Abstract
The study area, Odukpani is underlain by cretaceous sediments of the Calabar Flank. The main aquifers are fractured black shales that are inter-bedded with calcareous sandstones. The aquifers range from confined to unconfined. Water samples were taken from boreholes and shallow hand dug wells for chemical characterization with standard laboratory techniques. Results from the chemical analysis show that the waters are mostly alkaline with the exception of two mildly acidic samples. They depicted low electrical conductivity values and a total dissolved solids range from 13.1 to 341 mg/l which indicate fresh water. Cations levels occur in the order, Ca2+ ˃ Na+ ˃ Mg2+ ˃ K+ ˃ Fe2+ while anions occur as the following order, HCO3− ˃ SO42− ˃ Cl− ˃ CO3− ˃ NO3−. Piper trilinear and stiff plots show that Ca2+ – Na+ – HCO3− is dominant water type. The waters are supersaturated with respect to calcite in five of the samples. The rest have calcite under-saturation. Areas of calcite super-saturations are likely undergoing precipitation of carbonate minerals in the aquifer matrices. Precipitation and dissolution of carbonate minerals within the aquifers are likely contributing to the modification of flow characteristics of the aquifer. Comparison of the water chemistry with standards set by regulatory bodies indicates that the waters are of good quality.
Public Interest Statement
The quality of natural waters in carbonate aquifers at a densely populated Odukpani area of Cross River State, Southern Nigeria were investigated and compared to United State Environmental Protection Agency (1994) standard limits for drinking purposes. Water quality assessment is essential for human health and advancement. The results of this research show that Hand dug wells and boreholes in the study area are slightly acidic to alkaline and soft to moderately hard. Geochemical modeling results show that the limestone-rich boreholes and hand dug well samples in the area have tendency to cause serious corrosion and negative health impacts. The relationships that link sodium and chloride, Calcium + Magnesium, bicarbonate +, and sulfate suggest that the quality of the water samples are highly related to the carbonate minerals. Generally, the results of the investigation indicated that the waters in the study area are within the recommended limits stipulated by United State Environmental Protection Agency (1994).
1. Introduction
In Nigeria, carbonate minerals are found throughout the country. They serve as raw materials for about 10 cement industries (Nigeria Ministry of Solid Mineral Development, Citation2000) however; their precipitation in groundwater systems affects the physical properties (porosity and permeability) of the subsurface rocks and thereby modifying flow patterns. As a result, it is important to study aquifers that consist of carbonate minerals in great detail.
Many studies have been conducted elsewhere on carbonate aquifer systems such as geochemical processes in the aquifer systems and their hydrogeological implications (Giuseppe, Sibel, & Flavia, Citation2013) but in Nigeria Azubuike, Edet, and Solomon (Citation2012) described the groundwater chemistry of the Oban Massif, southeastern Nigeria. Most other works focus on the descriptive sedimentology of carbonate rocks (Anthony & Sunday, Citation2013; Samuel, Maina, & Matera, Citation2010). The ability of carbonate minerals in aquifers to modify groundwater chemistry or flow patterns have not been discussed in detail in Nigeria except in the work of Edet, Nganje, Ukpong, and Ekwere (Citation2011).
In this present study, boreholes and shallow hand dug wells and from the carbonate aquifer systems of Odukpani areas were characterized employing hydrochemical data to determine their quality parameters. This study was also designed to hydrochemically characterize these aquifer systems with the aim of evaluating the impact of carbonate rocks on groundwater in the study area.
2. Study area
The study area, Odukpani is situated between latitudes 5°051 N and 5°101 N and longitudes 8°161E and 8°211E, covering an area extent of about 85 square kilometers. The study area includes Odukpani, New Netim, Ituk Eno, and Okoyong communities. It has an estimated population of about 50,000 people and serves as the headquarters of Odukpani local government area of Cross River State, Nigeria. It is bordered by Mbarakom to the north, Ikot Ekpo to the south, Esuk Otu in the west, and Agbung in the east.
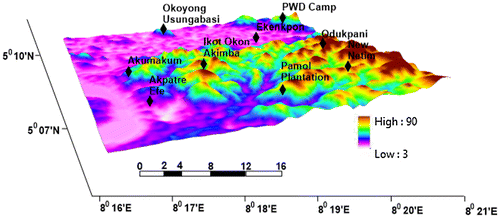
The study area has a relatively low relief (Figure ). The mean elevation ranges from 50 to 80 m above sea level. The two major rivers that drain the area are the Calabar River and the Aru Unyere River both of which empties into the Atlantic Ocean.
The two climatic conditions in the area are the wet season (from April to October) and dry season (from November to March). The mean annual rainfall is between 2,000 to 3,000 mm while temperature varies between 22 to36°C according to Iloeje (Citation1995).
Iloeje (Citation1995) also described the vegetation pattern in the area as tropical rainforest.
3. Geologic setting
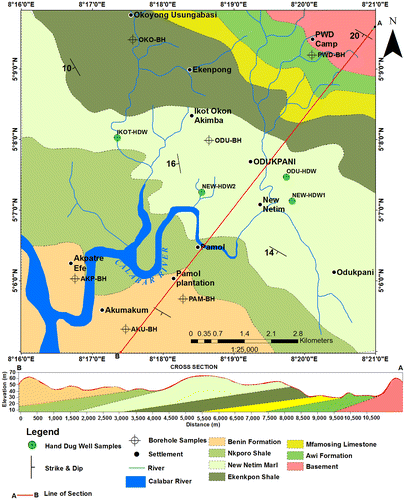
The geological map of the study area (Figure ) shows that Odukpani and environs consists of two main lithologic groups. These are the Precambrian Crystalline Basement and Cretaceous to Tertiary Sedimentary rocks. The basement rocks consists of migmatitic gneisses, banded gneisses, weakly foliated granites while sedimentary rocks consists of sandstones, shales, and limestones which have been generally grouped into Awi, Mfamosing, Ezeaku, Ekenkpon, New Netim Marl, Nkporo, and Ameki Formations, respectively, make up the Calabar Flank. Calabar Flank is a sedimentary basin that borders southeastern Nigeria’s continental margin. According to Reigers and Petters (Citation1987), the Calabar Flank is perpendicular to the major rift faults of the Benue Trough and structurally consists of NW–SE trending basement horsts (the Oban Massif and the Ituk-high) separated by a graben known as the Ikang Trough. The Cretaceous to Tertiary sediment that underlie the area are mainly (from the oldest to youngest), Awi Formation, Mfamosing Formation, Eze-Aku Formation, New Netim Marl, Nkporo Formation, and Ameki Formation. The Awi Formation (Aptian) which is the basal stratigraphic unit in the area consists of calcareous arkosic sandstones resting on the basement rock (Adeleye & Fayose, Citation1978). Mfamosing Formation (Albian) which overlies the Awi Formation consists of calcareous limestone with alternating succession of silty to sandy shales. Eze-Aku Formation (Cenomanian) which overlies the Mfamosing Formation consists of alternating succession dark gray shales with intercalation of limestones. Ekenkpon Shale consists of dark gray fissile shale with sandstone and siltstone intercalation. The Ekenkpon Shale is overlain by the Coniacian to Early Santonian New Netim Marl (Essien, Ukpabio, Nyong, & Ibe, Citation2005). Unconformably overlying the New Netim Marl is Campanian –Maastrichtian Nkporo Shale which consists of gray to bluish – black shales with bands of marly and silty to sandy shales. Ameki Formation is the Tertiary to Recent sediments overlying the Nkporo Shale (Odumodu, Citation1994). Examining the orientations, the beds have an average strike of 150°NW with dips of 10°–20°SW.
4. Hydrogeologic setting
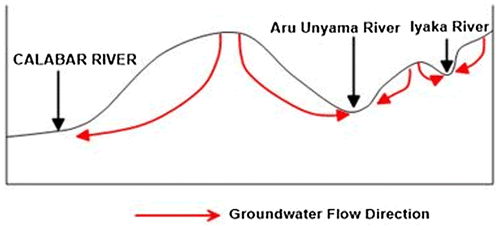
The hydrogeological setting of the area indicates that the water table configuration is directly related to the topography of the area. The water table is relatively shallow in low lying discharge areas. Aquifer types in the area ranges from confine to unconfined. The black, carbonaceous, weathered/ fractured shale with intercalations of marlstones and calcarous sandstones is the major aquifers of the area. This probably accounts for the few number of boreholes and relative abundance of hand dug wells at the discharge areas. The presence of springs and streams also suggest it is a sub-regional discharge area. The formation of springs in the area is due to the intersection of water table with the ground surface. Figure , schematically provides an idea about the groundwater flow direction in the area.
5. Materials and methods
The study procedure commenced with literature review and reconnaissance survey. This was followed by detailed geological mapping and hydrogeologic survey. The hydrogeological investigation involved water depth measurements with the aid of a calibrated tape and collection of water samples.
The analog geological map of the study area obtained from Nigeria Geological Survey Agency (NGSA) was georeferenced and digitize in ArcGis 10.2.2 software.
Three-dimensional map of the area was produced from SRTM data obtained from US Geological Survey (USGS) which was presented in UTM projection zone 32 N, Datum WGS84 using ArcScene software platform.
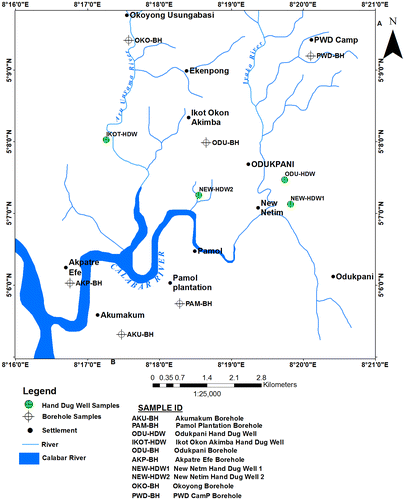
A total of 10 water samples were collected from boreholes and shallow hand dug wells (Table ) and (Figure ). Each sample was taken with polyethylene container of 1,000 cl. The containers were flushed several times with the water to be sampled, filled, and tightly closed, leaving only a small air bubble below the stopper. After sampling, each container was carefully labeled and sent to the Physical oceanography laboratory at University of Calabar Southeastern Nigeria for chemical analysis within 24 h of collection. The in situ measurements of total dissolved solid and electrical conductivity were carried out at the using Hanna (pH/Eh meter). The cations were determined by Buck model 210/211GF graphite furnace and 220 AAS while the anions like chloride were determined by volumetric, bicarbonate by titrimetric method according to procedures of APHA (Citation1998). For the identification of water types in the study area, the physio-chemical analysis data of the shallow hand dug wells and boreholes were used to generate piper and stiff diagrams using geochemical software (Rockworks15).
Table 1. Sample location table of the area
6. Results and discussions
The results obtained from the physico-chemical analysis of boreholes and shallow hand dug wells from the study area are summarized in Table .
Table 2. Results of chemical analysis of water samples
The pH values for the boreholes examined ranged from 6.0–8.5 while shallow hand dug wells measured varies between 6.8–8.5. The pH values shows marginally alkaline trends except BH3 (Odukpani) and HD1 (Odukpani) that are slightly acidic. According to the USEPA (Citation1994) guidelines, the range of desirable pH values for drinking water is 6.5–8.5. There are no boreholes and shallow hand dug well samples with pH values outside of the desirable ranges.
Electrical conductivity (EC) is a measure of the ability of water to conduct an electric current. It is used to estimate the amount of dissolved solids. In the study area, the values of conductivity ranged between 29.3–321 μS/cm for the borehole samples and 26.1–276 μS/cm for the shallow hand dug wells; with the highest value coming from Pamol Plantation (BH2) and New Netim (HW3) while lowest values was measured at Akmakum (BH1) and Odukpani (HW1). The observed TDS values in all the borehole samples fluctuate from 21.8–341 mg/l whereas shallow hand dug wells range from 13.1–138 mg/l. The permissible limit of TDS for drinking water is 2,000 mg/l. Hence, the observed TDS values in all the water samples are well within the fresh water limit. USEPA (Citation1994) classified all natural water with less than 61 mg/l CaCO3 as soft, 61–120 mg/l CaCO3 as moderately hard, 121–180 CaCO3 as hard, and more than 180 CaCO3 as very hard. In the study area, the concentration of CaCO3 measured in all the boreholes and shallow hand dug wells were found in the range of 11.4–61.7 mg/l and 6.8–78.7 mg/l which fall between soft and moderately hard water, respectively. The observed high total alkalinity values in New Netim HD4 and HD3 samples (Table ) are related to the main rock types in the area where limestone and marl are the most dominant formations.
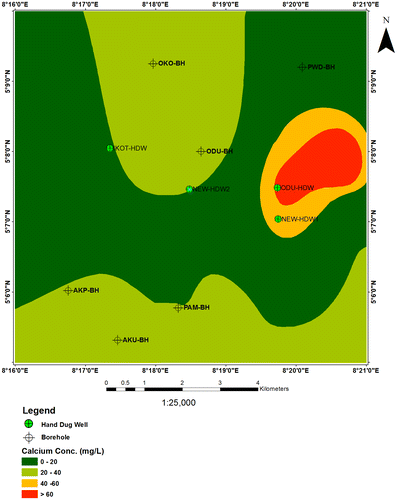
6.1. Calcium (Ca2+)
The calcium concentrations in water boreholes range from 5.2–36 mg/l and for shallow hand dug wells vary from 20.5–64.2 mg/l. Almost 42% of the boreholes and shallow hand dug well samples contain calcium concentrations higher than 30 mg/l, while about 10% of the boreholes show calcium concentrations less than 20 mg/l. Besides, HDW and HDW1 samples from Odukpani and New Netim show highest calcium values of 55.1 and 64.2 mg/l, respectively (Figure ).
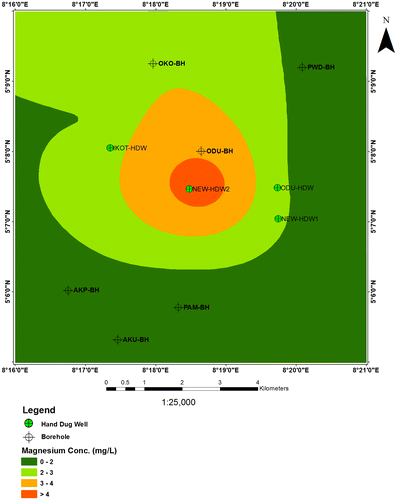
6.2. Magnesium (Mg2+)
In the study area, magnesium concentrations range between 0.7–4.3 mg/l for the borehole samples and 0.6–6.6 mg/l. Most of the shallow hand dug well samples show higher magnesium concentrations than the borehole samples. Among the shallow hand dug well samples, the highest magnesium (6.6 mg/l) concentration was observed in water samples from New Netim (Figure ). The sources of calcium in the water samples are attributed to the dissolution of the calcite and dolomite which is common in the area.
6.3. Sodium (Na+) and potassium (K+)
Sodium concentration in the investigated boreholes and shallow hand dug well samples are found in the range of 1.8–16.4 and 1.6–16.3 mg/l, respectively. The highest concentrations were observed in Pamol Plantation BH and New Netim HDW2. Most of the samples have sodium levels that are not in excess of the permissible limit of 200 mg/l stipulated by WHO (Citation2004). The potassium concentration of the boreholes and shallow hand dug well samples range from 0.2 – 2.4 and 0.2–2.0 mg/l. The sources of sodium and potassium in the water samples are attributed to the dissolution of silicate minerals.
6.4. Total iron (Fe3+)
The borehole samples showed a constant value of 0.1 mg/l for iron while no iron content of was detected from the shallow hand dug wells. The permissible limit of iron for drinking water is 0.3 mg/l (NSDWQ, Citation2007). Hence, the observed iron values in the borehole samples are below the appropriate limit. Iron in ground water may originate from a variety of mineral sources. The presence of high iron concentrations in the borehole samples may reflect siderite (FeCO3) dissolution.
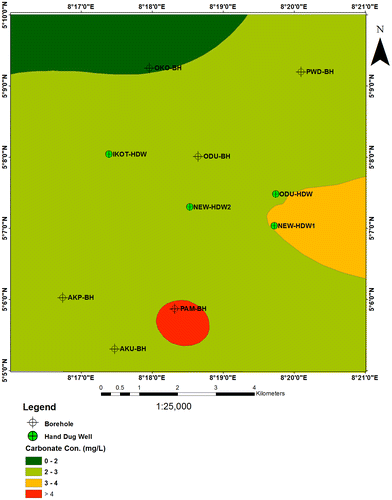
6.5. Carbonate (CO32−)
Carbonate is one component of alkalinity and its concentration is in a balance with bicarbonate between the pH range of 8.2 and 9.6. Carbonate concentration range from 2.8 to 3.9 mg/l for borehole samples and shallow hand dug wells, except Pamol Plantation BH that measures 7.8 mg/l (Figure ).
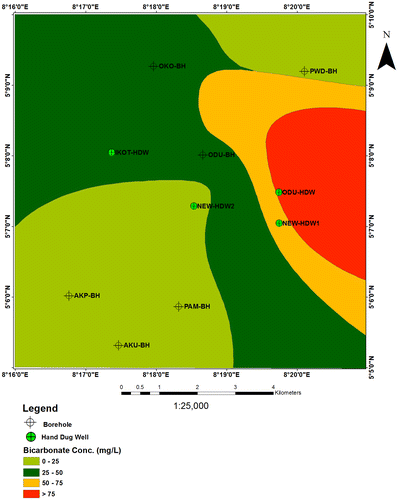
6.6. Bicarbonate (HCO3−)
The distribution of bicarbonate in study area varies between 9.4–64.7 mg/l for the borehole samples and 18.9–115.5mgl for the shallow hand dug wells (Figure ). The presence of high content of bicarbonate in Okoyong BH (60.8 mg/l), Odukpani BH (64.7 mg/l), New Netim HDW1 (99.1 mg/l), and Odukpani HDW (115.5 mg/l) may derived from dissolution of marlstone and carbonate rocks found in the area.
6.7. Chloride (Cl−) and sulfate (SO42−)
In this present work, the chloride contents of the borehole samples and shallow hand dug wells are very much within the safe range of 250 mg/l for drinking water as prescribed by WHO (Citation2004) (6.1 to 12.2 mg/L for the borehole samples and the shallow hand dug wells). The water can be considered as suitable for domestic purposes. The sulfate concentration in the borehole samples range from 1.5 to 16.1 mg/l but the shallow hand dug wells varies from 1.3 to 5.2 mg/l. The highest sulfate value was observed at Pamol Plantation BH (16.14 mg/l) but the rest of the samples are within the maximum allowable limits of 250 mg/l stipulated by USEPA (Citation1994) standard. The high concentration of sulfate is likely due to dissolution of gypsum found within the Benin Formation in the area.
6.8. Nitrate (NO3−)
The nitrate concentration in borehole samples and shallow hand dug wells are lower than 10 mg/l which is allowable limit for drinking water in all the 10 water samples measured. The maximum value of 2.0 mg/l was recorded in Akmakum BH (Table ). Elevated concentration above stipulated value by regulatory bodies may indicate pollution by feedlot run-off, sewage, or fertilizers. Based on the values; nitrate level in the study area has not reached pollution level.
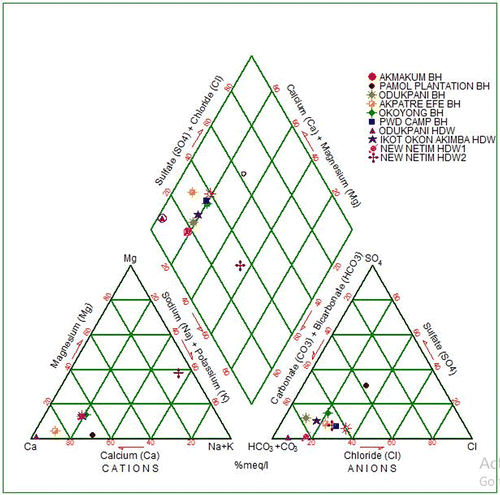
7. Hydro-chemical facies
Hydro-chemical facies are different zones that possess cation and anion concentration categories (Vikas, Kamra, Kumar, & Vishal, Citation2012). Hydro-chemical data of analyzed samples from the study area are plotted on a piper trilinear diagram (Figure ). The diagram reveals that 80% of Ca2+ – Na+ – HCO3− type of water dominated the study area, which may be attributed to the leaching of carbonate minerals through precipitation.
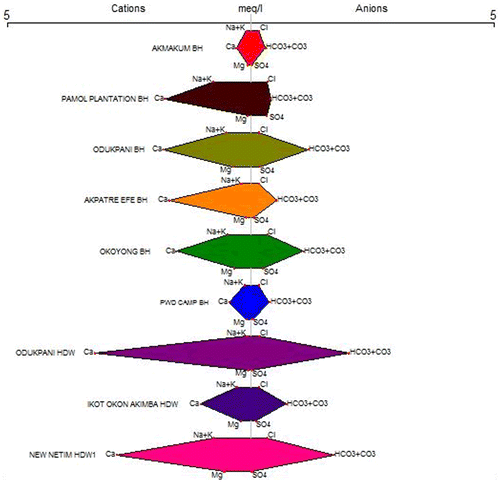
8. Stiff diagrams showing the results of the chemical analysis
The different water types of the boreholes and shallow hand dug wells were studied by plotting the concentrations of major cations and anions in the stiff diagrams (Figure ). The major cations and anions concentration of boreholes and shallow hand dug wells were dominated by calcium and bicarbonate belong to the group of Ca2+–HCO3− water type.
9. Calcite saturation indices
Solubility behavior of the waters was studied by calculating calcite saturation indices of the boreholes and shallow hand dug wells (Table ). The results of saturation indices suggest that Odukpani BH and HDW1, Okoyong BH, New Netim HD and HD samples are supersaturated with respect to calcite and dolomite; while Akmakum BH, Pamol Plantation BH, Ikot Okon Akimba HDW, Akpature Efe BH, and PWD Camp BH sampled waters were undersaturated with respect to gypsum and halite.
Table 3. Calcite saturation indices from Langelier
Works in literature showed that the permeability values within Calabar area vary from 6.08 × 10−1 to 9.43 × 10−3 cm/s for the sandy clay to gravelly clay, to 7.00 × 10−1 to 8.7 × 10−1 cm/s for coarse sand to gravelly sand whereas, the conduit volumes within rock matrix range between 0.95 × 104 to 1.35 × 104 m3 and continuous discharge measurement over a period of 18 months gave mean discharge value of 2.53 ms−1. The mean residence time of the flow is 10,200/2.7s or 70minutes (Eaton, Citation1950; Edet & Okereke, Citation1997; Youdeowei & Nwankwoala, Citation2010).
Areas of calcite supersaturated are likely to be experiencing carbonate precipitation or deposition within the matrices of the aquifer. If the void spaces are filled with carbonate materials, this will input negatively in the porosity/permeability of the aquifer. It will distort groundwater flow paths and increase flow pressure. Areas of calcite under saturation are likely to be experiencing higher rate of dissolution. The aquifer matrix in such locations will become more permeable with time. In this way, the precipitation/dissolution processes within the study area will modify the permeability regime of the aquifers with time. The flow is likely to be low in the this low-porosity carbonate aquifers, so it is likely that most of the water flows through fractures and channels in the area may have a short residence time.
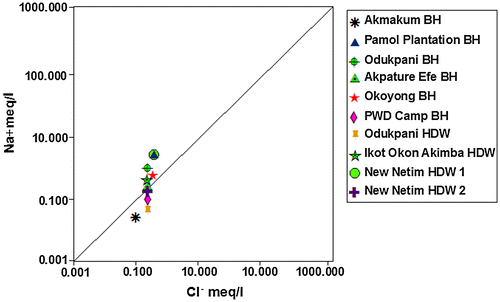
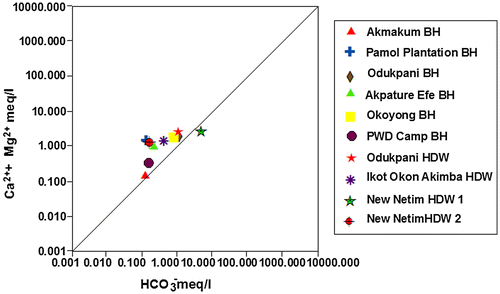
10. Cross plots
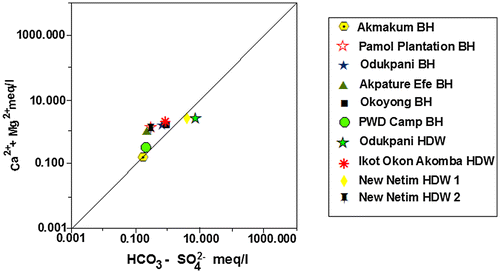
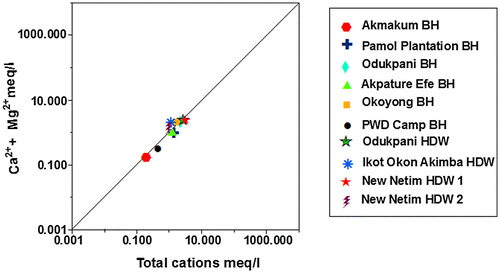
The interconnectivity between Na+ – Cl− has been used to describe the components of salinity and saline intrusions by different authors such as Dixon & Chiwell (Citation1992), Garcia, Hidalgo, & Blesa (Citation2001), and Edet et al. (Citation2011). The coexistent of both ions reveals dissolution of chloride content by evaporation mechanisms (Jalali, Citation2005). Figure shows that the dissolution of gypsum/halite in Akpature Efe Borehole and New Netim Hand Dug Well releases equal concentrations of Na+ – Cl− (Table ) which may be attributed to proximity of the aquifer to sea water intrusions as recorded by Amadi, Ofoegbu, & Morrison (Citation1989); Edet et al. (Citation2011) whereas, other groundwater samples shows no relationship between Na+ – Cl− concentrations indicating that sodium may be associated with other ions in the study area. The plot of Ca2+ + Mg2+ against HCO3− as shown in Figure below reveals excess of Ca2+ + Mg2+ over HCO3− indicating that sources apart from carbonate rocks may be contributing Ca2+ and Mg2+ in the study area. Figure revealed that almost all the measured groundwater samples are pointing above the 1:1 equiline except samples from Odukpani hand dug well and New Netim hand dug well 1 which is pointing below the 1:1 equiline and may also be contributed to congruent weathering of carbonate and incongruent weathering of silicates in the area. As indicated in Figure , the plot of Ca2+ + Mg2+ over Total Cations are typically more or less equal related as most of the measured samples are pointing to 1:1 equiline which is interpreted to be from dissolution of silicate and carbonate minerals, which has undergone various degrees of mixing–dilution and water–rock interaction. The groundwater chemistry in the study area is characterized by ion exchange and reverse ion exchange processes.
Table 4. Results cross plot based on measured samples
11. Conclusion
This work presents the water quality status of the study area for the purpose of drinking water. The results of hydrochemical analysis show that groundwater in the study area are slightly acidic to alkaline and soft to moderately hard in nature. Order of abundance of major chemical species was Ca2+ ˃ Na+ ˃ Mg2+ ˃ K+ ˃ Fe2+ and HCO3− ˃ SO42− ˃ Cl− ˃ CO3− ˃ NO3−. The distribution of major cations and anions suggest that the composition of boreholes and shallow hand dug wells are influenced by water–rock interaction in the area to reach a final stage of evolution represented by the Ca2+ – HCO3− water type (i.e. ion exchange interaction). The results of the investigation indicated that the waters in the study area are supersaturated with respect to calcite and dolomite (supersaturated water has tendency to serious corrosion); and under saturated with respect to gypsum and halite. It was concluded that the most of the samples measured fall within the recommended limits of USEPA (Citation1994).
However, further study is recommended in the area of this study to determine the remedy for the pollution of groundwater by carbonate system in the study area.
This study is only a preliminary effort to spotlight the possible implication of carbonate system in groundwater around the study area.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Ogbonnaya Igwe
Ogbonnaya Igwe is an associate professor and leader of the Environmental Monitoring and Assessment Research Group, University of Nigeria, Nsukka. He is an environmental expat specializing on Sustainable Environmental Improvement and Disaster Prevention. A graduate of the Kyoto University Japan, he holds his PhD in Environmental Protection and Disaster Control. Igwe’s contribution in this work is 40%.
Stanley Ifediegwu
Stanley Ifediegwu is a graduate student under Igwe’s supervision. He has skills in modeling, water analysis, and environmental investigation. This work is part of his graduate research under Igwe. His contribution in this work is substantial (30%).
Daniel Ozoko
Daniel Ozoko is a hydro-geologist with many years of experience in aquifer investigation and evaluation. He is a senior lecturer in the University and a Fellow of many international and local bodies. He has mentored several graduate students most of whom work as either lecturers or company staff. He made substantial contribution (30%) in this work.
References
- Adeleye, D. R., & Fayose, E. A. (1978). Stratigraphy of the type section of the Awi Formation, Odukpani area Southeastern Nigeria. Journal of Mining and Geological Engineering, 15, 33–37.
- Amadi, P. A., Ofoegbu, C. O., & Morrison, T. (1989). Hydrogeochemical assessment of groundwater quality in parts of the Niger delta, Nigeria. Environmental Geology, 14(3), 195–202.
- Anthony, T. B., & Sunday, O. I. (2013). Evaluation of Albian limestone exposed at Dangote cement quarry, Tse-Kucha near Yandev, North Central Nigeria: A geochemical approach. International Journal of Science & Technology, 2, 847–856.
- APHA. (1998). Standard methods for the examination of water and waste water (p. 874). Washington: Author.
- Azubuike, S. E., Edet, A. E., & Solomon, J. E. (2012). Groundwater chemistry of the Oban Massif, Southeastern Nigeria. Revista Ambi and Agua- Interdisc. Journal of Applied Sciences, 7(1), 51–66.
- Dixon, W., & Chiwell, B. (1992). The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, Southeast Queensland, Australia. Journal of Hydrology, 135, 259–274.10.1016/0022-1694(92)90091-9
- Eaton, F. M. (1950). Significance of carbonates in irrigation waters. Soil Science, 39, 123–134.10.1097/00010694-195002000-00004
- Edet, A. E., & Okereke, C. S. (1997). Assessment of hydrogeological conditions in basement aquifers of Precambrian Oban massif, southeastern Nigeria. Journal of Applied Geophysics, 36, 195–204.10.1016/S0926-9851(96)00049-3
- Edet, A. E., Nganje, T. N., Ukpong, A. J., & Ekwere, A. S. (2011). Groundwater chemistry and quality of Nigeria: A status review. African Journal of Environmental Science and Technology, 5(13), 1152–1169.
- Essien, N. U., Ukpabio, E. J., Nyong, E. E., & Ibe, K. A. (2005). Preliminary organic geochemical appraisal of Cretaceous Rock unit in the Calabar Flank, Southern Nigeria. Journal of Mining and Geological Engineering, 41(2), 181–191.
- Garcia, M. G., Hidalgo, M. V., & Blesa, M. A. (2001). Geochemistry of groundwater in alluvial plain of Tucuman province, Argentina. Hydrogeology Journal, 9, 597–610.10.1007/s10040-001-0166-4
- Giuseppe, S., Sibel, E., & Flavia, F. (2013). Water quality assessment of carbonate aquifers in Southern Latium Region, Central Italy: A case study for irrigation and drinking purposes. Applied Water Science, 4, 115–128.
- Iloeje, N. P. (1995). A new geography of Nigeria. Ikeja: Longman Lagos.
- Jalali, M. (2005). Major ion chemistry of groundwaters in the Bahar area, Hamadan, western Iran. Environmental Geology, 47, 763–772.10.1007/s00254-004-1200-3
- Nigeria Ministry of Solid Mineral Development. (2000). An inventory of solid mineral potentials of Nigeria, prospectus for investors (p. 15). Abuja: Author.
- NSDWQ. (2007). Nigerian standard for drinking water quality (p. 30). Nigerian Industrial Standard NIS 554. Standard Organization of Nigeria.
- Odumodu, C. F. (1994). Subsidence and hydrocarbon maturation in the sediments of the Calabar Flank, Southeastern Nigeria. Unpublished M.Sc. Thesis. University of Calabar, Nigeria.
- Reigers, T. J. A., & Petters, S. W. (1987). Depositional environments and diagenesis of Albian Carbonates on the Calabar Flank. Journal of Petroleum Geology, 10(3), 283–294.10.1111/jpg.1987.10.issue-3
- Samuel, T. M., Maina, H. M., & Matera, O. N. (2010). Comparative assessment of limestone resources of Guyuk and Ashaka area for Industrial utilization. J. Phys. Sci. Inno., 2, 1–8.
- USEPA. (1994). Quality criteria for water. EPA 440/5-86-001. Washington, DC.
- Vikas, T. K., Kamra, S. K., Kumar, A., & Vishal, K. (2012). Hydro-chemical analysis and evaluation of groundwater quality for irrigation in Karnal District of Haryana State, India. International Journal of Environmental Science and Technology, 3(2), 756–766.
- WHO. (2004). Guidelines for drinking water quality (3rd ed., Vol. 1, p. 515). Geneva: Recommendation.
- Youdeowei, P. O., & Nwankwoala, H. O. (2010). Subsoil characteristics and hydrogeology of the export-processing zone, Calabar-Southeastern Nigeria. Journal of Applied Sciences and Environmental Management, 14(2), 11–15.