?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To determine the in vivo neuropharmacological and in vitro antioxidant activities of methanolic extract of Tetracera sarmentosa. Open field (OFT), hole cross (HCT), thiopental-induced sleeping time (TIST), elevated plus-maze (EPMT) tests were used to determine the neuropharmacological activity and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay, reducing power assay, total phenolic content tests were used to evaluate the in vitro antioxidant activity of T. sarmentosa. In the case of OFT and HCT, the extract showed a decrease in exploratory and locomotion activities at both dose levels (200 and 400 mg/kg body weight). In the thiopental-induced hypnosis test, 400 mg/kg dose of T. sarmentosa produced quick onset of sleep and prolonged duration of sleep than that of 200 mg/kg dose. T. sarmentosa extract showed the lessening percentage of entries of mice into the open arm and decreased percentage of time spent in open arm compared to the standard drug diazepam. In the case of DPPH scavenging activity, IC50 value of methanolic plant extract of T. sarmentosa is 151.56 μg/ml whereas the value of ascorbic acid is 23.53 μg/ml. In this current study, the phenolic content of T. sarmentosa was found to be 140.34 ± 1.56 GAE mg/gm dry extract. Results of this study revealed that methanolic extract of T. sarmentosa contains significant neuropharmacological and antioxidant activities.
Public Interest Statement
Day by day the use of synthetic drugs is increasing which is responsible for various side effects as well as sometimes for hazardous adverse effects. Synthetic drugs are also expensive. So it is the high time to explore indigenous medicinal plants for the discovery of natural drug molecules which will be safer for human being and less expensive than synthetic drugs. Our approach is to discover new medicinal plant which will be a great alternative way for the treatment of various diseases. In this experiment, we are evaluating the neuropharmacological and antioxidant effects of Tetracera sarmentosa.
Competing Interests
The authors declare no competing interest.
1. Introduction
Medicinal plants are the prominent source of secondary metabolites as well as active drug compounds. It plays a pivotal role for the discovery of new drug molecules. Additionally, medicinal plants contain different essential bioactive compounds such as antioxidants, alkaloids, flavonoids, saponins, steroids, terpenoids, polysaccharides, and so on which are the important part of modern and traditional medicines (Doughari, Ndakidemi, Human, & Benade, Citation2012; Mahboubi, Haghi, Kazempour, & Hatemi, Citation2013; Ming, Khang, Sai, & Fatt, Citation2003). Tetracera sarmentosa (Family: Dilleniaceae) is a scandent shrub which is generally found in Chittagong, Chittagong Hill Tracts, and Cox’s Bazar (Bangladesh Ethnobotany Online Database, Citationn.d.). It is also widely distributed through Southern China, India, Sri Lanka, Mayanmar, Thailand, Malaysia, and Indonesia (Flora of China, Citationn.d.). Traditionally, the root of this plant extract is taken for the treatment of rheumatism (Bangladesh Ethnobotany Online Database, Citationn.d.). In Sri Lanka, it is also used as a healing agent for the treatment of bone fracture. Antinociceptive activity of this plant was also found when aqueous leaf extract was tested on rats (Fernando, Ratnasooriya, & Deraniyagala, Citation2009). According to a research of chemical components of this plant by GC-MS, few essential oils were isolated from leaves of this plant. Among them hexadecanoic acid (41.59%), phytol (11.10%), and linoleic acid (5.08%) are notable chemical components (Da, Zhu, Zhao, Teng, & Gan, Citation2014). In this modern era, anxiety, stress, and depression are the most common form of mental disorder. Anxiety is a normal response to stress. But, when it becomes excessive, turns to a disastrous psychiatric disorder. Depression is an effect which shows reduction of mental interests and enjoyments through the absence of proper control. It may also show emotional and cognitive difficulties. It includes the central nervous system depression activity by showing sedative, hypnotic, and tranquilizer, and anxiolytic properties. Various methods like hole cross, open field, thiopental sodium-induced sleep, elevated plus-maze (EPM) tests are used to obtain the actual analytical result. Anxiety disorders affect about 40 million American adults age 18 years and older (about 18%). It is also the most common emotional disorders affecting people in all countries worldwide. It is reported that more than 20% of the adult population suffer from these conditions at some stage during their life (Abid, Hrishikeshavan, & Asad, Citation2006; Buller & Legrand, Citation2001; Titov, Andrews, Kemp, & Robinson, Citation2010; Yadav, Kawale, & Nade, Citation2008). Antioxidants provides great protection to the physiological system of our body through protecting cells from Reactive Oxygen Species (ROS), such as superoxide, peroxide, peroxynitrite, lipid peroxidation. On the contrary, antioxidants protect the cell from oxidative stress and notorious ROS that can cause great harm to the cell, destroy the cell, and causes various diseases, such as cardiovascular diseases, renal, hepatic diseases (Díaz-Muñoz, Álvarez-Pérez, & Yáñez, Citation2006; Rodrigo, Libuy, Feliú, & Hasson, Citation2013; Takimoto & Kass, Citation2007; Zweier & Talukder, Citation2006). In this study, methanolic plant extract of T. sarmentosa was evaluated to find its in vivo neuropharmacological and in vitro antioxidant activities.
2. Materials and methods
2.1. Plant material
The leaves of T. sarmentosa were collected from Hathazari area of Chittagong district, Bangladesh and authenticated by Dr. Shaikh Bokhtear Uddin, associate professor, Department of Botany, University of Chittagong. A voucher specimen has been deposited at the Department of Pharmacy, International Islamic University, Chittagong, Bangladesh.
2.2. Preparation of extract
The leaves were dried for a period of two weeks under shade and ground, followed by grounding to course powder. The ground leaves (250 gm) were soaked in sufficient amount of methanol (1:3) for one week at room temperature with an occasional shaking and stirring then filtered through a cotton plug followed by Whitman filter paper (NO. 1). The solvent was evaporated under reduced pressure at room temperature to yield semisolid. The extract was then preserved in a refrigerator till further use.
2.3. In-vivo neuropharmacological activity
2.3.1. Experimental animals
Swiss Albino mice weighing 25–30 gm of both male and female were collected from International Center for Diarrheal Diseases Research, Bangladesh (ICDDRB) and housed in polypropylene cages under controlled conditions. The animals were exposed to alternative 12:12 h light and dark cycle at an ambient temperature of 26 ± 2°C. Animals were allowed free access to drinking water and pellet diet, collected from ICDDRB, Dhaka. Mice were acclimatized for seven days in the laboratory environment prior to the study. The set of rules followed for animal experiment were approved by the institutional animal ethics committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh according to governmental guidelines (Zimmermann, Citation1983).
2.3.2. Experimental design
The animals were randomly divided into four groups and each group consisting of five mice. The test groups received methanolic leaf extract of T. sarmentosa at the doses of 200 and 400 mg/kg while positive control was treated with diazepam (1 mg/kg) and control with vehicle (1% Tween 80 in water).
2.3.3. Open field test
The method described by Gupta was slightly modified and used for screening depressive action of the test drugs on CNS in mice. The animals were divided into control, positive control, and test groups. The test groups received T. sermentosa methanolic leaf extracts at the doses of 200 and 400 mg/kg body weight orally whereas the control group received vehicle (1% Tween 80 in water) and reference standard drug Diazepam at the dose of 1 mg/kg (i.p). The floor of an OFT of half square meter was divided into a series of squares each alternatively colored black and white. The apparatus had a 40 cm height wall. The number of squares visited by the animals was counted for 3 min, at 0, 30, 60 and 90 min during the study period (Gupta, Dandiya, & Gupta, Citation1971).
2.3.4. Hole cross test (HCT)
The test was observed by the method described by Takagi et al. for screening CNS depressant activity in mice. The animals were divided into three groups—control, positive control, and test. The test groups received methanol extract of T. sarmentosa at the doses of 200 and 400 mg/kg body weight orally whereas the control group received vehicle (1% Tween 80 in water). A steel partition was fixed in the middle of a cage having a size of 30 × 20 × 14 cm. A hole of 3 cm diameter was made at a height of 7.5 cm in the center of the cage. The number of passages of a mouse through the hole from one chamber to other was counted for a period of 3 min on 0, 30, 60, 90, and 120 min after the oral treatment with test drugs. Diazepam was used in the positive control group as reference standard at the dose of 1 mg/kg (i.p) (Takagi, Watanabe, & Saito, Citation1971).
2.3.5. Thiopental sodium-induced sleeping time test
The experiment was conducted following the method described by Ferrini et al. The animals were randomly divided into three groups consisting of five mice each. The test groups received methanol extract from the leaves of T. sarmentosa at a dose of 200 mg/kg and 400 mg/kg (p.o) body weight while the standard group was treated with diazepam (1 mg/kg, p.o) and control group with vehicle (1% Tween 80 in water). Twenty minutes later, thiopental sodium (40 mg/kg, i.p) were administered to each mice to induce sleep. The animals were observed for the latent period (time between thiopental administrations to loss of righting reflex) and duration of sleep i.e. time between the loss and recovery of righting reflex (Ferrini, Miragoli, & Taccardi, Citation1974).
2.3.6. Elevated plus-maze (EPM test)
The elevated plus maze (EPM) is a rodent model of anxiety that is used as a screening test for putative anxiolytic or anxiogenic compounds and as a general research tool in neurobiological anxiety research. The EPM apparatus consists of two open arms (5 × 10 cm) and two closed arms (5 × 10 × l5 cm) radiating from a platform (5 × 5 cm) to form a plus sign figure. The apparatus was situated 40 cm above the floor (Lister, Citation1987). The open arms edges were 0.5 cm in height to keep the mice from falling and the closed-arms edges were 15 cm in height. Sixty minutes after administration of the test drugs, each animal was placed at the center of the maze facing one of the enclosed arms. During the 5 min test period, the number of open and enclosed arms entries was recorded (Pellow & File, Citation1986). Entry into an arm was defined as the point when the animal places all four paws onto the arm. The procedure was conducted in a sound attenuated room; observations made from an adjacent corner (Braida, Prana, & Hiberty, Citation2009; Braida et al., Citation2008).
2.3.7. Acute oral toxicity test
An acute oral toxicity test was performed according to the “Organization for Environmental Control Development” guidelines (OECD: Guidelines 420; Fixed Dose Method). Swiss Albino mice (n = 5) (both male and female) overnight fasted for 18 h were used for the study. Different doses of methanolic plant extract were administered orally into the mice. The maximum given dose was 600 mg/kg body weight. Then the animals were observed for the first three hours of administration and mortality recorded within 48 h. No mortality was observed at maximum dose which is 600 mg/kg.
2.3.8. Evaluation of antioxidant effect
A number of assays such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay, ferric reducing power assay, and total phenolic content (TPC) tests were performed for the evaluation of antioxidant properties of the plant extract.
2.3.9. DPPH free radical scavenging assay
Free radical scavenging activity of methanolic extract of T. sarmentosa was determined according to the Brand-Willium (Brand-Williams, Cuvelier, & Berset, Citation1995) method with a slight modification. This activity was determined spectrophotometrically by taking absorbance of DPPH at 517 nm. Then the % of free radical inhibition was calculated using following equation:
Lower the absorbance with high concentration of extract indicates potential antioxidant activity of test sample. Ascorbic acid was used as a reference standard.
2.3.10. Reducing power assay
Reducing power capacity of the methanolic extract of T. sarmentosa was determined according to Oyaizu (Citation1986) method. The amount of ferrous complex was determined spectrophotometrically by taking absorbance at 700 nm where plant extracts having excellent antioxidant property show greater absorbance with the higher concentration of extract solution. Ascorbic acid was used as a reference standard.
2.3.11. Total phenolic content (TPC) determination
TPC in the plant extract was determined by following the Folin-ciocalteu method (Slinkard & Singleton, Citation1977). TPC was determined spectrophotometrically by taking absorbance at 760 nm. Standard Gallic acid solution of different concentrations with same procedure were used to prepare a calibration curve by plotting the absorbance against their respective concentrations from where a standard equation was formulated to determine the unknown concentration of Gallic acid equivalent (GAE) phenolic concentration of the sample by putting the value of absorbance in the equation. Then the TPC was determined by following equation:
2.3.12. Statistical analysis
The data were expressed as mean ± standard error of mean (S.E.M.). Statistical comparisons were performed using one-way ANOVA followed by Dunnett’s multiple comparison test. The values obtained were compared with the vehicle control group and were considered statistically significant when p < 0.05.
3. Results
3.1. In-vivo neuropharmacological activity
3.1.1. Open field test
In the OFT, the extract showed a decrease in locomotion in the test animals at both dose levels (200 and 400 mg/kg body weight). The depressant activity was slowly reduced with time. The results were dose-dependent & statistically significant (Table ).
Table 1. CNS depressant activity of TSME in OFT test
3.1.2. HCT
In the animal treated with methanol extract at 400 mg/kg dose showed a dose-dependent reduction in the locomotor activity and at higher dose, it was comparable with that of standard drug diazepam. Diazepam was used as the standard drug in the experimental animals to evaluate the CNS depressant effect of the plant extract. The extract produced reduction in spontaneous motor activity, and this effect may be attributed to CNS depression, as depression of locomotor activity is common to most neuroleptics. The CNS was depressed till observation and the results were statistically significant (Table ).
Table 2. CNS depressant activity of TSME in hole cross test
3.1.3. Thiopental sodium-induced sleeping time test
In the thiopental-induced hypnosis test, the extract at doses, 200 and 400 mg/kg showed a significant reduction in the time of onset of sleep in a dose-dependent manner. The effect of the extract (200 and 400 mg/kg) on the onset of sleep were comparable to that of the standard. In our study, 400 mg/kg dose of TSME produced quick onset of sleep and prolonged duration of sleep than that of 200 mg/kg dose (Table ).
Table 3. Thiopental sodium-induced hypnosis test
3.1.4. Elevated plus-maze (EPM) test
The EPM test is probably the most widely used model of animal anxiety. A substance which has anxiolytic effect generally increases time and proportion of entrance into the open arms when treated animals are exposed to EPM. Our present results showed that the treatment with T. sarmentosa extract decreased the percentage of entries of mice into the open arm and percentage of time spent in open arm compared to standard drug diazepam. Reference drug diazepam showed significant anxiolytic effect than T. sarmentosa (Table ).
Table 4. EPM test for the evaluation of anxiety
3.2. Evaluation of in vitro antioxidant effect
3.2.1. DPPH free radical scavenging assay
The DPPH radical scavenging activity of T. sarmentosa is shown in Table . This activity was found to increase with increasing concentration of the extract. DPPH antioxidant assay is based on the ability of DPPH, a stable free radical, to decolorize in the presence of antioxidants. In Table , IC50 value of the extract was 151.56 μg/ml as compared to that of ascorbic acid (IC50 23.53 μg/ml) which is a well-known antioxidant.
Table 5. DPPH scavenging activity of Ascorbic acid and TSME
3.2.2. Reducing power capacity
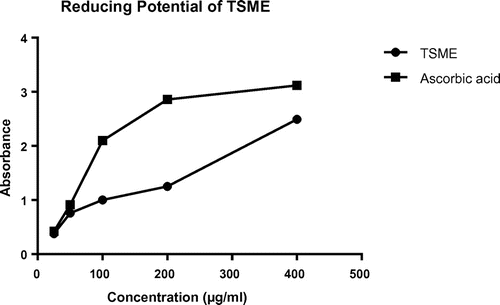
Reducing power is related with the antioxidant effect. Components containing reducing power decreases the oxidized intermediated of lipid peroxidation process. The amount of ferrous complex was determined spectrophotometrically by taking absorbance at 700 nm while the TSME displayed increased absorbance with the increasing concentrations of the extract solution demonstrating that the plant had significant reducing power capacity and the results have been shown in Figure . The extract increased the absorbance significantly, demonstrating the antioxidant potential of TSME. The plant extract showed activity with an absorbance of 2.49 and 1.00 at 400 and 100 μg/ml concentration, respectively, which was appeared to be comparable to the activity of the reference standard ascorbic acid that gave an absorbance of 3.12 and 2.10 at the same concentration.
3.2.3. Determination of TPCs
Phenolic compounds are important plant constituents, having excellent antioxidant properties due to their hydroxyl groups that act as free radical terminator. The TPC in the methanolic extract was expressed as the number of GAE. Final result of TPC in extract was expressed as mg GAE/gm of dry extract. In our current study, the TPC of TSME was found to be 140.34 ± 1.56 GAE mg/gm dry extract (Table ).
Table 6. Determination of total phenolic content as GAE mg/gm extract
4. Discussion
Anxiety and depression-related disorders are increasing day by day all over the world. In 1990, people suffered from anxiety or depression-related disorder were 416 million (World Health Organization, Citation2016). In 2013, it is almost increase by closely 50% which is 615 million (World Health Organization, Citation2016). Humanitarian emergencies and ongoing conflict add further to the need for scale up of treatment options. WHO estimates that, during emergencies, as many as 1 in 5 people are affected by depression and anxiety (World Health Organization, Citation2016). The CNS depressant activity obtained for extract was evidenced from the suppression of the number of squares traveled by the mice in the test group throughout the study period. For both OFT and HCT, reduced movements of mice were observed in the treatment group especially at 400 mg/kg dose, which indicates the sedative action of the test extract. In TIST, the extract at both doses showed a significant reduction in the time of onset of sleep in a dose-dependent manner. EPM is a popular test for anxiolytic-related behavioral assessment. Compared to the reference drug diazepam test extract showed minor anxiolytic action at highest dose which is 400 mg/kg dose. On experimenting, it was found that IC50 value of the extract was 151.56 μg/ml as compared to that of ascorbic acid (IC50 23.53 μg/ml) which is a well-known antioxidant in the case of DPPH radical scavenging assay. Reducing power test of this plant extract showed a promising activity with an absorbance of 2.49 at 400 μg/ml compared to reference standard ascorbic acid that gave an absorbance of 3.12 at the same concentration. In our current study, the TPC of TSME was found to be 140.34 ± 1.56 GAE mg/gm dry extract which demonstrated strong reduction capability.
Many research showed that plant containing phenols, flavonoids, saponins, and tannins are useful in many CNS disorders (Bhattacharya & Satyan, Citation1997). Phytochemical investigations also showed the presence of phenols, flavonoids, saponins, and steroids in this plant (Mungole & Chaturvedi, Citation2011). So might be this phytoconstituents are responsible for its antioxidant activity. The oxidative imbalance by ROS such as superoxide, peroxide, peroxynitrite, nitric oxide, lipid peroxidation plays a key role in anxiety development. Studies in both humans and animals have shown a strong correlation between anxiety and ROS. It has been suggested that a medicine or therapy specifically focus in reducing ROS production may have a beneficial effect in reducing anxiety. However, the neurobiological pathways underlying the effect of oxidative stress on anxiety symptoms are not fully comprehended. The challenge now is to identify the oxidative stress mechanisms likely to be involved in the induction of anxiety symptoms and produce a therapy or medicine which may prevent this disorder. Antioxidants are great source to neutralize the free radicals in lipid chains by contributing a hydrogen atom usually from a phenolic hydroxyl group, which in turn converts phenolic groups into stable free radicals that do not initiate or propagate further oxidation of lipids (Uttara, Singh, Zamboni, & Mahajan, Citation2009). These findings suggest that the plant could be useful as a therapeutic agent for anxiety-related disorders. Further investigations are needed to elucidate the prime constituents of this plant.
6. Conclusion
From the above experiments, it could be concluded that methanolic extract of T. sarmentosa contains significant neuropharmacological and antioxidant activities. To elucidate the exact mechanism action and bioactive compounds responsible for the neuropharmacological and antioxidant activites of this plant extract, further pharmacological studies must be performed.
Ethical approval
The set of rules followed for animal experiment were approved by the institutional animal ethics committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh according to governmental guidelines.
Funding
The authors received no direct funding for this research.
Acknowledgments
The authors are grateful to Department of Pharmacy, International Islamic University Chittagong for giving all facilities to carry out the whole project successfully. Authors also convey their gratitude to the committee of Animal Research Branch of the International Centre for Diarrheal Disease and Research, Bangladesh (ICDDRB) for providing experimental rats.
Additional information
Notes on contributors
Mohammad Nazmul Alam
Mohammad Nazmul Alam is the corresponding author of this experiment. He has completed his Master of Pharmacy degree with Thesis from the Department of Pharmaceutical Sciences, North South University and Bachelor of Pharmacy (Honors) degree from the Department of Pharmacy, International Islamic University Chittagong. His research interest is the exploration of Pharmacological activities, such as in vivo neuropharmacological effect, in vitro antioxidant effect of different plant extracts. He is also doing research work on Alzheimer’s disease, Isoprenaline-induced cardiovascular dysfunction in aged rats, high fat diet-induced obesity in rats, CCl4-induced cirrhotic liver in rats. His work includes prevention of inflammation, oxidative stress, cardiac remodeling, obesity, dyslipidemia, fibrosis using various natural products and safer chemical compounds for living creatures.
References
- Abid, M., Hrishikeshavan, H. J., & Asad, M. (2006). Pharmacological evaluation of Pachyrrhizus Erosus (L.) seeds for central nervous system depressant activity. Indian Journal of Physiology and Pharmacology, 50, 143–151.
- Bangladesh Ethnobotany Online Database. (n.d.). Tetracera sarmentosa. Retrieved from February 21, 2016, from http://www.ebbd.info/tetracera-sarmentosa.html
- Bhattacharya, S. K., & Satyan, K. S. (1997). Experimental methods for evaluation of psychotropic agents in rodents: I-Anti-anxiety agents. Indian Journal of Experimental Biology, 35, 565–575.
- Braida, B., Prana, V., & Hiberty, P. C. (2009). The physical origin of Saytzeff’s rule. Angewandte Chemie International Edition, 48, 5724–5728.10.1002/anie.v48:31
- Braida, D., Limonta, V., Capurro, V., Fadda, P., Rubino, T., Mascia, P., Zani, A., Gori, E., Fratta, W., Parolaro, D., & Sala, M. (2008). Involvement of κ-opioid and endocannabinoid system on salvinorin a-induced reward. Biological Psychiatry, 63, 286–292.10.1016/j.biopsych.2007.07.020
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology., 28, 25–30.10.1016/S0023-6438(95)80008-5
- Buller, R., & Legrand, V. (2001). Novel treatments for anxiety and depression: Hurdles in bringing them to the market. Drug Discovery Today, 6, 1220–1230.10.1016/S1359-6446(01)02043-8
- Da, F., Zhu, H., Zhao, X., Teng, J., & Gan, J. (2014). Analysis of the chemical components of essential oils from leaves of Tetracera sarmentosa by GC-MS. Medicinal Plant, 5, 27–28.
- Díaz-Muñoz, M., Álvarez-Pérez, M. A., & Yáñez, L. (2006). Correlation between oxidative stress and alteration of intracellular calcium handling in isoproterenol-induced myocardial infarction. Molecular and Cellular Biochemistry, 289, 125–136.10.1007/s11010-006-9155-1
- Doughari, J. H., Ndakidemi, P. A., Human, I. S., & Benade, S. (2012). Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. Journal of Ethnopharmacology, 141, 1041–1050.10.1016/j.jep.2012.03.051
- Fernando, T., Ratnasooriya, W. D., & Deraniyagala, S. A. (2009). Antinociceptive activity of aqueous leaf extract of Tetracera sarmentosa L. in rats. Pharmacognosy Research, 1, 381–386.
- Ferrini, R., Miragoli, G., & Taccardi, B. (1974). Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittel Forschung, 24, 2029–2032.
- Flora of China [FOC]. (n.d.). Tetracera sarmentosa (Vol. 12, page 331). Author. Retrieved February 22, 2016, from http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242351569
- Gupta, B. D., Dandiya, P. C., & Gupta, M. L. (1971). A psycho-pharmacological analysis of behaviour in rats. The Japanese Journal of Pharmacology, 21, 293.10.1254/jjp.21.293
- Lister, R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl), 92, 180–185.
- Mahboubi, M., Haghi, G., Kazempour, N., & Hatemi, A. R. (2013). Total phenolic content, antioxidant and antimicrobial activities of Blepharis edulis extracts. Songklanakarin Journal of Science and Technology, 35, 11–16.
- Ming, K. J., Khang, G. N., Sai, C. L., & Fatt, C. T. (2003). Recent advances in traditional plant drugs and orchids. Acta Pharmacologica Sinica, 24, 7–21.
- Mungole, A., & Chaturvedi, A. (2011). Hibiscus sabdariffa L. a rich source of secondary metabolites. International Journal of Pharmaceutical Sciences Review and Research, 6, 83–87.
- Oyaizu, M. (1986). Studies on product of browning reaction prepared from glucose amine. The Japanese Journal of Nutrition and Dietetics, 44, 307–315.10.5264/eiyogakuzashi.44.307
- Pellow, S., & File, S. E. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacology Biochemistry and Behavior, 24, 525–529.10.1016/0091-3057(86)90552-6
- Rodrigo, R., Libuy, M., Feliú, F., & Hasson, D. (2013). Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Disease Markers, 35, 773–790.10.1155/2013/974358
- Slinkard, K., & Singleton, V. L. (1977). Total phenol analyses: Automation and comparison with manual methods. American Journal of Enology and Viticulture, 28, 49–55.
- Takagi, K., Watanabe, M., & Saito, H. (1971). Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. The Japanese Journal of Pharmacology, 21, 797–810.10.1254/jjp.21.797
- Takimoto, E., & Kass, D. A. (2007). Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension, 49, 241–248.
- Titov, N., Andrews, G., Kemp, A., & Robinson, E. (2010). Characteristics of adults with anxiety or depression treated at an internet clinic: Comparison with a national survey and an outpatient Clinic. PLoS ONE, 5(5), e10885.10.1371/journal.pone.0010885
- Uttara, B., Singh, A. V., Zamboni, P., & Mahajan, R. (2009). Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology, 7, 65–74.10.2174/157015909787602823
- World Health Organization. (2016, April). Investing in treatment for depression and anxiety leads to fourfold return. Washington, DC: Lancet Psychiatry.
- Yadav, A. V., Kawale, L. A., & Nade, V. S. (2008). Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian Journal of Pharmacology, 40, 32–36.
- Zimmermann, M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109.10.1016/0304-3959(83)90201-4
- Zweier, J. L., & Talukder, M. A. H. (2006). The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research, 70, 181–190.10.1016/j.cardiores.2006.02.025