Abstract
Although essential oils from Douglas fir are popular topical skincare products, research regarding their biological effects on human skin cells is scarce. Here, we studied the biological activity of a commercially available Douglas fir (Pseudotsuga menziesii) essential oil (DEO) in a human dermal fibroblast model of chronic inflammation and fibrosis induced by stimulation with cytokines. Chemical analysis of DEO indicated that its major chemical components (i.e. >5%) were beta-pinene (23%), sabinene (17%), terpinolene (14%), delta-3-carene (11%), and alpha-pinene (9%). We analyzed the effect of DEO on the levels of 17 important protein biomarkers associated with inflammation, immune system modulation, and tissue remodeling. DEO exhibited significant anti-proliferative activity in human fibroblasts. DEO also significantly inhibited the production of vascular cell adhesion molecule 1, collagen III, and plasminogen activator inhibitor 1. We also observed that DEO robustly modulated global gene expression levels in diverse ways. In particular, DEO affected the expression of genes involved in immune modulation and cancer signaling. This study provides the first evidence of biological activity of DEO in human dermal fibroblasts. Our results suggest that DEO may modulate immune responses and tumor signaling processes. Further research about the biological and pharmacological mechanisms of DEO action is recommended.
Public Interest Statement
Essential oils are popular worldwide for skincare purposes. Our study examined the biological effects of Douglas fir essential oil (DEO) in a human skin disease model. The effects of DEO were determined by measuring the levels of biomarkers that are linked to inflammation, immune function, and wound healing. The effects of DEO on genome-wide gene expression were also studied. DEO showed strong anti-proliferative and immune modulatory activities. Notably, DEO affected critical genes and pathways associated with immune modulation and cancer signaling. The findings from this study suggest that DEO is biologically active in human skin cells and it potentially modulates immune response and cancer biology. Thus, this study provides an important stepping stone for further research on DEO and its health benefits in humans.
Competing Interests
Xuesheng Han is employee of dōTERRA, where the studied compound DEO was manufactured.
1. Introduction
Douglas fir (Pseudotsuga menziesii) essential oil (DEO) is typically composed of beta-pinene, sabinene, terpinolene, delta-3-carene, alpha-pinene, and several other aromatic substances in smaller amounts. DEO has been reported to possess antimicrobial and antifungal properties (Johnston, Karchesy, Constantine, & Craig, Citation2001; Tesevic et al., Citation2009). Although DEO has gained popularity in skincare usage, scientific studies of its biological effects on human skin cells are limited. Therefore, we sought to evaluate the biological activity of a commercially available DEO in a human fibroblast model of chronic inflammation and fibrosis. In particular, we studied the effect of DEO on 17 important protein biomarkers that are critical for inflammation, immune modulation, and tissue remodeling. We also analyzed the impact of DEO on genome-wide gene expression profile. This study provides the first evidence of biological activity of DEO in human skin cells. Our data suggest that DEO may modulate immune responses and tumor signaling processes.
2. Materials and methods
All experiments were conducted in HDF3CGF, a biologically multiplexed activity profiling (BioMAP) system, which includes a cell culture of human dermal fibroblasts designed to model chronic inflammation and fibrosis in a robust and reproducible way. The system consists of three components: a cell type, stimuli to create the disease environment, and a set of biomarker (protein) readouts to examine the effect of treatment on disease environment (Berg et al., Citation2010). The methodologies used in this study are similar to those previously described (Han & Parker, Citation2017a, 2017b; Kunkel et al., Citation2004).
2.1. Cell cultures
Primary human neonatal fibroblasts were prepared as previously described (Bergamini et al., Citation2012) and were plated under low serum conditions for 24 h before stimulation with a mixture of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interferon (IFN)-ϒ, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF). The cell culture and stimulation conditions for the HDF3CGF assays have been described in detail elsewhere and were performed in a 96-well plate (Bergamini et al., Citation2012; R Development Core Team, Citation2011).
2.2. Protein-based readouts
An enzyme-linked immunosorbent assay (ELISA) was used to measure the biomarker levels of cell-associated and cell membrane targets. Soluble factors in the supernatants were quantified using either homogeneous time-resolved fluorescence detection, bead-based multiplex immunoassay, or capture ELISA. The adverse effects of the test agents on cell proliferation and viability (cytotoxicity) were measured using the sulforhodamine B (SRB) assay. For proliferation assays, the cells were cultured and quantified after 72 h (which is optimal for the HDF3CGF system), and the detailed procedure has been described in a previous study (Bergamini et al., Citation2012). Measurements were performed in triplicate wells, and a glossary of the biomarkers used in this study is provided in Supplementary Table S1.
Quantitative biomarker data are presented as the mean log10 relative expression level (compared to the respective mean vehicle control value) ±standard deviation of triplicate measurements. Differences in biomarker levels between DEO- and vehicle-treated cultures were tested for significance with the unpaired Student’s t-test. A p value <0.05 outside the significance envelope and with an effect size of at least 10% (more than 0.05 log10 ratio units) was considered statistically significant.
2.3. RNA isolation
Total RNA was isolated from cell lysates using the Zymo Quick-RNA miniprep kit (Zymo Research Corp., Irvine, CA, USA) according to the manufacturer’s instructions. RNA concentration was determined using a NanoDrop ND-2000 system (Thermo Fisher Scientific, Waltham, MA, USA). The RNA quality was assessed using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and an Agilent RNA 6000 Nano kit. All samples had a A260/A280 ratio between 1.9 and 2.1 and RNA integrity number score >8.0.
2.4. Microarray analysis for genome-wide gene expression
The effect of DEO (0.0037%, v/v) on the expression of 21,224 genes in the HDF3CGF system after 24 h of treatment was examined. Samples for microarray analysis were processed by Asuragen Inc. (Austin, TX, USA) according to the company’s standard operating procedures. Biotin-labeled cRNA was prepared from 200 ng total RNA using an Illumina TotalPrep RNA amplification kit (Thermo Fisher Scientific, Waltham, MA, USA) and one round of amplification. The cRNA yields were quantified using ultraviolet spectrophotometry, and the distribution of the transcript sizes was assessed using the Agilent Bioanalyzer 2100. Labeled cRNA (750 ng) was used to probe Illumina human HT-12 v4 expression bead chips (Illumina, Inc., San Diego, CA, USA). Hybridization, washing, staining with streptavidin-conjugated cyanine-3, and scanning of the Illumina arrays were carried out according to the manufacturer’s instructions. The Illumina BeadScan software was used to produce the data files for each array; the raw data were extracted using Illumina BeadStudio software.
The raw data were uploaded into R (R Development Core Team, Citation2011) and analyzed for quality-control metrics using the beadarray package (Dunning, Smith, Ritchie, & Tavare, Citation2007). The data were normalized using quantile normalization (Bolstad, Irizarry, Astrand, & Speed, Citation2003), and then re-annotated and filtered to remove probes that were non-specific or mapped to intronic or intragenic regions (Barbosa-Morais et al., Citation2010). The remaining probe sets comprised the data-set for the remainder of the analysis. The fold-change expression for each set was calculated as the log2 ratio of DEO to the vehicle control. These fold-change values were uploaded onto Ingenuity Pathway Analysis (IPA, Qiagen, Redwood City, CA, USA, www.qiagen.com/ingenuity) to generate the networks and pathway analyses.
2.5. Reagents
DEO (provided by dōTERRA, Pleasant Grove, UT, USA) was diluted in dimethyl sulfoxide (DMSO) to 8X the specified concentrations (final DMSO concentration in culture media was no more than 0.1% [v/v]); 25 μL of each 8X solution was added to the cell culture to a final volume of 200 μL. DMSO (0.1%) served as the vehicle control. Chemical analysis of DEO by gas chromatography–mass spectrometry indicated that its major chemical constitutes (i.e. >5%) are beta-pinene (23%), sabinene (17%), terpinolene (14%), delta-3-carene (11%) and alpha-pinene (9%).
3. Results and discussion
3.1. Biological activity of DEO in pre-inflamed human dermal fibroblasts
We analyzed the biological activity of DEO in the dermal fibroblast cell system HDF3CGF, which features the microenvironment of inflamed human skin cells with boosted inflammatory and immune responses. DEO biological activity was assessed at four different concentrations (0.011, 0.0037, 0.0012, and 0.00041%, v/v). DEO was overly cytotoxic at 0.011% concentration, and thus, effects of only three other concentrations were further analyzed. The expressions of several biomarkers were significantly altered by the exposure of cells to 0.0037% DEO (Figure ).
Figure 1. Bioactivity profile of Douglas fir essential oil (DEO, 0.0037% v/v) in human dermal fibroblast system HDF3CGF.
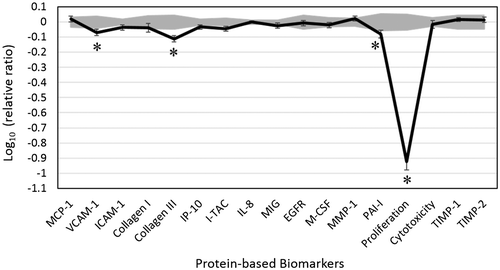
Furthermore, DEO showed significant anti-proliferative activity in dermal fibroblasts. DEO significantly inhibited increase in the production of vascular cell adhesion molecule 1 (VCAM-1), collagen-III, and plasminogen activator inhibitor 1 (PAI-1). VCAM-1 mediates adhesion of monocytes and T cells to endothelial cells, and is considered as an inflammatory biomarker. Collagen-III is an extracellular matrix protein that belongs to the family of fibrillar collagen found in extensible connective tissues. Collagen-III is involved in cell adhesion, cell migration, and tissue remodeling. PAI-1 is critically involved in tissue remodeling and fibrinolysis. The inhibitory effect of DEO on these protein molecules suggests that DEO may possess immunomodulatory property, and thus, may affect wound healing.
Terpinolene, a major active component of DEO, downregulates the expression of serine/threonine protein kinase AKT1 in K562 cells and inhibits cell proliferation (Okumura, Yoshida, Nishimura, Kitagishi, & Matsuda, Citation2012). Reports showed that terpinolene exhibited anticancer and antioxidative effects in rat brain cells, and was therefore suggested to be a potent anti-proliferative agent for brain tumor cells (Aydin, Türkez, & Taşdemir, Citation2013). More recently, the anti-inflammatory effect of terpinolene has been demonstrated in a rat model of chronic inflammation (Macedo et al., Citation2016).
3.2. Effect of DEO on genome-wide gene expression
To better understand the effect of DEO on human cells, we studied the effect of 0.0037% DEO (the highest tested concentration that was not overly cytotoxic to these cells) on RNA levels of 21,224 genes in the HDF3CGF system. We observed that DEO affected expression of a diverse set of human genes. Among the 118 genes whose expression levels were significantly altered by DEO (with a log2 fold-change ratio of expression over vehicle control ≥|1.5|), the majority (81 out of 118 genes) were significantly downregulated, whereas the rest were significantly upregulated (Table S2). A cross-comparison of the protein and gene expression data revealed that collagen III expression was inhibited by DEO at both protein and gene levels.
IPA showed that exposure to DEO significantly affected many canonical signaling pathways from literature-validated databases (Figure ). Many of these pathways are involved in cell cycle control, cancer biology, and immune response. For example, the four most-matched pathways (and genes in these pathways) were inhibited overall by DEO (Figure , Tables S3–S6). These findings indicate that DEO might potentially modulate immune response and cancer biology.
Figure 2. Top 20 canonical pathways that match with the profile of biological effects of Douglas fir essential oil (DEO, 0.0037%, v/v) on gene expression in the HDF3CGF system.
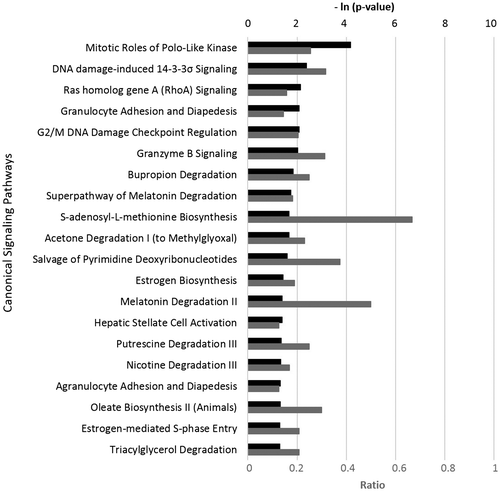
Several earlier studies have suggested that terpinolene might be a potential anticancer agent (Aydin et al., Citation2013; Okumura et al., Citation2012). Studies of other essential oils that have relatively high content of β-pinene and/or sabinene (which are also abundant in DEO) showed promising anticancer and immunomodulatory effects of these compounds (da Silva et al., Citation2016; Krifa et al., Citation2015; Sertel, Eichhorn, Plinkert, & Efferth, Citation2011). Further research is required to explore the biological mechanisms of action of DEO.
The present study has several limitations. Although the disease model was designed to simulate disease biology of chronic inflammation and fibrosis, the in vitro study results cannot be directly correlated with the more complex human system. The impact of DEO on gene expression was only evaluated after short-term intervention. Therefore, the effect of DEO on genome-wide gene expression in longer term remains elusive. Nonetheless, the study provides evidence of the biological effect of DEO on human skin cells based on protein and gene expression data, and will likely stimulate further research into its mechanisms of action.
4. Conclusions
To the best of our knowledge, this is the first study to evaluate the biological activity of DEO in human skin cell culture. DEO significantly inhibited cell proliferation and the production of VCAM-1, collagen-III, and PAI-1. Genome-wide gene expression profile changes indicated that DEO robustly impacted genes and pathways that are critical for cancer signaling, DNA damage response, and immune modulation. These data suggest that DEO may modulate immune responses and cancer signaling. Further investigations into the biological and physiological mechanisms of action of DEO are recommended.
Supplemental data
Supplemental data for this article can be accessed here https://doi.org/10.1080/23312025.2017.1336886.
Funding
This study was funded by dōTERRA (Pleasant Grove, UT, USA) and was conducted at DiscoverX (Fremont, CA, USA).
Supplemental_material.docx
Download MS Word (698.4 KB)Acknowledgment
We thank Editage (www.editage.com) for English language editing.
Additional information
Notes on contributors
Xuesheng Han
Han's group is specifically interested in the efficacy and safety of essential oils and their active components. Our studies of essential oils in both in vitro and clinical settings utilize a variety of experimental approaches, including analytical, biological, biochemical, and biomedical methodologies. The research work discussed in this paper represents one part of a large research project, which was designed to extensively examine the impact of essential oils on human cells. This study, along with others, will further the understanding of the health benefits of essential oils for a wide research audience. Besides essential oils, we are also interested in studying the health benefits of herbal supplements and skin care products. Han holds a PhD in Biological Sciences and is an elected Fellow of the American College of Nutrition.
References
- Aydin, E., Türkez, H., & Taşdemir, S. (2013). Anticancer and antioxidant properties of terpinolene in rat brain cells. Arhiv Za Higijenu Rada I Toksikologiju, 64, 415–424. doi:10.2478/10004-1254-64-2013-2365
- Barbosa-Morais, N. L., Dunning, M. J., Samarajiwa, S. A., Darot, J. F. J., Ritchie, M. E., Lynch, A. G., & Tavaré, S. (2010). A re-annotation pipeline for Illumina BeadArrays: Improving the interpretation of gene expression data. Nucleic Acids Research, 38, e17. doi:10.1093/nar/gkp942 10.1093/nar/gkp942
- Berg, E. L., Yang, J., Melrose, J., Nguyen, D., Privat, S., Rosler, E., … Ekins, S. (2010). Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods, 61, 3–15. doi:10.1016/j.vascn.2009.10.001 10.1016/j.vascn.2009.10.001
- Bergamini, G., Bell, K., Shimamura, S., Werner, T., Cansfield, A., Müller, K., … Neubauer, G. (2012). A selective inhibitor reveals PI3 Kγ dependence of T(H)17 cell differentiation. Nature Chemical Biology, 8, 576–582. doi:10.1038/nchembio.957 10.1038/nchembio.957
- Bolstad, B. M., Irizarry, R. A., Astrand, M., & Speed, T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics, 19, 185–193.10.1093/bioinformatics/19.2.185
- da Silva, J. K. R., Pinto, L. C., Burbano, R. M. R., Montenegro, R. C., Andrade, E. H. A., & Maia, J. G. S. (2016). Composition and cytotoxic and antioxidant activities of the oil of Piper aequale Vahl. Lipids in Health and Disease, 15, 174. doi:10.1186/s12944-016-0347-8 10.1186/s12944-016-0347-8
- Dunning, M. J., Smith, M. L., Ritchie, M. E., & Tavare, S. (2007). Beadarray: R classes and methods for Illumina bead-based data. Bioinformatics, 23, 2183–2184. doi:10.1093/bioinformatics/btm311 10.1093/bioinformatics/btm311
- Han, X., & Parker, T. L. (2017a). Antiinflammatory activity of cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytotherapy Research, n/a–n/a. doi:10.1002/ptr.5822
- Han, X., & Parker, T. L. (2017b). Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharmaceutical Biology, 55, 1619–1622. doi:10.1080/13880209.2017.1314513 10.1080/13880209.2017.1314513
- Johnston, W. H., Karchesy, J. J., Constantine, G. H., & Craig, A. M. (2001). Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. Phytotherapy Research, 15, 586–588.10.1002/(ISSN)1099-1573
- Krifa, M., El Mekdad, H., Bentouati, N., Pizzi, A., Ghedira, K., Hammami, M., … Chekir-Ghedira, L. (2015). Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumor Biology, 36, 5165–5170. doi:10.1007/s13277-015-3170-3 10.1007/s13277-015-3170-3
- Kunkel, E. J., Plavec, I., Nguyen, D., Melrose, J., Rosler, E. S., Kao, L. T., … Berg, E. L. (2004). Rapid structure-activity and selectivity analysis of kinase inhibitors by BioMAP analysis in complex human primary cell-based models. ASSAY and Drug Development Technologies, 2, 431–442. doi:10.1089/adt.2004.2.431 10.1089/adt.2004.2.431
- Macedo, E. M. A., Santos, W. C., Sousa, B. P., Lopes, E. M., Piauilino, C. A., Cunha, F. V. M., … Almeida, F. R. C. (2016). Association of terpinolene and diclofenac presents antinociceptive and anti-inflammatory synergistic effects in a model of chronic inflammation. Brazilian Journal of Medical and Biological Research, 49. doi:10.1590/1414-431X20165103
- Okumura, N., Yoshida, H., Nishimura, Y., Kitagishi, Y., & Matsuda, S. (2012). Terpinolene, a component of herbal sage, downregulates AKT1 expression in K562 cells. Oncology Letters, 3, 321–324. doi:10.3892/ol.2011.491
- R Development Core Team. (2011). R: A language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
- Sertel, S., Eichhorn, T., Plinkert, P. K., & Efferth, T. (2011). Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1). HNO, 59, 1203–1208. doi:10.1007/s00106-011-2274-3 10.1007/s00106-011-2274-3
- Tesevic, V., Milosavljevic, S., Vajs, V., Djordjevic, I., Sokovic, M., Lavadinovic, V., & Novakovic, M. (2009). Chemical composition and antifungal activity of the essential oil of Douglas fir (Pseudosuga menziesii mirb. Franco) from Serbia. Journal of the Serbian Chemical Society, 74, 1035–1040.10.2298/JSC0910035T
