Abstract
Ascorbic acid (Vitamin C) has long been known for its anti-cancer properties and in the present study the effects of ascorbic acid (AsA) on osteogenic differentiation, apoptosis, and signaling pathways of the human G29 osteosarcoma cell line were studied. The expression of Runt-related transcription factor-2 (RUNX2) and osteocalcin genes were evaluated by real-time polymerase chain reaction (PCR). Osteoblastic maturation was assessed with alkaline phosphatase activity and mineralization with alizarin red deposition, and apoptosis with a caspase-2 apoptotic assay as well as the cell viability via the cytotoxicity assay. The possible role of the MAP kinase pathway (p44/42, p38, and p-JNK signaling pathway) was also studied. Our results showed that RUNX2 and osteocalcin gene expression, mineralization, cell viability, and metabolic activity levels were increased in cells treated with low concentrations of AsA with respect to untreated cells. At higher concentrations, AsA resulted in decreases in these parameters and induced apoptosis of the G292 osteosarcoma cells via downregulation of the MAPK pathway. The findings presented here support the ability of AsA to modulate the viability and differentiation of the G292 type of bone cancer cell with increases or decreases depending on the AsA concentration suggesting a need for further evaluation of the possible use of this vitamin in the regulation of bone cell cancer growth.
Public Interest Statement
The potential role of ascorbic acid (Vitamin C) in the treatment of various forms of cancer remains controversial despite numerous studies over several decades addressing this issue in animal and tissue culture models as well as human clinical trials. The concept that there is a critical concentration of ascorbic acid needed for optimal antitumor effects has gained wide acceptance although the mechanism by which this agent regulates cell growth is still not understood. In the in vitro studies presented here, the effects of ascorbic acid on an osteosarcoma cell line are shown to be dose-dependent; higher concentrations decreased the cellular metabolic activity and differentiation, and increased cytotoxicity and cell death. Although research is needed to translate these in vitro effects to effective use of ascorbic acid in clinical treatment of osteosarcoma, the consistency of these results to those in the literature with other cancer cell lines is supportive of continued investigations.
Competing Interests
The authors declare no competing interest.
1. Introduction
In the 1950s, vitamin C was originally hypothesized to be protective against cancer, but in the 1970s, Ewan Cameron and Linus Pauling suggested that it also had a therapeutic effect, reporting increased survival of patients with advanced cancer following high-dose IV vitamin C treatment (Pauling, Citation1980; Pauling et al., Citation1981; Pauling & Moertel, Citation1986). However, randomized controlled trials in which high-dose vitamin C had been administered orally in anticancer therapy had often failed. This inconsistency has been attributed to the fact that in order to elucidate a response of vitamin C in the treatment of cancer, it is necessary to maintain relatively high plasma levels, which can often be challenging with oral doses (van der Reest & Gottlieb, Citation2016). The rebirth of the use of Vitamin C in cancer treatment occurred with substantial contribution to research on its pharmacokinetics (Padayatty et al., Citation2004) as well as studies on the ability of pharmacological concentrations of the vitamin to selectively evoke death of several types of cancer cells (Chen et al., Citation2005). A recent systematic review of published results of 5 randomized controlled trials, 12 phase I/II trials, 6 observational studies, and 11 case reports concluded that there is no “high-quality evidence” to suggest that the use of vitamin C (ascorbic acid) supplementation either orally or IV in cancer patients enhances chemotherapeutic effects or reduces its toxicity (Jacobs, Hutton, Ng, Shorr, & Clemons, Citation2015). Although there is a need for additional rigorous randomized controlled trials to establish the risks and benefits of vitamin C, there is also a need to further delineate the possible mode of action of this agent in various cancer cell types for design of therapeutic approaches that might need to be unique for certain cancerous conditions.
Vitamin C is a water-soluble, essential multifunctional micronutrient that acts as an anti-oxidant and is required in its reduced form (L-ascorbic acid) for many enzymatic reactions (Golde, Citation2003). It is an essential supplement for osteoblastic cell differentiation from mesenchymal stem cell precursors (Langenbach & Handschel, Citation2013). At low doses, it can increase cellular proliferation, collagen production, and hydroxylation of proline and lysine residues in collagen (Schwarz, Kleinman, & Owens,Citation1987). However, in higher concentrations, it can be cytotoxic (Duarte & Lunec, Citation2005) and inhibit prostaglandins of the two series (arachidonic acid derived), which have been correlated with inflammation and increased cell proliferation (ElAttar & Lin, Citation1992). It has also been suggested that vitamin C may promote oxidative metabolism by inhibiting the utilization of pyruvate for anaerobic glycolysis (Lohmann, Citation1987). This can be attributed to its pro anti-oxidant effect. Previous studies have demonstrated that the anti-proliferative activity of ascorbic acid (AsA) is due to the inhibition of expression of genes involved in cell division progression (Hitomi & Tsukagoshi, Citation1996; Ohno, Ohno, Suzuki, Soma, & Inoue, Citation2009). One recent study, (Valenti et al., Citation2014) has assessed the effects of various concentrations of AsA in the anti-proliferation and differentiation of MG-63 cells and expanded on earlier studies in which AsA showed a growth repressive effect depending on its concentration in this same cell line (Takamizawa et al., Citation2004). Although these cells are often used as a prototype of normal human osteoblastic cells, MG-63 cells are an osteosarcoma lineage with an abnormal gene expression profile, altered extracellular bone matrix synthesis, and atypical bone formation (Benayahu, Shur, Marom, Meller, & Issakov, Citation2002).
JNK and p38 are key mediators of stress and inflammation responses, while the ERKs cascade is mostly induced by growth factors (Hu, Feng, & Cheng, Citation2001). The JNK stress pathway participates in many different intracellular processes, including cell growth, differentiation, transformation, and apoptosis (Niu et al., Citation2015). Consequently, some studies have proposed the inhibition of these pathways as targets in cancer therapy (Fliedner et al., Citation2014). Human osteosarcoma cell lines that have shown resistance to conventional chemotherapy, such as cisplatin and methotrexate, have been shown to be very sensitive to agents that are associated with activation of stress-response JNK signaling pathway and JNK downstream target, AP-1 transcriptional factor. In particular, the MG-63 cell line as well as the human osteosarcoma cell line, G292, have been shown to exhibit inhibition of cell viability and induction of apoptosis in respond to BBMD3, a derivative of natural product berbamine, that is an activator of JNK/AP-1 signaling (Yang et al., Citation2013). In view of the similarities in the response of these osteosarcoma cells to BBMD3, we proposed that G292 cells would respond to AsA with some anti-tumor responses as previously reported for MG-63 cells (Takamizawa et al., Citation2004; Valenti et al., Citation2014).
In this paper, we report, for the first time the anti-metabolic and differentiation effects of AsA on the human osteosarcoma G292 cell line. Furthermore, we studied possible pathways involved with occurrence of apoptosis of the cells post treatment with AsA.
2. Materials and methods
2.1. Cell culture conditions
The human osteosarcoma-derived cell line G292, purchased from the American Type Culture Collection (Manassas, VA) was grown in 75-cm2 plastic culture flasks (Falcon, Oxnard, CA) containing minimum essential medium-alpha (MEM-α; Gibco Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco Life Technologies) and a 1% anti-mycotic solution (Gibco Life Technologies). Cultures were maintained at 37°C in a humidified incubator with 5% CO2. The medium was routinely changed every 2–3 days, and cells were passaged approximately every 72–96 h.
2.2. Cell metabolic activity, cell viability, and apoptotic activity
Cell metabolic activity was evaluated by a colorimetric assay based on the reduction of the tetrazolium salt sodium 3I-[1-phenylamino-carbonyl-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) (MTT) by mitochondrial dehydrogenase of viable cells to a formazan dye (Promega, Madison, WI, USA) as described previously (Fernandes, Barone, & Dziak, Citation2016). The MTT test was performed after 48 h of L-ascorbic acid (AsA) (Sigma-Aldrich, St. Louis, MO) exposure. Briefly, the cells were seeded in complete medium (MEM-α, 10% FBS, and 1% anti-mycotic) and after 80% confluence; the cells were treated with the varying concentrations (0, 62.5, 125, 250, 500, 1,000 μM) AsA for the indicated time. The cells were then incubated with 200-μl clear (without phenol red) MEM-α and 20-μl MTT assay reagent for an additional 3 h and with 200-μl DMSO at the end of the incubation period. The supernatants were then transferred to a new 96-well plate for recording the absorbance at 490 nm using a 96-well plate reader.
The cytotoxicity of AsA and a putative apoptosis effect on the G292 cells were assayed using a CellTox Green™ assay (Promega, Madison, WI) and an ApoLive-Glo™ Multiplex Assay kit (Promega, Madison, WI), respectively. Cytotoxicity was assayed in a 96-well plate format with 5,000 cells/well after 24-h incubation with the inhibitors/vehicle with the CellTox Green™ assay (Promega, Madison, WI), following the Express, No-step Addition at Seeding Method described by the manufacturer. Fluorescence (Ex510/Em532) was measured with a fluorescent plate reader (Biotek, USA).
For the measurements of viability and apoptosis using the Apo-Live-Glo™ Multiplex kit G292 cells of approximately 500/well were seeded in a flat 96-well micro-plate as triplicates with the control and experimental treatment groups of AsA. After a total of 7 days exposure to AsA, (with medium changed every 48 h in both control and AsA groups) old medium was removed from the wells and 100 μl of fresh medium was added. Based on the color of the phenol red indicator present in the medium, there was no indication of any significant changes in pH in any of the cultures under the experimental conditions used in these incubations. Twenty microliters of viability/cytotoxicity reagent containing both GF-AFC and bis-AAF-R110 substrates were added to each well, and briefly mixed by orbital shaking at 300–500 rpm for 30 s and then incubated at 37°C for 30–180 min. Fluorescence (viability) was measured at Ex400/Em505 using a fluorescent plate reader (Biotek, USA). Immediately after the readings were completed, 100 μl of Caspase-Glo 3/7 reagent was added to each well, and briefly mixed by orbital shaking at 300–500 rpm for 30 s and then incubated at room temperature for 30–180 min. Luminescence, which is proportional to the amount of caspase activity present in the samples, was measured using a Perkin Elmer Victor3TMV Wallac plate reader using the luminescence (1.0 s) protocol.
2.3. ALP activity and Alizarin red staining
Alkaline Phosphatase (ALP) activity was assessed with an ALP kit purchased from Sigma-Aldrich, following the manufacturer’s instructions as described previously (Fernandes et al., Citation2016). Briefly, the cells were seeded in the complete medium (MEM-α, 10% FBS, and 1% anti-mycotic) and after 80% confluence, the cells were treated with varying concentrations of ascorbic acid for the indicated time before the ALP activity was measured. ALP activity was normalized according to protein content of the cell sample lysate. The protein concentration of the cell lysate was measured with a bicinchoninic acid (BCA) Protein Assay Kit (Sigma-Aldrich) and the ALP activity was expressed as absorbance at 405 nm/mg protein.
To measure the level of calcium mineral deposition, alizarin red staining (AR-S) was performed on the cells that were treated with the various concentrations of ascorbic acid. After 3 weeks in culture, the cells were fixed with 70% ethanol, rinsed five times with deionized water, treated with 40 mM alizarin red solution for 10 min at pH 4.2, and then washed for 15 min with PBS. Cetylpyridinium chloride (CPC) extraction was used for destaining. AR-S was removed from the cell samples by the addition of CPC (10% w/v, pH 7.0) followed by incubation at room temperature with gentle shaking for 1 h. The absorbances of the CPC extractions were measured at 550 nm (Barres, Mota Anna, Greenberg, Almojaly, & Dziak, Citation2015).
2.4. Total RNA extraction and reverse transcription
After inducing the G292 cells with the varying concentrations of ascorbic acid treatment, the cells were scraped, and pellets were collected by centrifugation at 1,000× g for 10 min at 4°C for RNA extraction. Total RNA was extracted from each pellet using the RNeasy minikit (Qiagen, USA) with DNAse I treatment. First-strand cDNA was generated using the High-Capacity cDNA Archive Kit, with random hexamers, (Applied Biosystems PE, Foster City, CA, USA) according to the manufacturer’s protocol. The cDNA product was quantified using a NanoDrop 2000/2000c Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and then aliquoted in equal volumes and stored at −80°C.
2.5. Real-time PCR
Polymerase chain reaction (PCR) was performed in a total volume of 20 μl containing 1 × Premix Ex Taq™ (2×), 1 × Rox Reference Dye (50×) and 20 ng of cDNA. Probe sets for each gene (RUNX2 5′-F: AAGTGCGGTGCAAACTTTCT-3′ R: 5′-TCTCGGTGGCTGCTAGTGA-3′);(OPN osteocalcin—F GCAGAGTCCAGCAAAGGT; R CAGCCATTGATACAGGTAGC) were obtained from Assay-on-Demand Gene Expression Products (Applied Biosystems). Real-time PCR reactions were carried out in a two-tube system and in multiplex. The amplification conditions included 30 s at 95°C (initial denaturation), followed by 50 cycles at 95°C for 5 s (denaturation) and at 60°C for 31 s (annealing/extension). Thermocycling and signal detection were performed with ABI Prism 7000 Sequence Detector (Applied Biosystems). Signals were detected according to the manufacturer’s instructions. ΔΔcT values were then calculated with respect to the control. To normalize mRNA expression for sample-to-sample differences in RNA input, quality, and reverse transcriptase efficiency, the housekeeping gene GAPDH was amplified. Endogenous control gene was abundant and remained constant proportionally to total RNA among the samples.
2.6. Western blot
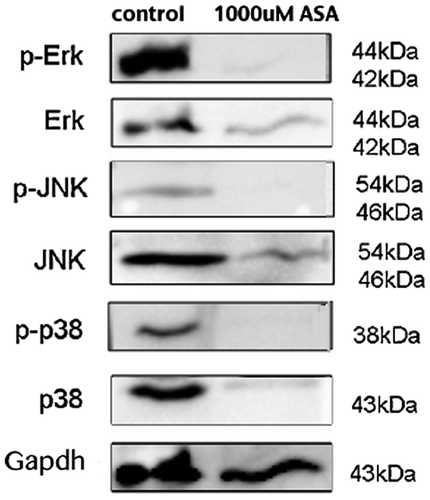
G292 cells were seeded at equal concentrations and incubated with 1,000 μM of ascorbic acid for 4 days. Control group cells were not treated. Cells were washed with cold PBS and lysed in RIPA buffer with protein inhibitor cocktail (Thermo Fisher Scientific, IL, USA). The protein concentration of the cell lysate was measured with a Bicinchoninic Acid (BCA) Protein Assay Kit (Sigma-Aldrich). Samples were run on a 15% SDS-PAGE gel and the proteins were transferred to nylon membranes. The membranes were blocked with 2% non-fat dry milk in TBS overnight at 4°C and then incubated with rabbit antibody to phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), 1:5000 (Cell Signaling Technology, MA, USA), rabbit antibody to p-JNK, 1:2000 (Cell Signaling Technology, MA, USA), and rabbit antibody to p-38, 1:2000 (Cell Signaling Technology, MA, USA) and housekeeping GAPDH.
2.7. Statistical analysis
Statistical analyses were performed using SPSS-17.0 software. Where indicated, experimental data were reported as mean ± standard deviation of triplicate independent samples. Data were analyzed using Student’s t-test and one-way analysis of variance, and Tukey’s HSD test was applied as a post hoc test if statistical significance was determined. A value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Effect of ascorbic acid on G29 cell metabolic, cell viability, and apoptotic activity
As shown in Figure (A), incubation of the G292 cells with 62.5 μM AsA significantly increased the metabolic activity of the cells compared to controls (0 μM AsA) but with higher concentrations an inverse relationship between AsA concentration and activity was observed with 1,000 μM AsA resulting in a significant decrease compared to the other groups.
Figure 1. (A) MTT assay: cell metabolic activity was assessed with various concentrations (62.5, 125, 250, 500, 1,000 μM) of ascorbic acid (AsA) added to the G292 cells; absorbance measurements were normalized to the controls (0 μM). 1,000 μM demonstrated significantly greater inhibition of cell activity (# = p < 0.05), whereas, 62.5 μM AsA demonstrated a significant increase in cell activity compared to controls (* = p < 0.05). (B) Cytotoxicity assay: cells treated with various concentrations of AsA were evaluated via the CellTox Green™ assay for viability at 0, 24, 48, and 72 h. 1,000 μM AsA resulted in significantly lower cell viability (# = p < 0.05), while 62.6 μM AsA led to significantly higher cell viability as compared to other groups (* = p < 0.05). (C) Cell viability and (D) Apoptosis (assessed with the Apo-Live-Glo™ Multiplex kit): Incubation with 1,000 μM AsA resulted in significantly lower cell viability and higher apoptosis, whereas 62.5 μM AsA led to significantly higher cell viability in comparison to other groups (# = p < 0.05; * = p < 0.05).
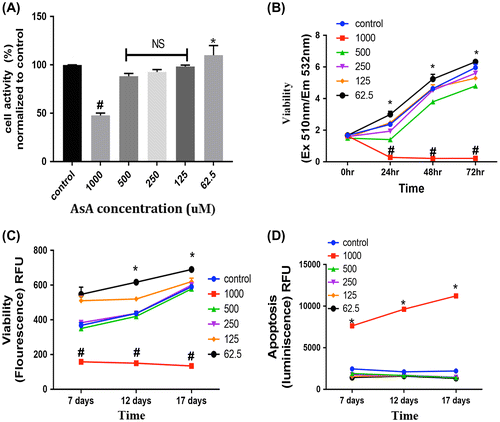
We measured cytotoxicity with a fluorescence plate format assay that uses a cyanine dye impermeant to live cells and stains DNA in dead cells. Using this assay, it was observed that 62. 5 μM AsA significantly increased the live G292 cells activity compared to controls, while higher concentrations of AsA reduced this parameter and the cells incubated with 1,000 μM AsA showed significantly less cellular activity compared to the other groups. These effects were observed at 24, 48, and 72 h of incubation with AsA (Figure (B)).
The graphs in (Figure (C) and (D)) depict the results of an ApoLive-Glo™ multiplex assay showing the cell viability of G292 cells treated with ascorbic acid. The viability data (Figure (C)) supports the results obtained with the other assays assessing cellular activity with 62.5 μM AsA significantly increasing this parameter compared to controls and the higher dose (1,000 μM AsA) decreasing viability. These effects were observed with incubation periods from 7 to 17 days with medium changes with fresh AsA every 3 days. The results of the apoptosis phase of the assay revealed that incubation with 1,000 μM AsA over this period of time significantly increases apoptosis of the G292 cells (Figure (D)).
3.2. Effects of ascorbic acid on G292 cell differentiation and mineralization
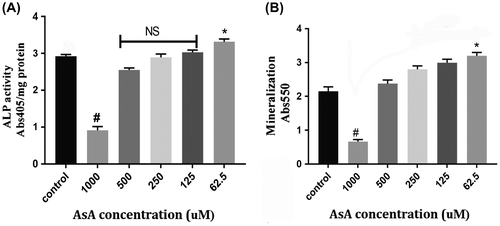
To assess the ability of AsA to induce G292 cell differentiation, ALP activity and the presence of mineralization, detected by alizarin red, were evaluated. After 8 days of incubation, with medium changes every 3 days, 62.5 μM AsA significantly increased ALP activity compared to controls (0 μM AsA), while 1,000 μM AsA significantly decreased this marker of differentiation and the other lower AsA concentrations tested (125, 250, 500 μM) had no significant effects (Figure (A)). After 10 days of treatment incubation with AsA, as shown in Figure (B), we observed a significant increase in mineralization with 62.5 μM AsA, and a significant decrease with 1,000 μM AsA and no significant effects in cells incubated with the other tested AsA concentrations.
Figure 2. (A) Alkaline phosphatase (ALP) activity: Various concentrations (0, 62.5, 125, 250, 500, 1,000 μM) AsA were tested for their effects on ALP activity after 8 days of incubation. Incubation with 62.5 μM AsA resulted in significantly higher ALP activity (* = p < 0.05), whereas doses of 1,000 μM AsA led to lower ALP activity (# = p < 0.05). (B) Alizarin Red (ARS) Mineralization assay: Quantitative results from ARS staining demonstrated significantly higher mineralization activity with 62.5 μM (* = p < 0.05), whereas 1,000 μM AsA demonstrated significantly lower mineralization activity (# = p < 0.05) after 10 days of incubation.
To further evaluate the role of AsA on osteogenic differentiation, we analyzed the effects of AsA at concentrations ranging from 0 to 1,000 μM on the transcription of Runt-related transcription factor-2 (RUNX2) and on the osteosarcoma-related gene osteocalcin. AsA concentrations of 62.5 and 125 μM induced a significant increase of RUNX2 mRNA expression with respect to controls after 72 h of culture with 62.5 μM producing the highest increase in this marker of differentiation (Figure (A)). Incubations with concentrations of 250 and 500 μM AsA resulted in no significant effect; however, with 1,000 μM AsA, RUNX2 mRNA expression was significantly decreased (p < 0.05) compared to controls (Figure (A)). Similar results on the expression of osteocalcin mRNA in these cells were obtained after 72-h incubations with concentrations of AsA from 62.5 to 1,000 μM (Figure (B)).
Figure 3. Real-time PCR [qPCR] (A and B): The effect of various concentrations (0, 62.5, 125, 250, 500, 1,000 μM) of AsA and the mRNA fold change of RUNX2 and osteocalcin (ocn) was evaluated in the G292 cells after 72-h incubation. 1,000 μM AsA-treated cells demonstrated significantly lower fold change (^ = p < 0.05), Both 125 and 62.5 μM AsA treated cells demonstrated significantly higher fold changes in RUNX2 and ocn in comparison to other groups (#, * = p < 0.05) with 62.5 AsA producing the greatest increases.
![Figure 3. Real-time PCR [qPCR] (A and B): The effect of various concentrations (0, 62.5, 125, 250, 500, 1,000 μM) of AsA and the mRNA fold change of RUNX2 and osteocalcin (ocn) was evaluated in the G292 cells after 72-h incubation. 1,000 μM AsA-treated cells demonstrated significantly lower fold change (^ = p < 0.05), Both 125 and 62.5 μM AsA treated cells demonstrated significantly higher fold changes in RUNX2 and ocn in comparison to other groups (#, * = p < 0.05) with 62.5 AsA producing the greatest increases.](/cms/asset/2bbf66d8-0ccf-4ef1-98b9-b24e3649c1ab/oabi_a_1288335_f0003_b.gif)
3.3. Role of MAPK pathway on the effects of ascorbic acid on G292 osteosarcoma cells
Western blot analysis revealed that the protein levels of phospho-ERK1/2 MAPK, phospho-pJNK, p-38, and phospho-p38 in the AsA-treated group (1,000 μM) were lower than those in control groups in G292 cells (Figure ).
4. Discussion
Several studies in the literature, recently reviewed by van der Reest and Gottlieb (Citation2016) have suggested the role of L-ascorbic acid, also referred to as Vitamin C, as an anti-tumor agent. In this report, we provide, for the first time, evidence demonstrating that ascorbic acid has an inhibitory and apoptotic effect on G292 osteosarcoma cells. We found that high-dose AsA can inhibit the growth of G292 osteosarcoma significantly, whereas low-dose AsA significantly increases differentiation and cell metabolic activity. Furthermore, higher doses AsA decreased osteogenic differentiation gene expression in a dose-dependent manner and inhibited G292 activity via the MAPK pathway.
Previous studies have demonstrated that AsA in low doses could stimulate differentiation and proliferation of MG-63 osteosarcoma cells, whereas in high doses could induce apoptosis (Valenti et al., Citation2014). In our study, we extended this work to study the effect of AsA on G292 osteosarcoma cell line since the G292 cell line is a more stable cell line and less aggressive (proliferates slowly) as compared to the MG-63 cell line (Lucero et al., Citation2013). In the G292 cells studied here, it was observed that the cell metabolic activity, ALP activity and mineralization increased with the lower dose AsA and decreased with high doses of AsA. Furthermore, apoptosis also was shown to increase in G292 cells treated with higher doses of AsA. Apoptosis was measured in these studies with the ApoLive-Glo™ Multiplex Assay that measures both the number of viable cells as a marker of cytotoxicity and caspase activation as a marker of apoptosis to determine the mechanism of cell death (Telford, Komoriya, & Packard, Citation2002). The first part of the assay measures the activity of a protease marker of cell viability. The live cell protease activity is restricted to intact viable cells and is measured using a fluorogenic, cell-permeant, peptide substrate (glycyl-phenylalanyl-amino fluorocoumarin; GF-AFC). The substrate enters intact cells, where it is cleaved by the live-cell protease activity to generate a fluorescent signal proportional to the number of living cells that then becomes inactive upon loss of cell membrane integrity and leakage into the surrounding culture medium. The second part of the assay uses the Caspase-Glo® Assay technology to detect caspase activation, a key biomarker of apoptosis. The Caspase-Glo® Assay provides a luminogenic caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD, in a reagent optimized for caspase activity, luciferase activity, and cell lysis. It should be noted here that the MTT assay also employed in our studies correlated well with the viability part of the ApoLive-Glo™ Multiplex assay, although the latter gave more subtle results. This assay essentially assessed the cell membrane integrity and is therefore more sensitive than the MTT assay that measures mitochondrial enzymes as an indication of cell metabolic activity (Fernandes et al., Citation2016).
ALP activity is a marker of osteoblastic differentiation. In the present study, low doses of AsA increased ALP activity, whereas high doses decreased this activity, indicating that high dose of AsA can inhibit osteoblastic differentiation which is in agreement with the study of Valenti et al. (Citation2014), where they reported that low doses of AsA could increase osteoblast differentiation and high doses could induce decreases in this parameter. Moreover, in our studies here, the mineralization potential of G292 cells increased with low doses of AsA, but not at high doses of AsA with a decrease at 1,000 μM. Furthermore, RUNX2 and osteocalcin genes that are associated with osteogenic differentiation were elevated in G292 cells treated with low doses of AsA (62.5–125 μM), however, there was a significant decrease in these levels at a concentration of 1,000 μM AsA suggesting that AsA, at high doses, can inhibit terminal maturation of osteoblasts. Moreover, high doses of AsA treated osteosarcoma demonstrated a rounded morphology of the cells, which has recently been known to be associated with cells with damaged DNA undergoing mitosis which could provide an association between the process of cell cycle arrest and cell death as reported by Kubara et al. (Citation2012).
Our data suggest that cell death induced by AsA in human osteosarcoma cells is an apoptotic process via the downregulation of the MAPK signaling pathways, consistent with reports from previous studies on cell death and survival in osteosarcomas (Niu et al., Citation2015).
The MAPK pathway plays a key role in extracellular signal transduction to initiate cellular responses (Burotto, Chiou, Lee, & Kohn, Citation2014). MAPK pathways can relay, amplify and integrate signals from a diverse range of stimuli and elicit appropriate physiological responses, including cellular proliferation, differentiation, development, inflammatory responses, and apoptosis (Na, Kim, & Park, Citation2012). Studies have demonstrated that the MAPK signaling pathways activate and phosphorylate the osteoblast-specific transcription factor Cbfa1 (RUNX2) in MC3T3-E1 cells and play an important role in the regulation of osteoblast-specific gene expression (Xiao et al., Citation2000). Other studies suggest an important role for the extracellular signal-regulated kinase (ERK)–MAPK pathway in the regulation of osteoblast differentiation and fetal bone development (Ge, Xiao, Jiang, & Franceschi, Citation2007). Additionally, the role of p-38 and JNK signaling pathway contributes to cell proliferation and apoptosis. JNK appears to be an intrinsic component of the mitochondrial-dependent death pathway during stress-induced apoptosis. Suggestion of JNK involvement in apoptosis came from the observation that Jnk1−/− jnk2−/− mice were resistant to apoptosis induced by UV irradiation, anisomycin, and DNA-alkylating agent methyl methanesulfate (Baltriukiene, Kalvelyte, & Bukelskiene, Citation2007). The downregulation of p-44/42, p-38, and p-JNK by 1,000 μM AsA observed in our studies is consistent with a role of these components in actions of this agent on the G292 osteosarcoma cells.
There are emerging data that the pharmaceutically active form of AsA is its oxidized form, dehydroascorbate (DHA). Preclinical studies with a variety of cancer cells showed that pharmacological doses of ascorbate can act as a proxidant and produce hydrogen peroxide which results in toxic effects on cancerous cells without producing adverse effects on normal cell viability (Chen et al., Citation2005, Citation2008). In a study with colorectal cancer cells, it was reported that tumor cells with high GLUT1 glucose transporter expression along with KRAS or BRAF oncogene-induced glycolytic addiction are selectively susceptible to cytotoxic effects induced by the AsA (Yun et al., Citation2015). Likewise, it had been shown that increased endogenous levels of hydrogen peroxide with high concentrations of AsA decreased the tumor growth of pancreatic tumor cell lines (Espey et al., Citation2011). The potential role of peroxide-induced oxidative stress in the mechanism of AsA action in G292 cells was not addressed in these previous studies, or in this present study. However, this role should be pursued in future studies, particularly in a comparative analyses with other osteosarcoma cell lines in order to achieve a more thorough understanding of critical factors involved in cytotoxic effects in this type of tumor.
In conclusion, ascorbic acid has dose-related effects on G292 cells with high doses resulting in decreases in parameters of osteoblastic differentiation and maturation, and increases in apoptosis. The dose of AsA (1,000 μM) at which antiproliferative, antidifferentiation, and apoptotic effects on G292 cells were consistently observed here falls within the range of doses (600–4,000 μM) that have had such effects on other cell types in vitro (Belin et al., Citation2009; Chen et al., Citation2005, Citation2008; Valenti et al., Citation2014). Although more clinical studies are necessary to establish the in vivo efficacy of ASA as an anti-osteosarcoma therapeutic agent, pharmacokinetics conducted with this agent suggest that the doses effective here could be achieved with IV therapy (Padayatty et al., Citation2004). Therefore, these present studies provide a basis for further investigation on the use of AsA at high doses as an adjuvant to standard chemotherapy for some forms of osteosarcoma.
Funding
The authors received no direct funding for this research. Funding was from general departmental support.
Acknowledgments
The authors wish to thank Leandra Velasquez of the Department of Oral Biology at University at Buffalo for her technical assistance with the western blot studies described here as well as Dr Michelle Visser, Assistant Professor of Oral Biology at University of Buffalo, for use of some equipment and reagents in her laboratory as well as her advice on technical aspects of the western blot analysis employed here.
Additional information
Notes on contributors
Gabriela Fernandes
Gabriela Fernandes conducted this study as a postdoctoral fellow in the Department of Oral Biology at the University at Buffalo. She received her MS in Oral Sciences from that Institution and has also clinical training in dentistry having obtained a BDS from YCMM & RDF Dental College. Her research interests have been in tissue engineering and regulation of bone remodeling, both in normal bone as well as osteosarcomas.
Andrew W. Barone
Andrew W. Barone is presently pursuing a DDS at the University at Buffalo as well as conducting research in the laboratory of Rosemary Dziak. He has conducted studies on the use of a nanoscaffold in bone regeneration as well as with putative regulators of osteosarcomas.
Rosemary Dziak
Rosemary Dziak is a professor of Oral Biology at the University at Buffalo. Her research revolves around osteoblastic cell-mediated bone regulation and she has conducted research on the mechanism of action of many agents that have effects on pathological and physiological bone metabolism.
References
- Baltriukiene, D., Kalvelyte, A., & Bukelskiene, V. (2007). Induction of apoptosis and activation of JNK and p38 MAPK pathways in deoxynivalenol-treated cell lines. Alternatives to Laboratory Animals: ATLA, 35, 53–59. Retrieved from www.ncbi.nlm.nih.gov/pubmed/17411352
- Barres, L., Mota Anna, D. S., Greenberg, M., Almojaly, S., & Dziak, R. (2015). Effects of alendronate on human alveolar osteoblastic cells: Interactions with platelet-derived growth factor. International Journal of Oral Health Dentistry, 1, 2. doi:10.16966/2378-7090.108
- Belin, S., Kaya, F., Duisit, G., Giacometti, S., Ciccolini, J., & Fontés, M. (2009). Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS One, 4, e4409. doi:10.1371/journal.pone.0004409
- Benayahu, D., Shur, I., Marom, R., Meller, I., & Issakov, J. (2002). Cellular and molecular properties associated with osteosarcoma cells. Journal of Cellular Biochemistry, 84, 108–114. doi:10.1002/jcb.1270
- Burotto, M., Chiou, V. L., Lee, J. M., & Kohn, E. C. (2014). The MAPK pathway across different malignancies: A new perspective. Cancer, 120, 3446–3456. doi:10.1002/cncr.28864
- Chen, Q., Espey, M. G., Krishna, M. C., Mitchell, J. B., Corpe, C. P., Buettner, G. R., … Levine, M. (2005). Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the National Academy of Sciences of the United States of America, 102, 13604–13609. doi:10.1073/pnas.0506390102
- Chen, Q., Espey, M. G., Sun, A. Y., Pooput, C., Kirk, K. L., Krishna, M. C., … Levine, M. (2008). Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proceedings of the National Academy of Sciences, 105, 11105–11109. doi:10.1073/pnas.0506390102
- Duarte, T. L., & Lunec, J. (2005). Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radical Research, 39, 671–686. doi:10.1080/10715760500104025
- ElAttar, T. M. A., & Lin, H. S. (1992). Effect of vitamin C and vitamin E on prostaglandin synthesis by fibroblasts and squamous carcinoma cells. Prostaglandins, Leukotrienes and Essential Fatty Acids, 47, 253–257. doi:10.1016/0952-3278(92)90194-N
- Espey, M. G., Chen, P., Chalmers, B., Drisko, J., Sun, A. Y., Levine, M., & Chen, Q. (2011). Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radical Biology and Medicine, 50, 1610–1619. doi:10.1016/j.freeradbiomed.2011.03.007
- Fernandes, G., Barone, A., & Dziak, R. (2016). Effects of verapamil on bone cancer cells. Journal of Cell-Biology-&-Cell-Metabolism, 3, 13. Retrieved from http://www.heraldopenaccess.us/fulltext/Cell-Biology-&-Cell-Metabolism/Effects-of-Verapamil-on-Bone-Cancer-Cells-In-Vitro.pdf
- Fliedner, S. M., Engel, T., Lendvai, N. K., Shankavaram, U., Nölting, S., Wesley, R., … Lehnert, H. (2014). Anti-cancer potential of mapk pathway inhibition in paragangliomas—Effect of different statins on mouse pheochromocytoma cells. PloS One, 9, e97712. doi:10.1371/journal.pone.0097712
- Ge, C., Xiao, G., Jiang, D., & Franceschi, R. T. (2007). Critical role of the extracellular signal–regulated kinase–MAPK pathway in osteoblast differentiation and skeletal development. The Journal of Cell Biology, 176, 709–718. doi:10.1083/jcb.200610046
- Golde, D. W. (2003). Vitamin C in cancer. Integrative Cancer Therapies, 2, 158–159. doi:10.1177/1534735403002002009
- Hitomi, K., & Tsukagoshi, N. (1996). Role of ascorbic acid in modulation of gene expression. In Subcellular biochemistry (pp. 41–56). Springer US. doi: 10.1007/978-1-4613-0325-1_3
- Hu, J. Z., Feng, D. Y., & Cheng, R. X. (2001). Hunan yi ke da xue xue bao= Hunan yike daxue xuebao= [Expressions of p-MAPK, cyclin D1, p53 protein and their relationship in osteosarcoma]. Bulletin of Hunan Medical University, 26, 325–327.
- Jacobs, C., Hutton, B., Ng, T., Shorr, R., & Clemons, M. (2015). Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. The Oncologist, 20, 210–223. doi:10.1634/theoncologist.2014-0381
- Kubara, P. M., Kernéis-Golsteyn, S., Studény, A., Lanser, B. B., Meijer, L., & Golsteyn, R. M. (2012). Human cells enter mitosis with damaged DNA after treatment with pharmacological concentrations of genotoxic agents. Biochemical Journal, 446, 373–381. doi:10.1042/BJ20120385
- Langenbach, F., & Handschel, J. (2013). Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Research & Therapy, 4, 1. doi:10.1186/scrt328
- Lohmann, W. (1987). Ascorbic acid and cancer. Annals of the New York Academy of Sciences, 498, 402–417. doi:10.1111/j.1749-6632.1987.tb23777.x
- Lucero, C. M., Vega, O. A., Osorio, M. M., Tapia, J. C., Antonelli, M., Stein, G. S., … Galindo, M. A. (2013). The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. Journal of Cellular Physiology, 228, 714–723. doi:10.1002/jcp.24218
- Na, K. Y., Kim, Y. W., & Park, Y. K. (2012). Mitogen-activated protein kinase pathway in osteosarcoma. Pathology-Journal of the RCPA, 44, 540–546. doi:10.1097/PAT.0b013e32835803bc
- Niu, N. K., Wang, Z. L., Pan, S. T., Ding, H. Q., Au, G. H., He, Z. X., … Yang, T. (2015). Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3 K/Akt/mTOR signaling pathway. Drug Design, Development and Therapy, 9, 1555. doi:10.2147/DDDT.S74197
- Ohno, S., Ohno, Y., Suzuki, N., Soma, G. I., & Inoue, M. (2009). High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Research, 29, 809–815. Retrieved from http://ar.iiarjournals.org/content/29/3/809.long
- Padayatty, S. J., Sun, H., Wang, Y., Riordan, H. D., Hewitt, S. M., Katz, A., … Levine, M. (2004). Vitamin C pharmacokinetics: Implications for oral and intravenous use. Annals of Internal Medicine, 140, 533–537. doi:10.7326/0003-4819-140-7-200404060-00010
- Pauling, L. (1980). Vitamin C therapy of advanced cancer. The New England Journal of Medicine, 302, 694–695. doi:10.1056/NEJM198003203021219
- Pauling, L., Anderson, R., Banic, S., Basu, T. K., Kallistratos, G., Murata, A., … Siegel, B. V. (1981). Workshop on vitamin C in immunology and cancer. International Journal for Vitamin and Nutrition Research. Supplement= Internationale Zeitschrift fur Vitamin-und Ernahrungsforschung, 23, 209–219. Retrieved from http://europepmc.org/abstract/med/6180999
- Pauling, L., & Moertel, C. (1986). A proposition: Megadoses of vitamin C are valuable in the treatment of cancer. Nutrition Reviews, 44, 28–29. doi:10.1111/j.1753-4887.1986.tb07553.x
- Schwarz, R. I., Kleinman, P., & Owens, N. (1987). Ascorbate can act as an inducer of the collagen pathway because most steps are tightly coupled. Annals of the New York Academy of Sciences, 498, 172–185. doi:10.1111/j.1749-6632.1987.tb23760.x
- Takamizawa, S., Maehata, Y., Imai, K., Senoo, H., Sato, S., & Hata, R. I. (2004). Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biology International, 28, 255–265. doi:10.1016/j.cellbi.2004.01.010
- Telford, W. G., Komoriya, A., & Packard, B. Z. (2002). Detection of localized caspase activity in early apoptotic cells by laser scanning cytometry. Cytometry, 47, 81–88. doi:10.1002/cyto.10052
- Valenti, M. T., Zanatta, M., Donatelli, L., Viviano, G., Cavallini, C., Scupoli, M. T., & Dalle Carbonare, L. U. C. A. (2014). Ascorbic acid induces either differentiation or apoptosis in MG-63 osteosarcoma lineage. Anticancer Research, 34, 1617–1627. Retrieved from http://ar.iiarjournals.org/content/34/4/1617.long
- van der Reest, J., & Gottlieb, E. (2016). Anti-cancer effects of vitamin C revisited. Cell Research, 26, 269–270. doi:10.1038/cr.2016.7
- Xiao, G., Jiang, D., Thomas, P., Benson, M. D., Guan, K., Karsenty, G., & Franceschi, R. T. (2000). MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. Journal of Biological Chemistry, 275, 4453–4459. doi:10.1074/jbc.275.6.4453
- Yang, F., Nam, S., Zhao, R., Tian, Y., Liu, L., Horne, D. A., & Jove, R. (2013). A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling. Cancer Biology & Therapy, 14, 1024–1031. doi:10.4161/cbt.26045
- Yun, J., Mullarky, E., Lu, C., Bosch, K. N., Kavalier, A., Rivera, K., … Muley, A. (2015, December). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350, 1391–1396. doi:10.1126/science.aaa5004.