Abstract
We evaluated the lethality, uptake, depuration, accumulation, and effects of waterborne arsenic in Lanzhou catfish (Silurus lanzhouensis). The 96-h LC50 and safe concentrations (SC) for Lanzhou catfish were 12.88 and 1.288 mg/L, respectively. We evaluated the effects of chronic exposure to 0 mg/L (C), 1.288 mg/L (T1), 0.5 mg/L (T2), and 0.1 mg/L (T3) and measured depuration rates post-exposure. As accumulated in the target organs in the following order of concentration: gill > muscle > brain > liver, which is consistent with the variation in k1. The values for k1 and CAmax declined with a decrease in arsenic concentration in the different target organs, whereas the reverse was true for BCF. The CL,50(t) values decreased initially and then approached equilibrium status after 30 of exposure. The gill tissue had the highest depuration rates, followed by muscle, brain, and liver. The treatment groups exposed to lower arsenic concentrations treats had lower k2 values in the target organs, but higher depuration half-lives (t1/2) at lower arsenic concentrations. Our results demonstrate that the target organs of Lanzhou catfish are capable of regulating arsenic toxicity by way of internal regulation mechanisms, and the rate of arsenic uptake and depuration over time are concentration- and tissue-dependent.
Public Interest Statement
Arsenic (As) is a heavy metal and highly toxic to animals, plants, and humans. Arsenic toxicity has been evaluated for some aquaculture species, but until now, little was known about the mechanisms of arsenic toxicity in freshwater fishes, especially the fishes without scales. To assess the lethality, distribution, and kinetics of waterborne As, the study evaluated the uptake of As, its elimination and its distribution in the target organs of Lanzhou catfish. This is the first study to document bioaccumulation and depuration of As in the catfish.
Competing Interests
The authors declare no competing interest.
1. Introduction
Arsenic (As) is a heavy metal that is relatively common in the environment as a consequence of both anthropogenic and natural processes. Arsenic is classified as a top environmental chemical concern by Petrusevski, Sharma, Schippers, and Shordt (Citation2007). United States Food and Drug Administration (Citation1993) noted that 90% of total arsenic exposure is derived from fish and other seafood. But one of the major challenges for assessing the potential risk of heavy metals to aquatic organisms is predicting the time-dependent internal effective dose that causes the toxic effects. The response of an organism is not merely determined by the dose–response relationship, but is also affected by the ability for biological regulation or detoxification during long-term exposures (Schuler, Landrum, & Lydy, Citation2004; Vijver, van Gestel, Lanno, van Straalen, & Peijnenburg, Citation2004). A number of models have been developed to predict toxicity to aquatic organisms by integrating the mechanistic process between accumulated chemical dose and the induced dynamics of tissue damage and recovery. These include the time-integrated concentration toxicity model (TIC), the concentration–time toxicity model (CT), the uptake–depuration toxicity model (UD), (Liao & Lin, Citation2001), the damage assessment model (DAM) (Lee, Landrum, & Koh, Citation2002), and the biotic ligand model (BLM) (Tsai, Chen, Ju, & Liao, Citation2009). All these models are based on a well-established one-compartment bioaccumulation model.
Lanzhou catfish (Silurus lanzhouensis), commonly known as the Yellow River catfish, are native to China and are distributed throughout the Ningxia section of the Yellow River. The species is widely cultured in China and has a high market value. Due to water pollution, overfishing, construction of water conservancy facilities, and other factors, wild Lanzhou catfish populations have declined in abundance recently and species is now listed on the China Species Red List (Wang & Xie, Citation2009). The declines are largely Because of the value of this species, it has been the focus of considerable research in recent years in China. In recent years, we have discovered and cloned four antitoxicity-related genes specific for Lanzhou catfish. These genes may help us to understand the antitoxicity mechanisms used by aquatic animals and take measures to protect this rare species. At present, to our knowledge, little is known about the uptake and effects of arsenic in Lanzhou catfish and, likewise, little is known about the actual uptake and elimination processes or on the kinetics of arsenic.
We evaluated the uptake of As, its elimination and its distribution in the target organs of Lanzhou catfish exposed to As-contaminated water at environmentally relevant concentrations in the laboratory. This is the first study to document bioaccumulation and depuration of As in the Lanzhou catfish, and to assess the lethality, distribution, and kinetics of waterborne As.
2. Materials and methods
2.1. Fish and water quality
The number of 1200 Lanzhou catfish (S. lanzhouensis) (mean body length = 5.437 ± 1.21 cm and mean body weight = 4.32 ± 0.85 g wet wt) were obtained from the Ningxia Fisheries Research Institute, Yinchuan, China. These fish were tested for arsenic contamination and the levels were non detectable. The fish were also visibly free of any lesions, deformities, or disease. This study was performed with the approval of the local ethical committee and all the experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were carried out in 80-L indoor rectangular plastic aquaria filled with 60 L water. The experimental water was filtered and aerated tap water (pH = 7.3 ± 0.27, alkalinity = 186.65 ± 22.4 mg/L, and Cl−1 < 13 mg/L, K+, Na+ < 24.3 mg/L, Ca2+ < 29.5 mg/L, p < 0.01 mg/L, H2S, Hg, and As not detected). Dissolved oxygen in each tank was maintained at close to saturation (7.21 ± 0.1 mg/L) by aeration. The temperature was maintained at 24.4 ± 0.5°C using submerged heaters in each aquarium.
2.2. Acute toxicity assays
Laboratory bioassays were conducted to determine the 24-, 48-, 72-, and 96-h LC50 values for Lanzhou catfish exposed to sodium arsenite (NaAsO2). Thirty fish were randomly selected and transferred into each test aquarium. After a 7-d acclimation period, the catfishes were exposed to one of five logarithmically spaced concentrations of As. All treatments were conducted in triplicate and the control group consisted of catfishes held in the experimental aquaria without the addition of As. The exposure concentrations were chosen based on the results of a preliminary test. The nominal concentrations of arsenic we tested were 0 (control), 6.67, 10, 15, 22.5, and 33.75 mg/L. The measured arsenic concentrations were 0.67 ± 0.02, 9.96 ± 0.05, 15.06 ± 0.05, 22.55 ± 0.10, and 33.68 ± 0.08 mg/L.
To maintain water quality, the fish were not fed throughout the test and the entire arsenic solution was replaced daily in each tank. Gross mortality of fish in each tank was recorded every hour for the first 12 h and every 2 h thereafter for 96 h. Dead fish were removed every 1–2 h.
2.3. Chronic toxicity
Based on the safe concentration estimated from toxicity testing above, we evaluated the pattern of bioaccumulation and depuration at four arsenic concentrations: 0 mg/L (C), 1.288 mg/L (T1), 0.5 mg/L (T2), and 0.1 mg/L (T3). Thirty fish were stocked into each experimental tank and acclimated to laboratory conditions for 2 weeks before exposure. During the acclimation (14 d) and experimental periods (42 d), the fish were fed with water worms (bait was cultured in our laboratory, Hg and As not detected) once a day for 7 d at a rate of 0.5% of fish biomass. We removed feces every 4 h and collected any uneaten food 1 h after feeding. During the 21-d exposure period, the entire arsenic solution was replaced daily in each tank, the water level was checked in each aquarium every 6 h and distilled water was used to keep levels constant. At the end of the 21-d exposure period, the fish were held for 21 d to evaluate depuration.
To measure the tissue concentrations of arsenic, three fish were sequentially sampled from each aquarium on days 0, 4, 7, 14, 21, 28, 35, and 42. Each fish was dissected and the gill, muscle, brain, and liver of each individual was removed, weighed, cleaned with deionized water, and individually wrapped in a plastic bag and stored frozen (−20°C) until trace element analysis. Dissected tissues samples were lyophilized and then ground to fine powder in a grinder. A ~500 mg sample of the powder was digested with concentrated nitric acid (7 ml) and perchloric acid (1 ml) in an Acid Digestion Bomb (5 min at 160°C, 35 Bar, 1,000 Watts). After cooling, the samples were diluted to 50 ml with deionized water and evaporated with an Electric Hot Plate (100°C) to a 1-ml solution. We then added 5% ascorbic acid (5 ml) and 5% thiocarbamide (5 ml) and made the volume up to 25 ml with 5% hydrochloric acid. Samples were analyzed by an AFS-230E Atomic Fluorescence Spectrometer to determine As concentrations in all tissues. Concentrations were expressed as μg/g dry weight.
2.4. Data analysis
We used a computerized probit analysis program (United States Environmental Protection Agency, Citation2000) to calculate the LC50 values (24-, 48-, 72-, and 96-h) and upper and lower 95% confidence levels. The 96-h safe concentration (1/10th of the 96-h LC50 value) was calculated following the method of Zhou and Zhang (Citation1989). The uptake rate constant (k1) and the depuration rate constant (k2) of arsenic were calculated using nonlinear regression based on the chronic-toxicity data. The organ-specific bioconcentration factor (BCF) was calculated using the formula BCF = k1/(k2 + g) (Falusi & Olanipekun, Citation2007), the maximal content in the organism (CAmax) at the theoretic equilibrium was estimated using the formula CAmax = BCF × CW. Depuration half-life (t1/2) was calculated as t1/2 = ln2/k2.
We used an AUC-based TIC toxicity model to estimate the internal lethal body burden [CL,50(t)] associated with 50% mortality (Liao et al., Citation2004). All data are presented as the mean ± SE. We tested for differences between groups using a one-way ANOVA (α = 0.05) followed by Duncan’s multiple range test.
3. Results
3.1. Arsenic acute-toxicity analysis
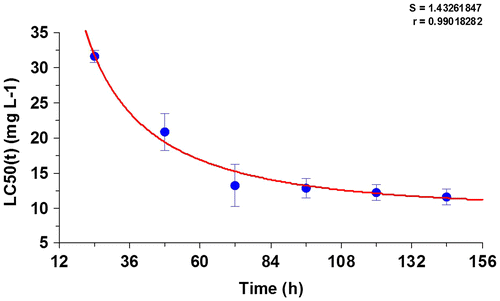
The LC50 values for the 24-, 48-, 72-, 96-, 120-, and 144-h exposures to arsenic ranged from 31.72 to 11.54 mg/L (Table ). The LC50(t) decreased as the duration of exposure increased. The safe concentration for a 96-h exposure to sodium arsenite was 1.288 mg/L in Lanzhou catfish.
Table 1. LC50 of arsenic in Lanzhou catfish at selected time intervals
3.2. As bioaccumulation and depuration
During the exposure phase, arsenic concentrations in the gill, muscle, brain, and liver varied significantly with time (F3,41 = 8.91, 7.29, 5.94, and 18.75, p < 0.01), between treatments (F3,41 = 35.09, 44.37, 502.28, and 43.95, p < 0.01), and with the interaction between treatment and time (F1,3 = 20.04, 28.79, 11.07, and 313.79; p = 0.02, 0.01, 0.05, and 0), respectively. After a 21-d exposure, As concentrations were much higher in the gill than in other target organs of Lanzhou catfish at each of the exposure concentrations (F(T1)3,79 = 555.66, F(T2)3,79 = 707.24, F(T3)3,79 = 559.28, p < 0.01). The order of accumulation was: gill > muscle > brain > liver. With the exception of the liver in T3 (F3,8 = 1767.69, p > 0.05), there was a significant difference in arsenic concentrations in each target organ between all three arsenic exposure treatment groups and the control at the end of the exposure period (Figure ).
Figure 1. Changes in As concentrations in the brain, gill, liver, and muscle during the exposure phase and recovery phase. Values represent the mean S.E. of triplicate subsamples. Values at a given time that are significantly different are indicated by different letters (p < 0.05).
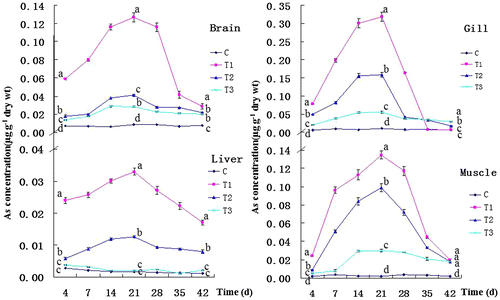
During the recovery interval, arsenic concentrations in the gill, muscle, brain, and liver varied significantly with time (F3,41 = 12.07, 12.76, 16.09, 9.44, p < 0.01), between treatments (F3,41 = 3.84, 152.78, 33.91, and 200.53; p < 0.01), and with the interaction between treatment and time (F1,3 = 3.08, 12.72, and 78.60; p = 0.18, 0.04, 0.003) in brain, muscle, and liver, except for the gill (F1,3 = 2.89; p = 0.19). After 21 d of recovery, arsenic concentrations had returned to normal levels in the gill of T1 (F3,8 = 922.7, p < 0.01) and liver of T3 (F2,6 = 414.62, p < 0.01) (Figure ).
3.3. Target organ As kinetics
The uptake rate constants (k1), the depuration rate constants (k2), and CAmax values, declined with a decrease in arsenic concentrations in the target organs (Table ). However, the BCF values and the depuration half-lives (t1/2) increased with a decrease in arsenic concentrations in the target organs (Table ). Thus, the rates of arsenic uptake and depuration over time are concentration- and tissue-dependent.
Table 2. Kinetic parameters of arsenic for Lanzhou catfish
The gill had higher k1 and BCF values than the remaining target organs in T1, T2, and T3. The k1 values ranged from 0.0219 to 0.1473 mL/g d and the BCF values ranged from 0.70 to 0.79 mL/g. Thus, As tends to accumulate primarily in the gills of Lanzhou catfish. The k1 values for the liver ranged from 0.0012 to 0.0054 mL/g d and the BCF values ranged from 0.18 to 0.20 mL/g, which were the lowest among the organs. Thus, the liver had the lowest capacity to uptake arsenic. The k2 values for the target organs ranged between 0.0061 and 0.2105 d−1, and were in the order gill > muscle > brain > liver. The depuration half-lives (t1/2) ranged from 3.3 to 22.8 d in T1, from 7.3 to 35.2 d in T2, and 25.1 to 113.6 d in T3. Among the target organs, the liver had the highest t1/2 value, indicating that it will take a longer time to eliminate arsenic than the other organs.
3.4. Prediction of the internal lethal body burden
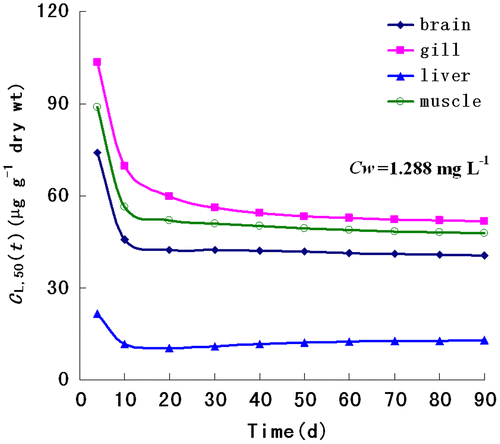
The optimal fit of the TIC toxicity model to the LC50(t) data are listed in Table . The regression coefficients (r2 = 0.98, p < 0.05) demonstrate the quality of fit for the TIC toxicity model (Figure ). Based on the TIC toxicity model, the internal lethal body burden [CL,50(t)] for each target organ at the site of action that causes 50% mortality was predicted assuming an exposure to 1.288 mg/L waterborne arsenic. The CL,50(t) values decreased initially and then approached an equilibrium after 30-d exposure (Figure ). The equilibrium CL,50(t) values in the gill, muscle, brain, and liver were 56.21, 50.93, 42.35, and 10.98 μg/g, respectively.
4. Discussion
Sures (Citation2003) speculated that teleosts are better adapted to resist metal and non-metal poisoning than are other vertebrates. Arsenic toxicity has been evaluated for some aquaculture species, but until now, little was known about the mechanisms of arsenic toxicity in catfish. We designed a toxicological model experiment to estimate the time–concentration-dependent tolerances of Lanzhou catfish to As toxicity and determine the mechanism of arsenic toxicity. The 96-h LC50 of arsenic to Lanzhou catfish was 12.88 mg/L, which is similar to that for rainbow trout Oncorhynchus mykiss (15.3 mg/L) (Tišler & Zagorc-Končan, Citation2002) and Medaka Oryzias latipes (14.6 mg/L) (Suhendrayatna, Ohki, Nakajima, & Maeda, Citation2002), but lower than for tilapia O. mossambicusis (28.68 mg/L: Liao et al., Citation2004; and 26.55 mg/L: Hwang & Tsai, Citation1993) and zebrafish Danio rerio (28.1 mg/L) (Tišler & Zagorc-Končan, Citation2002), but higher than for milk fish Chanos chanos (7.29 mg/L) (Chou, Liao, Lin, & Cheng, Citation2006). In general, the LC50 values of teleost species for arsenic are between 7 and 29 mg/L, depending on age, species, and environment conditions. We hypothesized that Lanzhou catfish would be more susceptible to arsenic uptake and toxicity than fish with larger scales, thereby making them a more suitable bioindicator for studying the accumulation and transformation of arsenic in freshwater organisms.
The TIC toxicity concept has a significant use in evaluating metal toxicity (Liao et al., Citation2004). We used a TIC toxicity model to predict the internal lethal body burden for each target organ at the site of action that causes 50% mortality [CL,50(t) values] in Lanzhou catfish when exposed to 1.288 mg/L waterborne arsenic concentration. The CL,50(t) values decreased initially and then approached an equilibrium after 30 d of exposure, suggesting that the target organs of Lanzhou catfish are capable of regulating arsenic toxicity by way of internal regulation. The AUC-based TIC model successfully predicted the trend in CL,50(t) values, which were consistent with the organ-specific toxic kinetic parameters.
As accumulated in the target organs of Lanzhou catfish in the following order of concentration: gill > muscle > brain > liver. Additionally, the sigmoid pattern of accumulation was steeper in profile in the gill than for the other target organs. The gills are an important site for the accumulation of many metals and organic pollutants. Furthermore, the gills are played a major role in regulating metal toxicity in fish, primarily by regulating against metal ion disruption (Daglish & Nowak, Citation2002). This explains why the gill is more sensitive and of value as a biomarker of exposure to waterborne arsenic in Lanzhou catfish.
Metals typically accumulate preferentially in the liver rather than muscle tissue (Liao et al., Citation2004; Storelli & Marcotrigiano, Citation2000). However, higher values have been reported in the muscle tissue in marine turtles (Storelli, Ceci, & Marcotrigiano, Citation1998) and in fish from the North Sea (De Gieter et al., Citation2002). In our study, the liver had the lowest capacity for arsenic accumulation, which may be a function of rapid arsenic methylation in this tissue. Inorganic arsenic methylation is the primary mechanism by which the body removes arsenic a process that occurs in the liver (Yamauchi & Yamamura, Citation1985). Thus, the rate of arsenic uptake was organ-specific and time-dependent in Lanzhou catfish. The capacity of the different target organs to accumulate arsenic varied depending on the exposure concentration. At the end of the recovery period, the majority of arsenic was cleared in individuals exposed to lower concentrations, except in the muscle. Interestingly, levels returned rapidly to normal during the recovery period in the gill of animals exposed to high concentrations of arsenic. This may have been a function of serious tissue damage, leading to increased cell membrane permeability, and excretion of arsenic by simple diffusion. Consistent with our observations, Calamari, Gaggino, and Pacchetti (Citation1982) reported a marked drop in tissue metal concentrations after the exposure was terminated. Taken together, these observations suggest that fish are able to regulate the concentrations of metals in their organs over time through the processes of absorption, excretion, detoxification, and storage (Chen, Yu, & Liu, Citation2001).
Subathra and Karuppasamy (Citation2008) and Kalay and Canli (Citation2000) demonstrated that the rate of Cu accumulation was higher than the rate of Cu elimination in fish. A similar pattern was reported by Liao et al. (Citation2004) for arsenic in tilapia. In contrast, our results suggest that the opposite is true in Lanzhou catfish. k2 was higher than k1, suggesting that Lanzhou catfish can more easily eliminate As in their body tissue. Thus, we speculate that Lanzhou catfish have a more effective mechanism for arsenic metabolism than other teleosts.
The k1 and k2 values decreased with an increase in waterborne arsenic concentrations. In contrast, Dang, Zhong, and Wang (Citation2009) concluded that k1 and k2 are not significantly affected by the concentration and exposure duration. Luoma and Rainbow (Citation2005) suggested that the assumption of a constant uptake rate might only be applicable in natural conditions or up to concentrations that are an order of magnitude higher than those seen in nature. The uptake rate is likely subject to saturation kinetics at very high concentrations because most of the metal ions pass through the gill membranes via channels or other facilitated diffusion processes (Green, Mirza, Wood, & Pyle, Citation2010). Tsai et al. (Citation2012) observed a decrease in the values of k1 and BCF with an increase in waterborne copper concentrations. Kraemer, Campbell, and Hare (Citation2008) showed that fish can alter their ability to uptake and eliminate metals over a longer time period or at higher concentrations in a field situation. Tsai, Huang, Chen, and Liao (Citation2012) suggested that if the tissue arsenic concentration exceeds the metal influx threshold (CIT) during short-term exposure conditions, the detoxification rate (kdex) will increase with the waterborne metal concentration, whereas if the reverse is true, the value of kdex will tend to be zero. The authors also demonstrated that BCF decreased as waterborne arsenic concentrations (Cw) increased (Cw > 5.1 μg/mL). McGeer et al. (Citation2003) comprehensively reviewed theoretical and experimental data describing BCF for Cu, Zn, Cd, Pb, and other metals, and found an inverse relationship between BCF and aqueous exposure concentrations. Liao et al. (Citation2003) noted that tilapia held in the field had a higher tendency to accumulate As (BCF = 143–421) than did fish from the same population in a 7-d lab bioaccumulation assay (BCF = 1.04–4.19). The lab group was exposed waterborne As concentrations that were ~30 times higher than the field group. Our results also showed an inverse relationship between BCF and exposure concentration. These observations are consistent with a number of studies that have developed metal bioaccumulation models for fish species. The relatively high value of BCF obtained from exposure to lower metal concentrations may result from the active regulation or acclimation by the organism to metals.
Among the various tissues (gill, liver, brain, and muscle) we evaluated for depuration of arsenic in Lanzhou catfish, the half-life was lowest for the gill, followed by brain, muscle, and liver. According to Anderson and Spear (Citation1980), the gills of pumpkin seed sunfish exhibit monophasic elimination of accumulated Cu. Metals that are accumulated in the gills may either be transferred back to water (adsorbed metals) or transferred to other tissues, particularly the liver, for detoxification (Subathra & Karuppasamy, Citation2008). This may explain why metals have a shorter biological half-life in the gills than in the liver. Together, these observations support the view that higher BCF values indicate that species or organ is more effective at the removal of pollutants (Kara & Zeytunluoglu, Citation2007). Muscle tissue is thought to be particularly inefficient at metal elimination. For example, muscle Cu concentrations of Mystus vittatus were not significantly lowered during a 30-d depuration period (Subathra & Karuppasamy, Citation2008). This lack of elimination by fish muscle explains why 90% of total arsenic in food comes from fish and other seafood.
Within an individual fish, the kinetics of metal accumulation and release are expected to be very complex because physical and chemical parameters, water temperature, salinity, diet, fish species, and many other parameters may affect the rate of metal accumulation and release in aquatic animals (Lemus and Chung, Citation1999).
5. Conclusion
Our results demonstrate that the target organs of Lanzhou catfish are capable of regulating arsenic toxicity by way of internal regulation mechanisms, and the rate of arsenic uptake and depuration over time are concentration- and tissue-dependent.
Funding
This work was supported by the External Cooperation Projects of Ningxia.
Additional information
Notes on contributors
Zongqiang Lian
Xudong Wu, PhD, is a professor in the Ningxia Engineering Research Center for Fisheries, Yinchuan, China. He is mainly engaged in environmental toxicology, aquatic animal protection genetics, and fish breeding research. Together with her research team members Zongqiang Lian has extensive publications on the environmental toxicology, aquatic animal protection.
References
- Anderson, P. D., & Spear, P. A. (1980). Copper pharmacokinetics in fish gills—1 kinetics in pumpkinseed sunfish, lepomis gibbosus, of different body sizes. Water Research, 14, 1101–1105.10.1016/0043-1354(80)90159-1
- Calamari, D., Gaggino, G. F., & Pacchetti, G. (1982). Toxicokinetics of low levels of Cd, Cr, Ni and their mixture in long-term treatment on Salmo gairdneri Rich. Chemosphere, 11, 59–70.10.1016/0045-6535(82)90094-7
- Chen, C. M., Yu, S. C., & Liu, M. C. (2001). Use of Japanese medaka (Oryzia latipes) and tilapia (Oreochromis mossambicus) in toxicity tests on different industrial effluents in Taiwan. Archives of Environmental Contamination and Toxicology, 40, 363–370.10.1007/s002440010184
- Chou, B. Y., Liao, C. M., Lin, M. C., & Cheng, H. H. (2006). Toxicokinetics/toxicodynamics of arsenic for farmed juvenile milkfish Chanos chanos and human consumption risk in BFD-endemic area of Taiwan. Environment International, 32, 545–553.10.1016/j.envint.2006.01.004
- Daglish, R. W., & Nowak, B. F. (2002). Rainbow trout gills are a sensitive biomarker of short-term exposure to waterborne copper. Archives of Environmental Contamination and Toxicology, 43, 98–102.10.1007/s00244-002-1184-5
- Dang, F., Zhong, H., & Wang, W. X. (2009). Copper uptake kinetics and regulation in a marine fish after waterborne copper acclimation. Aquatic Toxicology, 94, 238–244.10.1016/j.aquatox.2009.07.011
- De Gieter, M., Leermakers, M., Van Ryssen, R., Noyen, J., Goeyens, L., & Baeyens, W. (2002). Total and toxic arsenic levels in north sea fish. Archives of Environmental Contamination and Toxicology, 43, 406–417.10.1007/s00244-002-1193-4
- Falusi, B. A., & Olanipekun, E. O. (2007). Bioconcentration factors of heavy metals in tropical crab (carcinus sp) from River Aponwe, Ado-Ekiti, Nigeria. Journal of Applied Sciences and Environmental Management, 11, 51–54.
- Green, W. W., Mirza, R. S., Wood, C. M., & Pyle, G. G. (2010). Copper binding dynamics and olfactory impairment in fathead minnows (Pimephale promelas). Environmental Science & Technology, 44, 1431–1437.10.1021/es9023892
- Hwang, P. P., & Tsai, Y. N. (1993). Effects of arsenic on osmoregulation in the tilapia Oreochromis mossambicus reared in seawater. Marine Biology, 117, 551–558.10.1007/BF00349765
- Kalay, M., & Canli, M. (2000). Elimination of essential (Cu, Zn) and nonessential (Cd, Pb) metals from tissues of a freshwater fish Tilapia zilli. Turkish Journal of Zoology, 24, 429–436.
- Kara, Y., & Zeytunluoglu, A. (2007). Bioaccumulation of toxic metals (Cd and Cu) by Groenlandia densa (L.) Fourr. Bulletin of Environmental Contamination and Toxicology, 79, 609–612.10.1007/s00128-007-9311-7
- Kraemer, L. D., Campbell, P. G. C., & Hare, L. (2008). Modeling cadmium accumulation in indigenous yellow perch (Perca flavescens). Canadian Journal of Fisheries and Aquatic Sciences, 65, 1623–1634.10.1139/F08-081
- Lee, J. H., Landrum, P. F., & Koh, C. H. (2002). Prediction of time-dependent PAH Toxicity in Hyalella azteca using a damage assessment model. Environmental Science & Technology, 36, 3131–3138.10.1021/es011202d
- Lemus, M. J., & Chung, K. S. (1999). Effect of temperature on copper toxicity, accumulation and purification in tropical fish juveniles Petenia kraussii. Caribbean Journal of Science, 35, 64–69.
- Liao, C. M., & Lin, M. C. (2001). Acute toxicity modeling of rainbow trout and silver sea bream exposed to waterborne metals. Environmental Toxicology, 16, 349–360.10.1002/(ISSN)1522-7278
- Liao, C. M., Chen, B. C., Singh, S., Lin, M. C., Liu, C. W., & Han, B. C. (2003). Acute toxicity and bioaccumulation of arsenic in tilapia (Oreochromis mossambicus) from a blackfoot disease area in Taiwan. Environmental Toxicology, 18, 252–259.10.1002/(ISSN)1522-7278
- Liao, C. M., Tsai, J. W., Ling, M. P., Liang, H. M., Chou, Y. H., & Yang, P. T. (2004). Organ-specific toxicokinetics and dose? Response of arsenic in tilapia Oreochromis mossambicus. Archives of Environmental Contamination and Toxicology, 47, 502–510.10.1007/s00244-004-3105-2
- Luoma, S. N., & Rainbow, P. S. (2005). Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environmental Science & Technology, 39, 1921–1931.10.1021/es048947e
- McGeer, J. C., Brix, K. V., Skeaff, J. M., DeForest, D. K., Brigham, S. I., Adams, W. J., & Green, A. (2003). Inverse relationship between bioconcentration factor and exposure concentration for metals: Implications for hazard assessment of metals in the aquatic environment. Environmental Toxicology and Chemistry, 22, 1017–1037.10.1002/etc.v22:5
- Petrusevski, B., Sharma, S., Schippers, J. C., & Shordt, K. (2007). Arsenic in drinking water (Thematic Overview Paper 17). IRC International Water and Sanitation Centre.
- Schuler, L. J., Landrum, P. F., & Lydy, M. J. (2004). Time-dependent toxicity of fluoranthene to freshwater invertebrates and the role of biotransformation on lethal body residues. Environmental Science & Technology, 38, 6247–6255.10.1021/es049844z
- Storelli, M. M., & Marcotrigiano, G. O. (2000). Total organic and inorganic arsenic from marine turtles (Caretta caretta) beached along the Italian Coast (South Adriatic Sea). Bulletin of Environmental Contamination and Toxicology, 65, 732–739.10.1007/s0012800184
- Storelli, M. M., Ceci, E., & Marcotrigiano, G. O. (1998). Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimen beached along the Adriatic Sea (Italy). Bulletin of Environmental Contamination and Toxicology, 60, 546–552.10.1007/s001289900660
- Subathra, S., & Karuppasamy, R. (2008). Bioaccumulation and depuration pattern of copper in different tissues of Mystus vittatus, related to various size groups. Archives of Environmental Contamination and Toxicology, 54, 236–244.10.1007/s00244-007-9028-y
- Suhendrayatna, Ohki, A., Nakajima, T., & Maeda, S. (2002). Studies on the accumulation and transformation of arsenic in freshwater organisms I. Accumulation, transformation and toxicity of arsenic compounds on the Japanese Medaka, Oryzias latipes. Chemosphere, 46, 319–324.10.1016/S0045-6535(01)00084-4
- Sures, B. (2003). Accumulation of heavy metals by intestinal helminths in fish: An overview and perspective. Parasitology, 126, S53–S60.10.1017/S003118200300372X
- Tišler, T., & Zagorc-Končan, J. (2002). Acute and chronic toxicity of arsenic to some aquatic organisms. Bulletin of Environmental Contamination and Toxicology, 69, 421–429.
- Tsai, J. W., Chen, W. Y., Ju, Y. R., & Liao, C. M. (2009). Bioavailability links mode of action can improve the long-term field risk assessment for tilapia exposed to arsenic. Environment International, 35, 727–736.10.1016/j.envint.2009.01.014
- Tsai, J. W., Ju, Y. R., Huang, Y. H., Deng, Y. S., Chen, W. Y., Wu, C. C., & Liao, C-M. (2012). Toxicokinetics of tilapia following high exposure to waterborne and dietary copper and implications for coping mechanisms. Environmental Science and Pollution Research, 20, 3771–3780.
- Tsai, J. W., Huang, Y. H., Chen, W. Y., & Liao, C. M. (2012). Detoxification and bioregulation are critical for long-term waterborne arsenic exposure risk assessment for tilapia. Environmental Monitoring and Assessment, 184, 561–572.10.1007/s10661-011-1988-8
- United States Environmental Protection Agency. (2000). Understanding and accounting for method variability in whole effluent toxicity applications under the national pollutant discharge elimination system (EPA833-R-00-003).
- United States Food and Drug Administration. (1993). Guidance document for arsenic in shellfish. Washington, DC: Author. Retrieved from http://www.cfsan.fda.gov/%7Efrf/guid-as.html
- Vijver, M. G., van Gestel, C. A., Lanno, R. P., van Straalen, N. M., & Peijnenburg, W. J. (2004). Internal metal sequestration and its ecotoxicological relevance: A review. Environmental Science & Technology, 38, 4705–4712.10.1021/es040354g
- Wang, S., & Xie, Y. (2009). China species red list, Vol. 2: Vertebrates part 1 (pp. 294–295). Beijing: Higher Education Press.
- Yamauchi, H., & Yamamura, Y. (1985). Metabolism and excretion of orally administrated arsenic trioxide in the hamster. Toxicology, 34, 113–121.10.1016/0300-483X(85)90161-1
- Zhou, Y. X., & Zhang, Z. S. (1989). Biological Aquatic toxicity test method (pp. 11–27). Beijing: China Agriculture Press.