer, P. (1896). Mitt. Zool. Stn (12th ed., p. 303). Neapel.
- Mir, A. H., Sexena, M., & Malla, M. Y. (2013). An acute oral toxicity study of methanolic extract from Tridex procumbens in Sprague Dawley’s Rats as per OECD guidelines 423. Asian Journal of Plant Science, 3, 16–20.
- Nabukenya, I., Rubaire-Akiiki, C., Mugizi, D., Kateregga, J., Olila, D., & Hoglund, J. (2014). Sub-acute toxicity of aqueous extracts of Tephrosia vogelii, Vernonia amygdalina and Senna occidentalis in rats. Natural Products Chemistry & Research, 2, 143. doi:10.4172/2329-6836.1000143
- Onyeyilli, P. A., Iwuoha, C. L., & Akinniyi, J. A. (1998). Chronic toxicity study of Ficus platyphtlla blume in rats. West African Journal of Pharmacology and Drug Research, 14, 27–30.
- Ping, K. Y., Darah, I., Chen, Y., Sreeramanan, S., & Sasidharan, S. (2013). Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Research International, 182064. doi:10.1155/2013/182064
- Priyadarshini, L., Mazumder, P. B., & Choudhury, M. D. (2014). Acute toxicity and oral glucosetolerance test of ethanol and methanol extracts of antihyperglycaemic plant Cassia alata Linn. Journal of Pharmacy and Biological Sciences, 9, 43–46.
- Rajalakshmi, A., Jayachitra, A., Gopal, P., & Krithiga, N. (2014). Toxicity analysis of different medicinal plant extracts in swiss albino mice. Pharmacology and Toxicology, 1(2), 1–6.
- Sengupta, M., Sharma, G. D., & Chakraborty, B. (2011). Hepatoprotective and immunomodulatory properties of aqueous extract of Curcuma longa in carbon tetra chloride intoxicated Swiss albino mice. Asian Pacific Journal of Tropical Biomedicine, 1, 193–199.10.1016/S2221-1691(11)60026-9
- Singh, S., & Rana, S. V. S. (2007). Amelioration of arsenic toxicity by L-Ascorbic acid in laboratory rat. Journal of Environmental Biology, 28, 377–384.
- Smith, J. E. (1995). Comparative hematology. In E. Beutler, M. A. Lichtman, B. S. Coller, & T. J. Kipps (Eds.), Williams hematology (5th ed., pp. 77–85). New York, NY: McGraw-Hill.
- WHO. (2003). Retrieved from http://www.who.int/mediacentre/factsheets/2003/fs134/en/
