Abstract
Saliva is considered as the front-line of non-invasive diagnostics as novel biomarkers continue to emerge for an array of systemic diseases. Biomarker development pipeline relies heavily on pre-analytical process such as saliva collection, handling, transport and storage. The aim of this study was to systematically evaluate the applicability of MAWI Cell Stabilization (MCS) buffer to transport and store saliva samples at room temperature for downstream applications. Human and bacterial genomic DNA (gDNA) and total protein in saliva samples with and without MCS buffer were quantified for a week at three time-points at room temperature. Based on our findings, MCS buffer was able to preserve human gDNA and total protein within the testing time-points. While bacterial gDNA was accurately preserved, MCS buffer was unable to halt bacterial growth at room temperature. We have identified a non-alcohol-based, non-lytic buffer that could maintain the integrity of both genomic materials and proteins in saliva samples. MCS buffer offers a method to potentially transport and store saliva samples at room temperature, accelerating the translation of salivary assays in remote/rural and resource limited settings.
Clinical significance
We have identified a non-alcohol-based, non-lytic buffer that could maintain the integrity of both genomic materials and proteins in saliva samples.
MCS buffer could potentially substitute the need of cold transport, opening salivary testing in remote and/or resource limited settings.
This functionality enables increased accessibility of samples at a global scale, thus, expends assay coverage and potentially aid in medical discovery at both local and global levels.
PUBLIC INTEREST STATEMENT
Increased recognition of the association between oral and systemic diseases turned the attention to saliva as a promising diagnostic fluid. The composition of whole saliva includes a large number of organic and inorganic biomolecules that facilitate important biological, chemical and physiological functions. These biomolecules can be utilised to monitor and assist in disease detection and progression. However, saliva biomolecules are known to be affected by storage and transport conditions. These factors must be carefully accounted for when utilising saliva as a diagnostic media. This study describes a method to preserve saliva biomolecules for disease management.
1. Introduction
Saliva as a diagnostics medium carries significant advantages due to the ease of collection, non-invasive sampling as well as the ability to accommodate multiple sampling in a given session. However, one of the drawbacks of using saliva as diagnostic fluid for clinical applications is attributed to the low concentration of analytes (Lim, Totsika, Morrison, & Punyadeera, Citation2017, Malamud, Citation2011; Pfaffe, Cooper-White, Beyerlein, Kostner, & Punyadeera, Citation2011). As an example, human C-reactive protein is present at ng/mL levels in blood, whereas the concentration of the same molecule in saliva is in the pg/mL range (Desai & Mathews, Citation2014). The advancement of cutting-edge proteomic and genomic technologies over the past decades has enabled salivary analytes to be detected in a clinically meaningful manner (Iorgulescu, Citation2009; Malamud, Citation2011; Pfaffe et al., Citation2011). The evolution of modern molecular biology research tools continues to instigate new applications of saliva in disease diagnosis, prognosis as well as treatment monitoring for a number of oral and systemic diseases (Lima, Diniz, Moimaz, Sumida, & Okamoto, Citation2010, Rathnayake, Gieselmann, Heikkinen, Tervahartiala, & Sorsa, Citation2017). In recent years, saliva-based assays has grown exponentially, resulting in the development of saliva assays ranging from allergy monitoring to cancer detection (Lim et al., Citation2016; Nunes, Mussavira, & Bindhu, Citation2015; Ovchinnikov et al., Citation2012, Citation2014; Zhang, Dimeski, & Punyadeera, Citation2014; Zhang et al., Citation2013).
Unlike other biospecimens, saliva secretion is real-time and its functions are affected by both internal and external factors. As an example, growth hormone levels are affected by diurnal fluctuations and found to be elevated in the saliva samples collected in the morning (Idris, Wan, Zhang, & Punyadeera, Citation2017). Saliva biomolecular composition is also affected by the type of saliva collection formats (e.g. whole-mouth unstimulated saliva, acid-stimulated saliva, mechanically stimulated saliva, buccal swab and oral rinse), daily oral hygiene treatments as well as nutrients intake (Kolenbrander et al., Citation2002, Lucs, Saltman, Chung, Steinberg, & Schwartz, Citation2013). To reduce the variability in saliva sample collection, corporations such as MAWI DNA Technologies (Hayward, CA, USA), Oasis Diagnostics® Corporation (Vancouver, WA, USA), DNA Genotek Incorporation (Ottawa, ON, Canada) and Norgen Biotek Corporation (Thorold, ON, Canada) have dedicated in developing robust saliva collection and preservation kits (Lim, Sun, Tran, & Punyadeera, Citation2016). The use of commercial saliva collection kits ensures uniform collection in the hands of trained personnel as well as untrained individuals, reducing variations between saliva samples. In addition, certain saliva collection devices such as buccal swabs are able to assist individuals (e.g. children, elderly, xerostomia patients and cancer patients after radiation treatment) who are unable to produce whole mouth unstimulated saliva.
While saliva collection devices eliminate variations from sample to sample collection, saliva transportation and storage are two of the key factors that may influence down-stream analysis. As an example, the protein concentration in saliva samples is 10–15 times lower compared with the plasma samples (Nunes et al., Citation2015). It is therefore crucial that the saliva samples be stored at −80°C freezer or collected on dry ice to reduce protein degradation (Esser et al., Citation2008). In addition, freeze-thawing saliva samples have also been shown to have detrimental effects on the analytes present in saliva. As such, saliva collection kits usually contain preservation buffer to help minimise the degradation of biomolecules in saliva samples, especially during sample transportation and unavoidable freeze-thaw processing prior to analysis. With progressively more saliva assays in the development pipeline, saliva preservation is highly important due to multi-site transportations. This functionality enables increased accessibility of samples at a global scale. Thus, expends assay coverage and potentially aid in medical discovery at both local and global levels. In this study, we will investigate a modern saliva preservation buffer (Mawi Cell Stabilization (MCS) buffer, Mawi DNA Technologies LLC, CA, USA), to evaluate the achievability of saliva storage at room temperature for transportation purposes.
We aim to test the potential applicability of MCS buffer in preserving both bacterial and human (g)DNA, total protein amount and protein structure integrity in saliva samples stored at room temperature for a week. Our results demonstrate that MCS buffer was able to preserve human genomic DNA (gDNA) and total protein within the testing time-points. While bacterial gDNA was preserved, the buffer did not manage to halt bacterial growth. In addition, the protein structure integrity also remains to be further tested albeit MCS buffer was able to preserve the protein structure of human Galectin-3 for at least 24 h at room temperature.
2. Materials and methods
2.1. Study cohort and sample collection
This study was approved by the Queensland University of Technology (HREC no. 1400000617) Medical Ethical Institutional Board and informed consent was obtained from all participants. All methods in this study were performed in accordance with the relevant guidelines and regulations. We have recruited normal healthy controls (n = 10) from the general population based on a stringent recruitment criteria to minimise baseline variations that may potentially affect the experimental endpoints. All volunteers (between 20 and 30 years of age) were self-reported to be in good general health with no underlying diseases, not receiving local and/or systemic antibodies and no history of smoking and drinking habits.
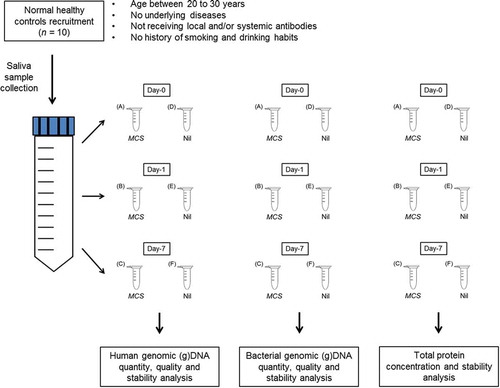
Saliva collections were carried out as per our previously published research (Lim et al., Citation2016). Each sample was further aliquoted into a set of six 1.5 mL Eppendorf tubes and labelled according to their incubation time period at room temperature: (a) Day-0—MCS, (b) Day-1—MCS, (c) Day-7—MCS, (d) Day-0—Nil, (e) Day-1—Nil, (f) Day-7—Nil (total, n = 60). In each set, MCS buffer was added into (a), (b) and (c) and phosphate-buffered saline (PBS) was added into (d), (e) and (f) with a 1:20 volume (Figure ). Samples from Day-0 act as baseline and were stored at −80°C immediately after preparation, while samples from Day-1 to Day-7 were kept at room temperature for 24 h and a week, respectively, before storing at −80°C.
Figure 1. The work flow for saliva samples collection and storage.
Saliva samples were collected in 50 mL Falcon tube and aliquoted into 1.5 mL Eppendorf tubes. Saliva preservation buffer (Mawi Cell Stabilization, MCS) was added to (a), (b) and (c) while 1× PBS was added to (d), (e) and (f) as mock. Samples (a) and (d) were used as baseline; samples (b) and (e) were incubated in room temperature for 24 h; samples (c) and (f) were incubated in room temperature for a week.
2.2. gDNA extraction
The Qick-gDNA™ MiniPrep Kit (Cat. No. D3025, Zymo Research, Irvine, CA, USA) was used with minor modifications to the manufacturer’s protocol to isolate human gDNA from whole saliva. To achieve a higher gDNA yield, 95 µL of DNA/RNA Shield (Cat. No. R1100-50, Zymo Research, Irvine, CA, USA) and 5 µL of (20 mg/mL) proteinase K (Cat. No. 76225, Affymetrix, Santa Clara, CA, USA) were added to 200 µL of whole saliva. In our previous study, we have established that the incorporation of enzymatic-mechanical lysis to the Maxwell® 16 LEV Blood DNA Kit (Cat. No. AS127A, Promega, Madison, WI, USA) protocol was able to provide high bacterial gDNA yield and purity (Lim et al., Citation2017). Hence, bacterial gDNA were extracted as per our previous work (Lim et al., Citation2017). The concentration and purity of the gDNA isolated from each sample was measured using NanoDrop® (Model No. ND-1000, ThermoFisher Scientific, Waltham, MA, USA).
2.3. Quantitative PCR (qPCR) assays
To determine the quantity and quality of human and bacterial gDNA in saliva samples, standard qPCR were used. Human β-globin gene (F-5ʹ CAACTTCATCCACGTTCACC 3ʹ and R-5ʹ GAAGAGCCAAGGACAGGTAC 3ʹ) was used as a primer to amplify human gDNA while bacterial 16S rRNA gene primer set (1114F-5ʹ CGGCAACGAGCGCAACCC 3ʹ and 1221R-5ʹ CCATTGTAGCACGTGTGTAGCC 3ʹ) was used to amplify bacterial gDNA. The reaction consisted of 5 μL of 2 × iTaq™ Universal SYBR® Green Supermix (Bio-Rad Laboratories, CA, USA), 200 nM of forward and reverse primers for each of the respective genes and 20 ng of gDNA template. The total reaction volume (10 μL) was subjected to qPCR amplification using the conditions of an initial denaturing stage at 95°C for 10 min and followed by 30 cycles of a minute at 60°C. HeLa cell line (Cat. No. 4007s, New England Biolabs, MA, USA) and Escherichia coli gDNA was used as a positive controls. The qPCR amplicons were further confirmed by gel electrophoresis.
2.4. Total protein quantification using bicinchoninic acid (BCA)
Pierce™ BCA Protein Assay Kit (Cat. No. 23225. Thermo Fisher Scientific, MA, USA) was used to quantify total protein according to the manufacturer’s protocol. An eight-point albumin standard curve was generated and saliva samples were diluted with 1× PBS at a 1:12.5 dilution ratio. Total volume of 25 µL of standards and samples was loaded in a duplicate fashion and the absorbance was measured at 562 nm using a Microplate Spectrophotometer (Benchmark Plus, Cat. No. 10314, Bio-Rad Laboratories Incorporate, CA, USA).
2.5. Human Galectin-3 enzyme-linked immunosorbent assay (ELISA)
Human Galectin-3 DuoSet ELISA (Cat. No. DY1154, R&D System Incorporate, MN, USA) was used to quantify Galectin-3 in human saliva according to the manufacturer’s protocol. Amicon Ultra centrifuge filter units, Ultra-15 MWCO 30 kDa (Cat. No. Z717185-8EA, Sigma-Aldrich Corporation, MO, USA) was used to create a Galectin-3 depleted saliva matrix. Recombinant Galectin-3 was spiked into this saliva matrix to generate an eight-point standard curve. Saliva samples were diluted with 1% Bovine serum albumin (BSA) in 1× PBS at a 1:99 dilution ratio. Total volume of 100 µL of standards and samples was loaded in a duplicate fashion and the absorbance was measured at 450 nm using a Microplate Spectrophotometer.
2.6. Statistical analysis
The statistical analysis was carried out by using GraphPad Prism (GraphPad Software Incorporate, CA, USA). Since the data generated in this study are not normally distributed, the non-parametric Wilcoxon matched-paired signed rank test was used when comparing two different variables, while Friedman test was used when comparing more than two different variables.
3. Results
3.1. The quantity, quality and stability of isolated gDNA from saliva samples
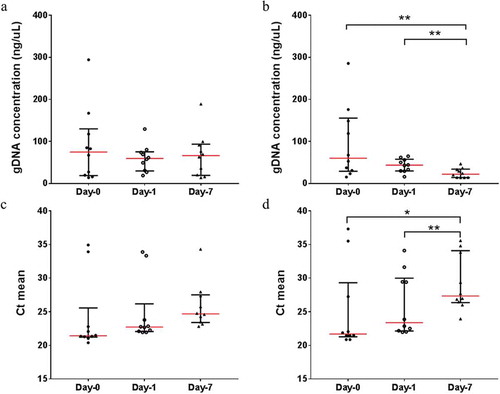
The human gDNA concentrations isolated from saliva samples stored in MCS buffer did not significantly vary from Day-0 (baseline) to Day-7 at room temperature (Figure ). Significant ongoing gDNA deterioration could be observed in samples without MCS buffer (from Day-0 to Day-7, 74.14% average gDNA loss) (Figure ). However, the human gDNA concentration between samples with and without the MCS buffer did not vary significantly prior to Day-7 (Supplementary data 1). The stability of human gDNA was tested using qPCR, targeting the human β-globin gene and the results are consistent. The average threshold cycle (Ct) means increases significantly from Day-0 (Ct = 25.01) and Day-1 (Ct = 26.00) to Day-7 (Ct = 29.16) when samples are stored at room temperature without MCS buffer (Figure ). The average Ct means for samples with MCS buffer showed no signs of degradation across the three time-points (Ct = 24.06, 24.76 and 25.85, respectively) when stored at room temperature (Figure ). In parallel, the average Ct means comparison between samples with and without MCS buffer is not significant across the three time-points prior to Day-7 (Supplementary data 2).
Figure 2. The scatter plots represents the human gDNA concentration and the mean threshold cycle (Ct) for human β-globin gene from saliva samples with (a and c) and without (b and c) MCS buffer at Day-0 (baseline), Day-1 and Day-7 at room temperature.
Significant differences are denoted with * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, respectively.
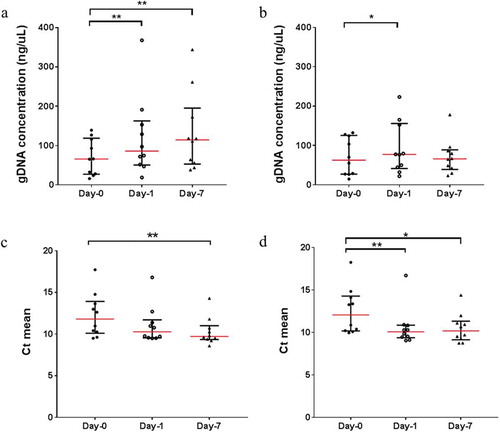
Unlike the extracted human gDNA, the bacterial gDNA concentration increased significantly across the three time-points, albeit in the presence of MCS buffer (Figure ). This occurrence may be due to the non-lytic nature of the MCS buffer, promoting bacterial growth in saliva samples stored at room temperature. Similarly, the bacterial gDNA concentration in saliva samples without MCS buffer increased significantly from Day-0 to Day-1 and reached a plateau at Day-7 (Figure ). This indicates that while there are bacterial growths in saliva samples without the MCS buffer, bacterial gDNA may also be simultaneously deteriorating due to the absence of preservation buffer, resulting in the bacterial gDNA concentration to remain constant at Day-7. When comparing the bacterial gDNA concentration between samples with and without MCS buffer, there are no significant differences at Day-0 and Day-1 (Supplementary data 3). However, at Day-7, the bacterial gDNA concentration decreased significantly in samples without the MCS buffer (Supplementary data 3). Similarly, the stability of bacterial gDNA was tested using qPCR targeting the bacterial 16S rRNA gene. Although there are significant differences in the average Ct means between Day-0 and Day-7 for both samples with (Ct = 12.24 and 10.32, respectively) and without (Ct = 12.55 and 10.52, respectively) MCS buffer (Figure and , respectively), the average Ct means comparison between samples with and without MCS buffer is not significant across the three time-points (Supplementary data 4).
Figure 3. The scatter plots represent the bacterial gDNA concentration and the mean threshold cycle (Ct) for bacterial 16S rRNA gene from saliva samples with (a and c) and without (b and c) MCS buffer at Day-0 (baseline), Day-1 and Day-7 at room temperature.
Significant differences are denoted with * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, respectively.
3.2. Total protein concentrations
The total protein concentrations did not vary significantly in the saliva samples with MCS buffer across the three time-points (Figure ). In contrast, the total protein concentrations decrease significantly in the saliva samples without MCS buffer across the three time-points (Figure ). This trend is more obvious when the total protein levels are compared between samples with and without the MCS buffer across the three time-points (Supplementary data 6). Initially, the total protein concentrations in the saliva samples without the MCS buffer were significantly higher in Day-0 compared with the saliva samples with MCS buffer but continued to drop from Day-0 to Day-7 (30.57% average total protein loss).
Figure 4. The scatter plots represent the total protein level and human Galectin-3 concentration from saliva samples with (a and c) and without (b and c) MCS buffer at Day-0 (base-line), Day-1 and Day-7 at room temperature.
Significant differences are denoted with * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, respectively.
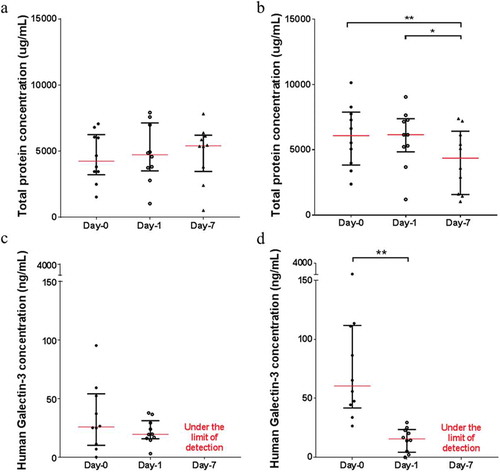
The protein structure integrity was also tested by quantifying human Galectin-3 in saliva samples. Based on our results, MCS buffer managed to preserve Galectin-3 in saliva samples at room temperature for at least 24 h (Figure ). In saliva samples without MCS buffer, the concentration of Galectin-3 decreased significantly (from Day-0 to Day-1, 82.7% average Galectin-3 loss) (Figure ). The Galectin-3 concentrations in the saliva samples with and without MCS buffer at Day-7 were under the limit of detection and therefore were taken out of the analysis. Similar to the total protein concentrations, samples without MCS buffer had a higher Galectin-3 concentration at Day-0 compared to samples with MCS buffer but decreased significantly after 24 h (Supplementary data 7).
4. Discussion
There are number of studies highlighting the influence of commercial saliva collection devices and processing protocols on the quantification of salivary biomolecules (Chen et al., Citation2016; Lazarevic, Gaïa, Girard, François, & Schrenzel, Citation2013; Lim et al., Citation2017, Mohamed, Campbell, Cooper-White, Dimeski, & Punyadeera, Citation2012, Vesty et al., Citation2017). However, there is a paucity of data and knowledge with respect to the influence of the preservation buffers when transporting saliva samples at room temperature. While recent studies demonstrate that existing saliva collection devices are able to preserve biomolecules in saliva at room temperature over an extended period of time, the emphasis has been mainly on the detection of human genomic material (Burrows, Ristow, & D’Amato, Citation2017, Chiang et al., Citation2015; Garbieri, Brozoski, Dionísio, Santos, & Neves, Citation2017; Nunes et al., Citation2012; Speicher & Johnson, Citation2014).
The MCS buffer is a proprietary non-alcohol-based, non-lytic saliva preservation buffer that allows cells to remain intact and potential viable at room temperature. The objective is to enable the analysis of genomic materials as well as proteins from the same sample. To date, there are no comparable saliva preservation buffer in the market that would cater to both genomic materials and proteins in the same samples. Such functionality would be of great benefit to the advancement of salivary research, thus our decision to perform this preliminary pilot study.
We found that MCS buffer was able to preserve both human and bacterial gDNA for a week at room temperature without significantly affecting the gDNA yield and integrity. In contrast, saliva samples without the MCS buffer suffered significant loss of human gDNA. While the MCS buffer was able to preserve bacterial gDNA in saliva, it did not manage to halt the bacterial growth. Our data suggests that the MCS buffer could potentially substitute the need of cold transport, opening salivary testing in remote and/or resource-limited settings (Chiang et al., Citation2015; Nunes et al., Citation2012).
The standard protocol for saliva transportation involves storing samples together with dry ice in a ventilated polystyrene box to ensure proper cryopreservation. While dry ice is regularly supplied in research institutes and medical hospitals, they are fairly inaccessible in rural and remote environments. Moreover, dry ice is classified as dangerous goods that require professional handling for international transportation. Based on our findings, MCS buffer should be considered as an alternative for saliva transportation due to its accessibility and safe handling while providing the same benefits as dry ice. In addition, MCS buffer is packaged as a bottled buffer that can easily be incorporated into multiple saliva collection formats such as whole-mouth natural saliva, buccal swab and oral rinse. Unlike other saliva collection devices, MCS buffer does not restrict the volume of saliva collection as the buffer is not “built-into” the devices and therefore could be re-adjusted according to needs.
Salivary proteins are commonly used as biomarkers to detect, monitor and predict oral and other systemic conditions (Foo et al., Citation2012, Citation2013; Mohamed et al., Citation2012; Yu et al., Citation2016; Zhang et al., Citation2014, Citation2013, Citation2010). This is attributable to the fact that 20–30% of the human proteins are also found in human saliva (Castagnola et al., Citation2011; Pfaffe et al., Citation2011). Unlike genomic materials, protein structure and functionality are affected by freezing. Since saliva consists of 99% water, small ice crystals and a relatively large surface area of ice–liquid interface are formed during fast freezing, which damages protein molecules upon contact (Cao et al., Citation2003; de Almeida et al., Citation2008; Francis, Hector, & Proctor, Citation2000). In addition, the recrystallization process during thawing further damages the proteins by exerting additional interfacial tension on the entrapped proteins (Cao et al., Citation2003; Francis et al., Citation2000). As such, there is a need for alternative saliva storage methods that are convenient and easily available for population-based screening. We observed that when saliva samples were stored at room temperature in MCS buffer, there was minimal protein degradation as quantified using the BCA assay.
Galectin-3 is a well-known biomarker for cardiovascular disease prognosis and often found to be elevated in blood samples collected from chronic heart failure patients and some cancer patients (Anastasi et al., Citation2017; Coppin et al., Citation2017; Dos Santos et al., Citation2017; Hao, Li, & Li, Citation2017; Ho et al., Citation2012; Idikio, Citation2011; Nakajima et al., Citation2016). As such, Galectin-3 was selected as a model protein to determine the efficiency of MCS buffer in maintaining protein structure. In brief, epitopes exist on Galectin-3 protein as tertiary structures of amino acids that recognise specific antibodies with the same structural shape (Morris, Citation1996). However, epitopes are susceptible to pH and temperature changes (Morris, Citation1996; Wilson, Sun, Ozturk, & Wands, Citation1991). Denatured epitopes are not recognised by antibodies with the same specificity or affinity (Wilson et al., Citation1991). In the presence of MCS buffer, at room temperature, Galectin-3 concentrations did not differ significantly after 24 h compared to the baseline levels.
5. Conclusion
In the future, the ratio of MCS buffer to saliva samples should be optimised to reduce bacterial growth and better protein structure integrity preservation. The MCS buffer would also have to be tested with a larger sample cohort in an extensive longitudinal study before it could substitute the need of cold transport and storage. In addition, different transportation temperatures testing are required to account for all climate zones. We hope that our findings will contribute to those efforts to streamline saliva transportation and alternative storage methods for future saliva research.
Abbreviations
BCA, bicinchoninic acid; Ct, threshold cycle; ELISA, enzyme-linked immunosorbent assay; gDNA, genomic DNA; MCS, MAWI cell stabilization; qPCR, quantitative PCR.
Ethics
This study is approved by the Queensland University of Technology’s (HREC no.: 1400000617) Medical Ethical Institutional Board. All participants were informed consent for this study.
Competing interests
The authors declare that they have no competing interest.
Author’s contributions
YKL collected samples, compiled all epidemiological data on all subjects, conducted all experiments, data interpretation and wrote the manuscript and CP participated in study concept and design, study coordination and revised the manuscript critically for content. All authors have read and approved the content of this manuscript.
Consent
Not applicable.
Supplemental Material
Download MS Word (285.8 KB)Acknowledgement
We thank Mawi DNA Technology LLC for offering early access to the MAWI Cell Stabilization (MCS) buffer.
Supplementary material
Supplemental material for this article can be accessed here.
Additional information
Funding
Notes on contributors
Chamindie Punyadeera
Associate Professor Chamindie Punyadeera is a globally acknowledged pioneer in salivary diagnostics. She leads a world-class research laboratory in Australia, the Saliva Translational Research laboratory within the Queensland University of Technology. Her team focuses on developing novel diagnostic and prognostic biomarkers for cardiovascular diseases and head and neck cancers and linking oral health to systemic diseases. Her team collaborates closely with dentists, oral medicine specialist, surgeons, cardiologists, intensivists and large pharmaceutical companies in translating their research findings into a clinical setting. In this study, she and her PhD student, Yenkai Lim explored possible methods of preserving saliva biomolecules to accelerate the translation of salivary assays from bench to bedside.
References
- Anastasi, E., Gigli, S., Santulli, M., Tartaglione, S., Ballesio, L., Porpora, M. G., ... Manganaro, L. (2017). Role of Galectin-3 combined with multi-detector contrast enhanced computed tomography in predicting disease recurrence in patients with ovarian cancer. Asian Pacific Journal of Cancer Prevention, 18(5), 1277–1282.
- Burrows, A. M., Ristow, P. G., & D’Amato, M. E. (2017). Preservation of DNA from saliva samples in suboptimal conditions. Forensic Science International: Genetics Supplement Series, 6, e80–e81. doi:10.1016/j.fsigss.2017.09.050
- Cao, E., Chen, Y., Cui, Z., & Foster, P. R. (2003). Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnology and Bioengineering, 82(6), 684–690. doi:10.1002/bit.10612
- Castagnola, M., Picciotti, P. M., Messana, I., Fanali, C., Fiorita, A., Cabras, T., ... Scarano, E. (2011). Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngologica Italica, 31(6), 347–357.
- Chen, M., Wu, B., Chen, T., Liu, Z., Deng, Z., & Peng, L. (2016). The impact of different DNA extraction methods on the analysis of microbial diversity of oral saliva from healthy youths by polymerase chain reaction-denaturing gradient gel electrophoresis. Journal of Dental Sciences, 11(1), 54–58.
- Chiang, S. H., Thomas, G. A., Liao, W., Grogan, T., Buck, R. L., Fuentes, L., … Wong, D. T. W. (2015). RNAPro*SAL: A device for rapid and standardized collection of saliva RNA and proteins. Biotechniques, 58(2), 69–76. doi:10.2144/000114254
- Coppin, L., Vincent, A., Frénois, F., Duchêne, B., Lahdaoui, F., Stechly, L., … Pigny, P. (2017). Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Scientific Reports, 7, 43927. doi:10.1038/srep43927
- de Almeida, P. D. V., Gregio, A. M., Machado, M. A., de Lima, A. A., & Azevedo, L. R. (2008). Saliva composition and functions: A comprehensive review. The Journal of Contemporary Dental Practice, 9(3), 72–80.
- Desai, G. S., & Mathews, S. T. (2014). Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World Journal of Diabetes, 5(6), 730–738. doi:10.4239/wjd.v5.i6.730
- dos Santos, S. N., Sheldon, H., Pereira, J. X., Paluch, C., Bridges, E. M., El-Cheikh, M. C., ... Bernardes, E. S. (2017). Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget, 8(30), 49484–49501.
- Esser, D., Alvarez-Llamas, G., de Vries, M. P., Weening, D., Vonk, R. J., & Roelofsen, H. (2008). Sample stability and protein composition of saliva: Implications for its use as a diagnostic fluid. Biomarker Insights, 3, 25–27. doi:10.4137/BMI.S607
- Foo, J. Y., Wan, Y., Kostner, K., Arivalagan, A., Atherton, J., Cooper-White, J., … Punyadeera, C. (2012). NT-ProBNP levels in saliva and its clinical relevance to heart failure. PLoS One, 7(10), e48452. doi:10.1371/journal.pone.0048452
- Foo, J. Y., Wan, Y., Schulz, B. L., Kostner, K., Atherton, J., Cooper-White, J., … Punyadeera, C. (2013). Circulating fragments of N-terminal pro-B-type natriuretic peptides in plasma of heart failure patients. Clinical Chemistry, 59(10), 1523–1531. doi:10.1373/clinchem.2012.200204
- Francis, C. A., Hector, M. P., & Proctor, G. B. (2000). Precipitation of specific proteins by freeze-thawing of human saliva. Archives of Oral Biology, 45(7), 601–606. doi:10.1016/S0003-9969(00)00026-1
- Garbieri, T. F., Brozoski, D. T., Dionísio, T. J., Santos, C. F., & Neves, L. T. D. (2017). Human DNA extraction from whole saliva that was fresh or stored for 3, 6 or 12 months using five different protocols. Journal of Applied Oral Science, 25(2), 147–158. doi:10.1590/1678-77572016-0046
- Hao, M., Li, M., & Li, W. (2017). Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Molecular Medicine Reports, 15(1), 160–168. doi:10.3892/mmr.2016.6020
- Ho, J. E., Liu, C., Lyass, A., Courchesne, P., Pencina, M. J., Vasan, R. S., … Levy, D. (2012). Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. Journal of the American College of Cardiology, 60(14), 1249–1256. doi:10.1016/j.jacc.2012.04.053
- Idikio, H. A. (2011). Galectin-3 and Beclin1/Atg6 genes in human cancers: Using cDNA tissue panel, qRT-PCR, and logistic regression model to identify cancer cell biomarkers. PLoS One, 6(10), e26150. doi:10.1371/journal.pone.0026150
- Idris, F. P., Wan, Y., Zhang, X., & Punyadeera, C. (2017). Within-day baseline variation in salivary biomarkers in healthy men. OMICS: A Journal of Integrative Biology, 21(2), 74–80. doi:10.1089/omi.2016.0168
- Iorgulescu, G. (2009). Saliva between normal and pathological. Important factors in determining systemic and oral health. Journal of Medicine and Life, 2(3), 303–307.
- Kolenbrander, P. E., Andersen, R. N., Blehert, D. S., Egland, P. G., Foster, J. S., & Palmer, R. J. (2002). Communication among oral bacteria. Microbiology and Molecular Biology Reviews, 66(3), 486–505. doi:10.1128/MMBR.66.3.486-505.2002
- Lazarevic, V., Gaïa, N., Girard, M., François, P., & Schrenzel, J. (2013). Comparison of DNA extraction methods in analysis of salivary bacterial communities. PLoS One, 8(7), e67699. doi:10.1371/journal.pone.0067699
- Lim, Y., Sun, C. X., Tran, P., & Punyadeera, C. (2016). Salivary epigenetic biomarkers in head and neck squamous cell carcinomas. Biomarkers in Medicine, 10(3), 301–313. doi:10.2217/bmm.16.2
- Lim, Y., Totsika, M., Morrison, M., & Punyadeera, C. (2017). The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Scientific Reports, 7(1), 8523. doi:10.1038/s41598-017-07885-3
- Lim, Y., Wan, Y., Vagenas, D., Ovchinnikov, D. A., Perry, C. F. L., Davis, M. J., & Punyadeera, C. (2016). Salivary DNA methylation panel to diagnose HPV-positive and HPV-negative head and neck cancers. BMC Cancer, 16(1), 1–12. doi:10.1186/s12885-016-2785-0
- Lima, D. P., Diniz, D. G., Moimaz, S. A. S., Sumida, D. H., & Okamoto, A. C. (2010). Saliva: Reflection of the body. International Journal of Infectious Diseases, 14(3), e184–e188. doi:10.1016/j.ijid.2009.04.022
- Lucs, A. V., Saltman, B., Chung, C. H., Steinberg, B. M., & Schwartz, D. L. (2013). Opportunities and challenges facing biomarker development for personalized head and neck cancer treatment. Head & Neck, 35(2), 294–306. doi:10.1002/hed.v35.2
- Malamud, D. (2011). Saliva as a diagnostic fluid. Dental Clinics of North America, 55(1), 159–178. doi:10.1016/j.cden.2010.08.004
- Mohamed, R., Campbell, J.-L., Cooper-White, J., Dimeski, G., & Punyadeera, C. (2012). The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. Clinical and Translational Medicine, 1, 19. doi:10.1186/2001-1326-1-19
- Morris, G. E. (1996). Overview. In G. E. Morris (Editor), Epitope Mapping Protocols (pp. 1–9). Totowa, NJ: Humana Press.
- Nakajima, K., Heilbrun, L. K., Hogan, V., Smith, D., Heath, E., & Raz, A. (2016). Positive associations between Galectin-3 and PSA levels in prostate cancer patients: A prospective clinical study-I. Oncotarget, 7(50), 82266–82272. doi:10.18632/oncotarget.v7i50
- Nunes, A. P., Oli\veira, I. O., Santos, B. R., Millech, C., Silva, L. P., González, D. A., … Barros, F. C. (2012). Quality of DNA extracted from saliva samples collected with the Oragene™ DNA self-collection kit. BMC Medical Research Methodology, 12, 65. doi:10.1186/1471-2288-12-65
- Nunes, L. A., Mussavira, S., & Bindhu, O. S. (2015). Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochemia Medica, 25(2), 177–192. doi:10.11613/issn.1846-7482
- Ovchinnikov, D. A., Cooper, M. A., Pandit, P., Coman, W. B., Cooper-White, J. J., Keith, P., … Punyadeera, C. (2012). Tumor-suppressor gene promoter hypermethylation in saliva of head and neck cancer patients. Translational Oncology, 5(5), 321–326. doi:10.1593/tlo.12232
- Ovchinnikov, D. A., Wan, Y., Coman, W. B., Pandit, P., Cooper-White, J. J., Herman, J. G., & Punyadeera, C. (2014). DNA methylation at the novel CpG sites in the promoter of MED15/PCQAP gene as a biomarker for head and neck cancers. Biomarker Insights, 9, 53–60. doi:10.4137/BMI.S16199
- Pfaffe, T., Cooper-White, J., Beyerlein, P., Kostner, K., & Punyadeera, C. (2011). Diagnostic potential of saliva: Current state and future applications. Clinical Chemistry, 57(5), 675–687. doi:10.1373/clinchem.2010.153767
- Rathnayake, N., Gieselmann, D.-R., Heikkinen, A., Tervahartiala, T., & Sorsa, T. (2017). Salivary diagnostics—Point-of-care diagnostics of MMP-8 in dentistry and medicine. Diagnostics, 7(1), 7. doi:10.3390/diagnostics7010007
- Speicher, D. J., & Johnson, N. W. (2014). Comparison of salivary collection and processing methods for quantitative HHV-8 detection. Oral Diseases, 20(7), 720–728. doi:10.1111/odi.12196
- Vesty, A., Biswas, K., Taylor, M. W., Gear, K., Douglas, R. G., & Han, Y. (2017). Evaluating the impact of DNA extraction method on the representation of human oral bacterial and fungal communities. PLoS One, 12(1), e0169877. doi:10.1371/journal.pone.0169877
- Wilson, B. E., Sun, S., Ozturk, M., & Wands, J. R. (1991). Stability of monoclonal antibody-defined epitopes. Journal of Immunological Methods, 139(1), 55–64. doi:10.1016/0022-1759(91)90351-F
- Yu, J.-S., Chen, Y.-T., Chiang, W.-F., Hsiao, Y.-C., Chu, L. J., See, L.-C., … Hartwell, L. H. (2016). Saliva protein biomarkers to detect oral squamous cell carcinoma in a high-risk population in Taiwan. Proceedings of the National Academy of Sciences, 113(41), 11549–11554. doi:10.1073/pnas.1612368113
- Zhang, L., Xiao, H., Karlan, S., Zhou, H., Gross, J., Elashoff, D., … Wong, D. T. (2010). Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One, 5(12), e15573. doi:10.1371/journal.pone.0015573
- Zhang, X., Dimeski, G., & Punyadeera, C. (2014). Validation of an immunoassay to measure plasminogen-activator inhibitor-1 concentrations in human saliva. Biochemical Medicine, 24(2), 258–265. doi:10.11613/issn.1846-7482
- Zhang, X., Wan, Y., Cooper-White, J., Dimeski, G., Atherton, J., & Punyadeera, C. (2013). Quantification of D-dimer levels in human saliva. Bioanalysis, 5, 2249–2256. doi:10.4155/bio.13.190
