Abstract
48-year-old female with facial granulomatous nodules and fungal/bacterial infection after hyaluronic acid injection. She underwent anti-fungal/antibacterial therapy and local excision. The proposed mechanisms include inflammatory foreign body reaction and pathogen contamination. Providers must exercise caution with the use of facial fillers and demonstrate expertise in avoiding and managing potential complications.
Introduction
Over the past two decades, facial fillers have become an increasingly popular and widely used method of facial rejuvenation [Citation1,Citation2]. They provide a relatively safe, non-invasive way to achieve a more youthful appearance without the risks of surgery. Hyaluronic acid fillers have become the facial filler of choice because, overall, they are safe, effective, long lasting, less immunogenic and easy to store and apply [Citation3]. Due to the growing market and profitability, many other medical practitioners have begun to incorporate the use of fillers into their practice [Citation4]. Although fillers provide a non-surgical approach to the restoration of volume and youthfulness to the face, they are not harmless products. This is a clinical case of hyaluronic acid injection by a non-plastic surgeon that induced an unexpected adverse reaction resulting in subsequent infection and irreversible scarring.
Case report
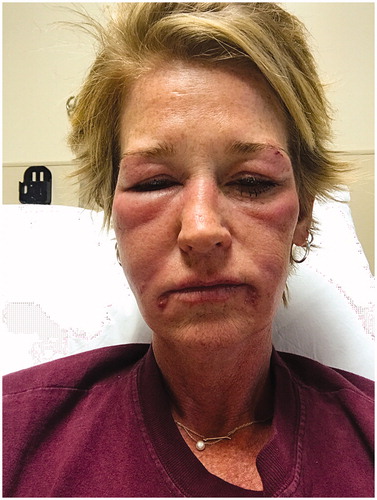
A 48-year-old woman presented with facial lesions ten months after injection with hyaluronic acid filler into her glabella, nasolabial folds and marionette lines performed by an outside dermatologist. Information about the exact product used and dosing was unavailable to us. One month after the filler injection, she developed a hypersensitivity reaction consisting in facial edema, erythema, itchiness and mild fever (). She was evaluated with computed tomography (CT) scan at an outside hospital which demonstrated preseptal cellulitis and soft tissue edema without evidence of abscess. This cellulitic reaction was treated with intravenous (IV) antibiotics and intramuscular steroids and resolved without complication. Seven months later, the patient used a brand-new suction powered facial pore cleansing device on her entire face. Thereafter, she developed enlarging ulcerated lesions only on areas previously injected with hyaluronic acid filler. The microscopic examination performed by her dermatologist revealed the presence of fungal hyphae. For this reason, the patient was referred to our institution for further management.
Figure 1. One month after the hyaluronic acid filler injection, patient developed a facial hypersensitivity reaction consisting in facial edema, erythema, itchiness and mild fever.
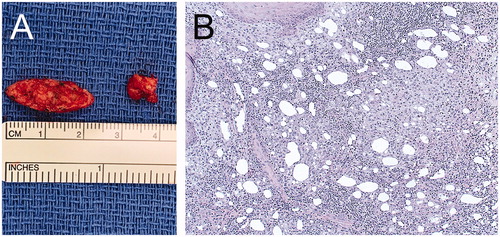
Upon admission, patient exhibited verrucous granuloma-like skin lesions on glabellar region, nasolabial folds and marionette lines (). CT face scan with IV contrast revealed abnormal cutaneous and subcutaneous heterogeneous enhancing consistent with phlegmon or early abscess formation (). She was treated with amphotericin B (5 mg/kg IV q24h for 2 weeks) guided by infectious disease consultants. In addition, local cultures of the lesions reported the presence of Eschericia coli, Enterococcus faecalis, and Staphylococcus epidermidis. Accordingly, vancomycin (15 mg/kg IV q12h) and meropenem (2 g IV q8h) were also added for 1 week. On post-admission day 15, we proceeded with definitive surgical debridement via wide local excision followed by wound care for healing by secondary intention (). On gross examination, the specimens appeared granulomatous in nature with liquefactive necrosis (). Final pathology reported the presence of squamous epithelium with chronic lymphohistiocytic inflammation and fibrinopurulent exudate including foreign body giant cells (). Grocott’s methenamine silver (GMS) stain with adequate positive and negative controls were negative for fungal infection.
Figure 2. A. Verrucous ulcerative skin lesions on nasolabial folds, marionette lines and glabellar region (not shown). B. Detail of the lesions on the left side of the face. C and D. Arrowheads show the abnormal heterogeneous enhancing soft tissue extending along the lateral left maxilla (1.6 cm AP by 1.0 cm transverse by 2.6 cm craniocaudal dimensions).
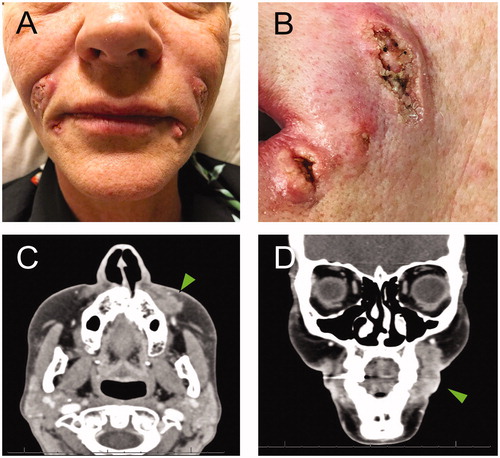
Figure 3. A and B. Intraoperative images showing the communication between nearby lesions and extension up to the infraorbital rim. C and D. Postoperative wounds that healed with regular dressing by secondary intention.
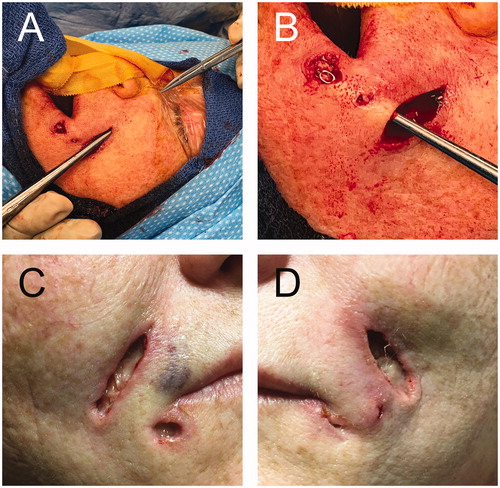
Figure 4. A. Intraoperative image showing the specimens from the left nasolabial fold and left marionette line. B. Hematoxylin-eosin stain showing lipogranulomas with granulomatous inflammation within the connective tissue stroma (20× magnification).
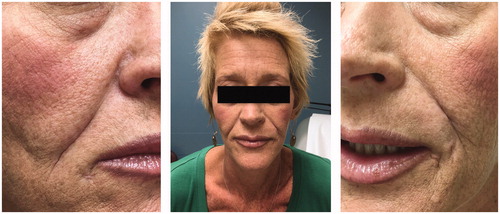
After significant clinical improvement, the patient was discharged with oral antibiotics and plans for close follow-up and future scar revision. Thus, based on culture results from intraoperative tissue samples, infectious disease team recommended doxycycline (100 mg by mouth twice a day for 14 days) and no further antifungal agents. She was seen most recently eight months post operatively. She showed no signs of ongoing infection and wounds were well healed. However, the deep scarring in the nasolabial folds has made the folds more prominent (). This is an aesthetic concern for the patient, but in an attempt to avoid direct excision or reinjection of foreign material locally, we have discussed alternatives which include laser of the affected area and partial scar revision.
Discussion
The most common filler complications include bruising, swelling, over- or under-volumization, and, more concerning, infection, vascular occlusion, and blindness [Citation5]. This case, in contrast, represents an unusual delayed inflammatory and infectious complication.
It is unclear what caused our patient’s ulcerative lesions. She had a complex presentation with multiple factors that contributed to the progression of her facial lesions. Based on her presentation and review of the literature, we hypothesize that she initially had an allergic type reaction. Even though the CT report attributed the event as preseptal cellulitis, she had other areas of inflammation at other injection sites as demonstrated in the photograph and a pattern mimicking angioedema (). Subsequently, the aggressive suction-assisted pore cleansing of the face could potentially create microscopic epidermal abrasions leading to inadvertent inoculation of pathogenic agents. Interestingly, she developed delayed inflammatory foreign body reaction lesions only in those areas where the hyaluronic acid filler was previously injected. In this regard, although the patient claims impeccable technique by the injecting provider, it is no possible to eliminate suboptimal technique at the time of injection. Accordingly, Mycobacterium facial infections due to the topical application of nonsterile ice prior to the filler injection has been described [Citation6,Citation7]. In addition, the patient states that the stock of fillers at her provider’s office was investigated without any evidence of contamination. This finding is consistent with several reports about the sterility of partially used hyaluronic acid fillers [Citation8,Citation9].
There have only been few reports of similar complications in the literature, and this is the only case exhibiting all three features: allergic, inflammatory, and infectious [Citation5–7,Citation10–12]. The potential underlying mechanisms for this rare unexpected course of hyaluronic acid filler injection include: 1) immediate and delayed hypersensitivity reactions, and 2) granuloma formation mediated by either inflammatory foreign body reactions or bacterial biofilm aggregation.
Several authors have described in detail type I histamine and IgE mediated hypersensitivity reactions as well as delayed type IV T- cell mediated hypersensitivity reactions as unusual complications of hyaluronidase injection [Citation13,Citation14]. The former can be seen in rare cases that rapidly progress to angioedema [Citation5,Citation10,Citation13–17].
In our patient, the clinical assessment of the lumps as well as their subsequent pathologic examination ( and ) support the diagnosis of granulomatous formation. It seems that the initial injection of filler triggers a physiologic foreign body reaction as a result of an influx of inflammatory cells [Citation5–14,Citation16,Citation18]. However, when there is a failure of effective phagocytosis of such inflammatory cells, the original self-limited response can progress to a severe inflammatory granulomatous process [Citation10,Citation13]. The underlying mechanism that induces this adverse transformation is currently not well understood [Citation13]. Such reactions may be also triggered by formation of bacterial biofilms which have similar presentation and are equally difficult to treat [Citation10,Citation19].
As final contributing factor in our case, the patient was transferred to our institution with diagnosis of fungal infection. Although no fungal growth was obtained in additional cultures after admission, appropriate treatment was established following the recommendation of infectious disease colleagues.
Ultimately, the combination of antibacterial and antifungal systemic treatments along with surgical excision eradicated any ongoing infection without further complication.
Conclusion
In summary, we report an unusual progression of rare complications of hyaluronic acid injection in a single patient. This type of cases requires a multidisciplinary approach with a combination of systemic and surgical management [Citation13,Citation15]. It is also important to emphasize the need for awareness and caution when injecting facial fillers as well as expertise in avoiding and managing potential complications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bass LS. Injectable filler techniques for facial rejuvenation, volumization, and augmentation. Facial Plast Surg Clin North Am. 2015;23(4):479–488.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23(4):423–432.
- Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19(3):141–150.
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesth Plast Surg. 2014;38(5):946–955.
- Vidič M, Bartenjev I. An adverse reaction after hyaluronic acid filler application: a case report. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27(3):165–167.
- Rodriguez JM, Xie YL, Winthrop KL, et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33(2):265–269.
- Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast. Reconstr Surg. 2013;132(4 Suppl 2):48S–58S.
- Al-Haddab M, Abduljabbar A, Somily AM. The sterility of partially used hyaluronic acid fillers after long storage. Dermatol Surg. 2017;43(7):967–970.
- Alharbi M. Review of sterility of reused stored dermal filler. J Cosmet Dermatol. 2019;18(5):1202–1205.
- Mamelak AJ, Katz TM, Goldberg LH, et al. Foreign body reaction to hyaluronic acid filler injection: in search of an etiology. Dermatol Surg. 2009;35(Suppl 2):1701–1703.
- Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42(2):232–239.
- Kim JH, Choi JS, Yun JH, et al. Foreign body reaction to injectable hyaluronic Acid: late granuloma formation. Ann Dermatol. 2015;27(2):224–225.
- Snozzi P, van Loghem J. Complication management following rejuvenation procedures with hyaluronic acid fillers-an algorithm-based approach. Plast Reconstr Surg Glob Open. 2018;6(12):e2061.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
- Signorini M, Liew S, Sundaram H, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers-evidence- and opinion-based review and consensus recommendations. Plast. Reconstr. Surg. 2016;137(6):961e–971e.
- Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 Pt 2):1616–1625.
- Cavallini M, Gazzola R, Metalla M, et al. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J. 2013;33(8):1167–1174.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23(4):447–458.
- Urdiales-Galvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510.