?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pneumonia is the major killer of children under five ages than any other illness in the world. Thus, the main aim of this study was to identify the prevalence and associated risk factors of pneumonia among under-five-year children using the data of the University of Gondar referral hospital. An institution-based cross-sectional study was conducted. A total of 270 children were selected by systematic sampling technique. The source of the data for this study was primary data. Data were entered and cleaned using Epi Info version 7 and exported to SPSS version 20 for analysis. Multinomial logistic regression models were used in this study. The prevalence of pneumonia among children under five was 18.5%. Multinomial logistic regression analysis result showed history of diarrhea (AOR = 2.130, 95% CI: 1.343–9.594), completely immunized (AOR = 0.131, 95% CI: 0.020−0.849, p value = .033), exclusive breastfeeding for at least six months (AOR = 0.108, 95% CI: 0.040−0.292, p value = 0.000), household history of ALRI (AOR = 3.142, 95% CI = 1.213–8.140, p value = 0.018), and crowded house (AOR = 3.908, 95% CI = 1.511–10.108, p value = 0.005) were statistically significantly associated with childhood pneumonia. The prevalence of pneumonia in this study is high. The results of the multiple multinomial logistic regression analysis models show that children who had a history of diarrhea for one month, immunization history, breastfeeding of the child, household history of ALRI, and children living in an overcrowded house were found to be statistically significant factors for pneumonia.
PUBLIC INTEREST STATEMENT
Pneumonia is the single leading cause of mortality in children under five and is a major cause of child mortality. Pneumonia kills more children less than five years of age than any other illness in the world. The prevalence of pneumonia and its consequences for children’s health, and especially for their growth and development, have made pneumonia an important public health problem, given the difficulty in implementing effective measures for controlling it. Childhood pneumonia is caused by a combination of host and environmental factors, but the most common factors are bacteria and virus. In low- and middle-income countries, pneumonia is frequently caused by bacterial pathogens in contrast to high-income countries where viral pathogens predominate. Viruses are the most common cause of pneumonia in young children, but the prevalence decreases with increasing age. It was more common during the rainy seasons and winter months.
1. Introduction
Pneumonia is an acute infection of the lung. It is the main killer of children under five age than any other diseases known to affect children and, also, more than the death of AIDS, malaria, and measles (WHO, Citation2006).
Pneumonia remains the leading infectious cause of death among children under five, killing approximately 2,400 children a day. Pneumonia accounted for around 16% of the 5.6 million under-five deaths, killing around 880,000 child5ren in 2016. Pneumonia causes 15% of all deaths in children under age five worldwide, 2% of which are newborns (UNCF, Citation2014).
Most of these deaths occurred in developing countries where access to care is limited and interventions that have improved care in developed countries are scarce (Izadnegahdar et al., Citation2013)
The under-five-year mortality rate was 55 per 1,000 live births, with pneumonia causing 22% of death (Owais et al., Citation2010). Sub-Saharan Africa had the maximum under-five death rate, having an average under-five death rate of 172 deaths per 1,000 live births (Rudan et al., Citation2004). In Ethiopia, pneumonia is one of the primary causes of death among under-five children in the country, contributing to 28% of death (CSA, Citation2016).
In a study conducted in Bangladesh, the overall prevalence of pneumonia among under-five children was 33.5%. According to the 2016 Ethiopian Demographic Health Survey report, the prevalence of acute respiratory infection in Ethiopia is 7% (CSA, Citation2016). In a study done in Wondo Genet district, Sidama zone, SNNPR, Ethiopia, the prevalence of pneumonia among under-five children was 33.5% (Abuka, Citation2017). In a study conducted in Este town, Ethiopia, the prevalence of pneumonia among under-five children was 16.1% (Fekadu et al., Citation2014). A study carried out in Gondar town, Ethiopia, confirmed that 12% of under-five children suffered from pneumonia.
In low-income countries, various studies conducted previously found out determinants like malnutrition (Fonseca, Kirkwood, Victora, Fuchs, Flores, Misago et al., Citation1996), low birth weight (Mahalanabis et al., Citation2002; Nira et al., Citation2013), breast-feeding (Dadi et al., Citation2014; Mahalanabis et al., Citation2002; Nira et al., Citation2013; Shibre, Citation2015), immunization status (Fonseca et al., Citation1990), infants delivered at home, solid fuel use, history of asthma, economic status, keeping large animals, home ventilation (Mahalanabis et al., Citation2002), overcrowding (Nira et al., Citation2013), living in a household with smoke (Karki et al., Citation2014), education level of the father (Dadi et al., Citation2014), children’s having history of diarrhea (Dadi et al., Citation2014), household history ALRI (Dadi et al., Citation2014), education level of the mother (Karki et al., Citation2014), place of delivery (Amauche, Citation2014), child age (Gabbad et al., Citation2014), and use of the HH during cooking (Fekadu et al., Citation2014) were found as possible risk factors associated with pneumonia among children.
The under-five pneumonia morbidity burden also costs the health services program as health services are passed on to cure high pneumonia morbidity cases. The prevalence of pneumonia and its consequences for children’s health, and especially for their growth and development, have made pneumonia an important public health problem, given the difficulty in implementing effective measures for controlling it. We used a different an advanced statistical model to fill the gap that other studies did not employ. There were no previous studies in this area that could determine the prevalence of the problem. Therefore, the main objective of this study is to determine the prevalence and associated risk factors of pneumonia among under-five year’s children.
2. Material and methods
2.1. Study design
An institutional-based cross-sectional study was employed among under-five-year children in the University of Gondar Referral Hospital.
2.2. Source of population
The source population for this study was all under-five children who visited the University of Gondar Referral Hospital.
2.3. Study population
The study population was all under-five children who visited the University of Gondar Referral Hospital during the data collection period.
2.4. Sample size determination
To determine the sample size, we use the following method. , where n = the desired sample size and Z = 95% standard normal deviation, which is usually set as 1.96.
p = The expected prevalence and is determined from the pilot survey that is taken from Gondar university hospital patients and among 45 random patients, and 15 of them were diagnosed as having pneumonia.
Therefore, p is equal to
That is, 33%
1–0.33 = 0.67, and let d = 0.05, degree of accuracy desired.
N is the monthly number of population surveys in the study period. The monthly number of population survey in the study period was 1,350.
But
The required sample size is 270.
2.5. Sampling techniques
The sampling technique used for this study was the systematic sampling technique. Hence, to select 270 patients among the 1,350 patients, systematic sampling technique is used, starting from one every fifth patient. The starting point is selected by using a simple random sampling technique among five patients.
2.6. Variables in the study
2.6.1. Dependent (response) variables
In this study, patient statuses of pneumonia (no pneumonia, pneumonia, and severe pneumonia) were used as a dependent variable.
2.6.2. Independent (predictor) variables
The independent variables are the age of the child, educational level of the mother, educational level of father, monthly income, place of residence, illness of diarrhea in one month, immunization history, child history of measles in one month, asthma history, place of delivery, cigarette smoker, a child carried on back during cooking, breastfeeding, HH history of ALRI, crowding status of the household, nutritional status weight for age, nutritional status height for age, the main material of roof the house, use of the HH during cooking, home characteristic ventilation the household, child keeping animals, and kind of toilet facility use.
2.7. Statistical models
To meet the objectives of this study, a multinomial logistic regression model was used. Therefore, the MLR model was used to assess the prevalence and associated factors of pneumonia among under-five-year children treated based on the data of Gondar university hospital.
2.7.1. Multinomial logistic regression model
Multinomial logistic regression is a simple extension of binary logistic regression that allows for more than two categories of the dependent or outcome variable. This type of regression is similar to logistic regression, but it is more general because the dependent variable is not restricted to two categories. Like binary logistic regression, multinomial logistic regression uses maximum likelihood estimation to evaluate the probability of categorical membership (Schwab, Citation2002).
2.7.2. Model selection
Model selection is estimating the performance of different models to choose the (approximate) best one. Methods such as forward, backward, and stepwise selection in logistic regression methods are not to be recommended. They give incorrect estimates of the standard errors and p-values and can delete variables that are critical to include. It is much better to compare models based on their results, reasonableness, and fit (as measured, e.g., by the Akaike Information Criterion (AIC) and Bayer’s Information Criterion (BIC)). The AIC and BIC criteria can be used to compare the suitability of competing models and thereby the desirability of retaining a variable in the model. AIC and BIC are defined as:
where p and n denote the number of parameters and the number of observations in the model, respectively. From a set of competing models, the best model is the one with the lowest value of AIC and BIC.
2.7.3. Test overall model fit
In testing the hypothesis that the model fits the data, the two common approaches are the likelihood-ratio statistic (G2) and Pearson’s and deviance chi-square statistics (x2) which are based on the comparison of the fitted and the observed counts.
2.7.4. The likelihood ratio test
The most common assessment of overall model fit in multinomial logistic regression is the likelihood ratio test, which is simply the chi-square difference between the null model (i.e., with the constant only) and the model containing the predictors (full model).
where L0 is the likelihood of the null model and L1 is the likelihood of the full model.
2.7.5. Pseudo R-square
Pseudo R squares are additional measures of goodness of fit for multinomial logistic regression.
There are several measures intended for the R-squared analysis for the OLS regression model, but none of them is an R-squared.
To estimate the coefficient of determination, we used three different methods, which are as follows:
2.7.5.1. McFadden’s
McFadden’s R2 is defined as:
where Mfull = model with predictors, Mintecept = model without predictors, and estimated likelihood.
2.7.5.2. Cox and Snell
Cox & Snell’s R2 is defined as
2.7.5.3. Nagelkerke
Nagelkerke's is defined as:
2.7.6. Wald test
Wald statistic is an alternative test that is commonly used to test the significance of individual logistic regression coefficients for each predictor. A Wald test is used to test the statistical significance of each coefficient () in the model. The statistic is defined as
,
where βj is the estimated coefficient for the first variable and
is its standard error.
2.8. Operational or standard definitions
Operational definitions for key concepts and measurements in the study are as follows:
No pneumonia: it is defined as a child with any signs of pneumonia, no pneumonia, or severe or very severe pneumonia.
Pneumonia: defined as a child with cough or difficult breathing having only fast breathing, but no lower chest wall in drawing or no signs of very severe pneumonia.
Severe pneumonia: defined as a young infant with cough or difficult breathing having fast breathing and/or lower chest wall in drawing or a child with cough or difficult breathing having only lower chest wall in drawing (WHO, Citation2005).
3. Result
3.1. Summary of descriptive statistics
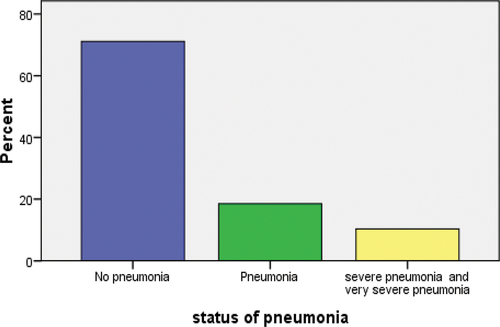
We analyzed the data among under five children from Gondar university hospital. From the sampled children, 71.1%, 18.5%, and 10.4% are no pneumonia, pneumonia, severe pneumonia, and very severe pneumonia, respectively.
The distribution is shown graphically in .
According to , 77.6% of the children whose fathers are illiterate have no pneumonia, 10.6% have pneumonia, and 11.8% have severe pneumonia. For primary (1–8) educational-level father group, 84.9% have no pneumonia, 11.3% have pneumonia, and 3.8% have severe pneumonia. Similarly, 79.2% of the children whose fathers have secondary (9–12) educational level have no pneumonia, 18.8% have pneumonia, and 2.1% have severe pneumonia.
Table 1. Percentage distribution status of pneumonia among under-five children within categories of explanatory variables
According to chi-square test results, age of the child by month, educational level of the mother, had measles, a child carried on back during cooking, weight for age, height for age, child keeping animals, and type of toilet facility use are not found to be significant predictors of pneumonia. Therefore, the p values of these variables are greater than .25, and therefore, they are not candidates for the multivariable model. From chi-square test results, educational level of father, monthly income, place of residence, had diarrhea, immunization history, infants delivered, cigarette smoker, breastfeeding history, history of ALRI, the crowded status of the household, the main material of the roof, and use of the household during cooking are statistically significant and, also, candidates for the multivariable model because the p values of these variables are less than .25 ().
Table 2. Univariate test result for likelihood ratio and Pearson’s chi-square test
4. Associated risk factors of pneumonia among under-five children
A child who had a history of diarrhea during the last one month (AOR = 2.130, 95% CI: 1.343–9.594, p value = 0.017) was 2.130 times more likely to get pneumonia as compared to a child who does not have a history of diarrhea.
This study also revealed that completely immunized children (AOR = 0.131, 95% CI: 0.020−0.849, p value = 0.033) were .131 times less likely to develop pneumonia as compared to children who were partially immunized.
Children whose parents practiced exclusive breastfeeding for at least six months (OR = 0.108, 95% CI: 0.040−0.292, p value = 0.000) were .108 times less likely to develop pneumonia than those whose parents practiced mixed breastfeeding.
The odds of pneumonia among children under five living in an overcrowded house (OR = 3.908, 95% CI = 1.511–10.108, p value = 0.005) were 3.908 times higher than among participants not living in an overcrowded house.
The odds for children with a household history of ALRI were 3.142 (OR = 3.142, 95% CI = 1.213–8.140, p value = 0.018) higher than for their counterparts without a household history of ALRI ().
Table 3. Parameter estimates of a model for multiple multinomial logistic regression analysis
Table 4. Model fit mummery statistics
Table 5. Pseudo R-square for model
Accordingly, the deviance-based chi-square test (χ2 = 199.276, df = 30, p-value <0.000) shows that the fit is adequate, meaning that at least one of the independent variables is significantly related to the response variable. The AIC values for the null model and the full model are 401.512 and 262.237, whereas the BIC values are 408.709 and 3.77.386, respectively. Since the full model has smaller values of AIC and BIC, we can conclude that it is a better fit for the data.
Nagelkerke's R2 will normally be higher than the Cox and Snell’s and McFadden’s measure. The Nagelkerke’s R2 that does range from 0 to 1 is a more reliable measure of the relationship and is the most reported of the R-squared estimates. In this case, it is 0.670, indicating a moderately strong relationship of 67% between the predictors and the prediction of the response variable.
5. Discussion
The main aim of this study was to identify the prevalence and determinants of pneumonia among under-five-year children using the data of the University of Gondar referral hospital. We found that the prevalence of pneumonia among under-five children in the current study was 18.5%. In our study, the prevalence of pneumonia is almost twice the prevalence of ARI (7.0%) among similar children reported by EDHS (CSA, Citation2016) and Debre Birhan District, North-East Ethiopia (5.5; Shibre, Citation2015). The possible reason for the difference in the prevalence of pneumonia might include the time of data collection, the assessment method used, and the difference in the level of advancement as well as the aggregation of risk factors.
In our study, the prevalence of pneumonia was higher than the prevalence of pneumonia reported by studies conducted in Arisi zone, Ethiopia (17.7%; Lema et al., Citation2019), Este town, Ethiopia (16.1%; Fekadu et al., Citation2014), Jimma zone (28.1%; Andualem et al., Citation2019), and Wondo Genet District (33.3%), Ethiopia (Abuka, Citation2017). These differences might be due to differences in study settings, environmental factors, the basic infrastructure of study households, and socio-demographic characteristics of mothers/caregivers. Our finding is also consistent with that of a community-based cross-sectional study in Jimma zone (28.1%; Andualem et al., Citation2019) and Wondo Genet District (33.3%) in Ethiopia (Abuka, Citation2017).
Children who had a history of diarrhea during the last month were 2.130 times more likely to develop pneumonia as compared to children who did not have a history of diarrhea. This result is supported by a study from Brazil and Ethiopia (Victora et al., Citation1994; Shibre, Citation2015; Dadi et al., Citation2014). This study also revealed that completely immunized children were .131 times less likely to develop pneumonia as compared to children who were partially immunized. Similar results were reported by Hemagiri et al. (Citation2014).
Children whose parents practiced exclusive breastfeeding for at least six months were .108 times less likely to develop pneumonia than those whose parents practiced mixed breastfeeding. This result is in agreement with K et al. (Citation2014); Fonseca, Kirkwood, Victora, Fuchs, Flores, Misago et al. (Citation1996); and Nira et al. (Citation2013).
In this study, children with a household history of ALRI predicted higher odds of a child having pneumonia compared to the absence of a child with a household history of ALRI. This finding is consistent with those of studies conducted in the Oromia zone, Ethiopia (Dadi et al., Citation2014); Kenya (Onyango et al., Citation2012); and India (Bhat & Manjunath, Citation2013).
In this study, children living in overcrowded houses were found to be a predictor of childhood pneumonia. This result supports findings from studies from Indonesia (Nira et al., Citation2013), northeast Brazil (Jackson et al., Citation2013), Canada (Banerji et al., Citation2009), and India (Goel et al., Citation2012).
6. Conclusions
The prevalence of pneumonia in this study area is high. The results of the multiple multinomial logistic regression analysis models show that children who had a history of diarrhea during the last one month, immunization history, breastfeeding of the child, household history of ALRI, and children living in an overcrowded house were found to be statistically significant factors for pneumonia. Therefore, children who have a concomitant illness like diarrhea may have a lowered immunity, making them more susceptible to diseases like pneumonia. Immunization has enormous potential to reduce the burden of childhood deaths from pneumonia in developing countries. Especially, complete immunization is a marker for increased access to health care services and better child care practices. Pneumonia can be reduced by promoting breastfeeding for the first six months. ALRIs are easily transmitted from household contacts to children. ARIs are very contagious and easily transmitted. Crowded houses may increase the probability of transmission of infectious disease from one family member to another family member. Historically, in developed countries, death from pneumonia has been reduced by improvement in living conditions and air quality.
Lists of acronyms
Ethical Approval
Ethical clearance was obtained from the college review board of the University of Gondar, College of Natural and Computational Science. A formal letter of cooperation was written for Gondar university hospital.
Consent
Consent for publication is secured from study participants. Consent from study participants was obtained prior to the data collection process. Information and confidentiality have been maintained by enrolling data collectors. People were not being forced to participate in the research, which is the fundamental principle of voluntary participation in research ethics.
Consent for publication
Not applicable.
Data Availability
The dataset analyzed during the current study is available from the corresponding author.
Authors’ contributions
Dessie Melese has contributed to conceptualization of the research problem, research concept and design, collection, and/or assembly of data, data analysis, and interpretation and writing of the article. Asrat Atsedwyen, Kassim Mohammed, Mequanent Wale Bisrat Misganaw, and Moges Zerihun have played a great role in re-vision of the research design, manuscript write-up, and editing the entire manuscript. Finally, all the authors have read and approved the final manuscript.
Acknowledgements
We thank all study participants and pediatric staff at UGH who gave full collaboration during the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Dessie Melese Chekole
Mr Dessie Melese is a lecturer at the University of Gondar, College of Natural and Computational Sciences, Department of Statistics, Gondar, Ethiopia. He graduated from the University of Gondar in statistics (B.Sc. in statistics and M.Sc. in biostatistics). From August 2011 to June 2016, he served as a junior statistician at Central Statistics Agency, Gondar Branch, Ethiopia. Currently, he works as a lecturer at the University of Gondar, Department of Statistics. His responsibilities are teaching different courses of statistics, consulting and advising students on academic issue and on their senior research project, working on research and community service in both team and individual levels, strongly participating in different national and international conferences, and giving/participating different advanced statistical software trainings.
References
- Abuka, T. (2017). Prevalence of pneumonia and factors associated among children 2-59 months old in Wondo Genet district. Sidama zone, SNNPR, Ethiopia: Current pediatric research.
- Amauche, F. (2014). Determine the risk factors that affect the prevalence of ARIs in under-five children in Enugu, Southeast Nigeria.
- Andualem, Z., Adane, T., Tigabu, A., Yallew, W. W., Wami, S. D., Dagne, H., Azanaw, J., Guyasa, G., Nigussie Azene, Z., & Endalew, M. (2019). Pneumonia among under-five children in Northwest Ethiopia: Prevalence and predictors a community-based cross-sectional study.
- Banerji, A., Greenberg, D., White, L. F., Macdonald, W. A., Saxton, A., Thomas, E., Sage, D., Mamdani, M., Lanctôt, K. L., Mahony, J. B., Dingle, M., & Roberts, A. (2009). Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children. Pediatric Infectious Disease Journal, 28(8), 697–15. https://doi.org/10.1097/INF.0b013e31819f1f89
- Bhat, R., & Manjunath, N. (2013). Correlates of acute lower respiratory tract infections in children under 5 years of age in India. The International Journal of Tuberculosis and Lung Disease, 17(3), 418–422. https://doi.org/10.5588/ijtld.12.0117
- CSA (2016). Ethiopia demographic and health survey, Addis Ababa. Ethiopia, and Rockville, Maryland, USA: CSA and ICF.
- Dadi, A. F., Kebede, Y., & Birhanu, Z. (2014). Determinants of pneumonia in children aged two months to five years in urban areas of Oromia Zone, Amhara Region, Ethiopia. Open Access Library Journal, 1(8), 1. http://dx.doi.org/10.4236/oalib.1101044
- Fekadu, G. A., Terefe, M. W., & Alemie, G. A. (2014). Prevalence of pneumonia among under-five children in Este Town and the surrounding rural Kebeles, Northwest Ethiopia: A community-based cross-sectional study. Science Journal of Public Health, 2(3), 150–155. https://doi.org/10.11648/j.sjph.20140203.12
- Fonseca, W., Kirkwood, B. R., Victora, C. G., Fuchs, S. R., Flores, J. A., & Misago, C. (1990). Risk factors for childhood pneumonia among the urban poor in Fortaleza. Brazil.
- Fonseca, W., Kirkwood, B. R., Victora, C. G., Fuchs, S., Flores, J., & Misago, C. (1996). Risk factors for childhood pneumonia among the urban poor in Fortaleza, Brazil: A case-control study. Bulletin of the World Health Organization, 74(2), 199.
- Gabbad, A. A., Alrahman, G. M. A., & Elawad, M. A. (2014). Childhood pneumonia at Omdurman Paediatric Hospital, Khartoum, Sudan. International Journal of Multidisciplinary and Current Research, 2.
- Goel, K., Ahmad, S., Agarwal, G., & Goel, P. (2012). A cross-sectional study on the prevalence of acute respiratory infections (ARI) in under-five children of Meerut District. India Community Med Heal Educ, 2(9), 2–5. doi:10.4172/2161-0711.1000176
- Hemagiri, K., Sameena, A., Aravind, K., Khan, W., & Vasanta, S. (2014). Risk factors for severe pneumonia in under-five children – A hospital-based study. Int J Res Health Sci, 2(1), 47–57.
- Izadnegahdar, R., Cohen, A. L., Klugman, K. P., & Qazi, S. A. (2013). Childhood pneumonia in developing countries. The Lancet Respiratory Medicine, 1(7), 574–584. https://doi.org/10.1016/S2213-2600(13)70075-4
- Jackson, S., Mathews, K. H., Pulanić, D., Falconer, R., Rudan, I., Campbell, H., & Nair, H. (2013). Risk factors for severe acute lower respiratory infections in children – A systematic review and meta-analysis. Croatian Medical Journal, 54(2), 110–21. https://doi.org/10.3325/cmj.2013.54.110
- K, H., B, S. A. R., K, A., Khan, W., SC, V., & Article, O. (2014). Risk factors for severe pneumonia in under-five children – A hospital-based study. http://www.ijrhs.com
- Karki, S., Fitzpatrick, A., & Shrestha, S. (2014). Risk factors for pneumonia in children under 5 years in a teaching hospital in Nepal. Kathmandu University Medical Journal, 12(4), 247–252. https://doi.org/10.3126/kumj.v12i4.13729
- Lema, B., Seyoum, K., & Atlaw, D. (2019). Prevalence of community-acquired pneumonia among children 2 to 59 months old and its associated factors in Munesa District. Oromia Region, Ethiopia: Arsi Zone.
- Mahalanabis, D., Gupta, S., Paul, D., Gupta, A., Lahiri, M., & Khaled, M. (2002). Risk factors for pneumonia in infants and young children and the role of solid fuel for cooking: A case-control study. Epidemiology & Infection, 129(1), 65–71. https://doi.org/10.1017/S0950268802006817
- Nira, N. K., Pramono, D., & Naning, R. (2013). Risk factors of pneumonia among under five children in Purbalingga District, Central Java Province. Tropical Medicine Journal, 3(2). http://dx.doi.org/10.22146/tmj.5864
- Onyango, D., Kikuvi, G., Amukoye, E., Omolo, & Omolo, J. (2012). Risk factors of severe pneumonia among children aged 2–59 months in western Kenya. The Pan African Medical Journal, 13.
- Owais, A., Tikmani, S. S., Sultana, S., Zaman, U., Ahmed, I., Allana, S., & Zaidi, A. K. (2010). Incidence of pneumonia, bacteremia, and invasive pneumococcal disease in Pakistani children. Tropical Medicine & International Health, 15(9), 1029–1036.
- Rudan, I., Tomaskovic, L., Boschi-Pinto, C., & Campbell, H. (2004). Global estimate of the incidence of clinical pneumonia among children under five years of age. Bulletin of the World Health Organization, 82(12), 895–903.
- Schwab, J. A. (2002). Multinomial logistic regression: Basic relationships and complete problems.
- Shibre, G. (2015). Assessment of the prevalence and associated factors of pneumonia in children 2 to 59 months old, Debre Birhan District. North-East Ethiopia: Addis Ababa University.
- UNCF. (2014). Pneumonia is a leading single disease killing under-five children.
- Victora, C. G., Fuchs, S. C., Flores, J. A. C., Fonseca, W., & Kirkwood, B. (1994). Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics, 93(6), 977–985. https://doi.org/10.1542/peds.93.6.977
- WHO. (2005). WHO pocketbook of hospital care for children: Guidelines for the management of common illness with limited resources.
- WHO. (2006). The world health report 2006: Working together for health, World Health Organization.