Abstract
We report the combination of osteocutaneous radial forearm free flap and extensive cervicothoracic flap to reconstruct a large through-and-through cheek and mandibular defect. In patients with difficult clinical settings, this approach reduces operative time and complications without compromising the functional and cosmetic outcomes.
Introduction
The considerable variability of surgical defects following head and neck ablative procedures demands the implementation of a broad armamentarium of reconstructive options [Citation1,Citation2]. Thus, random flaps have restricted mobility, and therefore, they are more suitable for coverage of relatively small defects [Citation3,Citation4]. Although axial flaps can overcome these limitations, they often appear too bulky and display color and texture mismatch with respect to tissues of recipient regions [Citation5]. In addition, axial flaps may cause significant functional impairments and unfavorable cosmetic outcomes at the donor sites [Citation6].
In recent decades, microsurgical flaps have become routine tools for head and neck reconstruction [Citation7]. However, the presence of multiple comorbidities, poor nutritional status, systemic inflammatory conditions, immune system disorders and coagulopathies, among others may generate adverse medical settings for these time-consuming and technically demanding surgical procedures [Citation8]. These concerns are especially crucial in cases where multiple tissues need to be replaced (i.e. skin, bone and intraoral lining).
The cervicofacial flap, an extended version of Mustarde’s rotation flap [Citation9], has been described for the reconstruction of midface soft tissue defects especially on cheek, temple and inferior eyelid [Citation1–12]. Its cervicothoracic variant includes the dissection of the superior thoracic wall to expand the arc of rotation [Citation13]. Cervicofacial and cervicothoracic flaps are timesaving flaps that provide excellent skin color, thickness and texture match with cosmetically acceptable scars and minimal donor site morbidity [Citation10–13].
In this article, we describe the use of an extensive cervicothoracic rotation flap and an osteocutaneous radial forearm free flap to reconstruct a composite through-and-through cheek and mandibular defect caused by the resection of a squamous cell carcinoma lesion.
Case report
A 62-year-old Indian male who presented to our clinic for evaluation of a persistent intraoral lesion. The lesion progressively increased in size for 9 months and became painful. Patient denied any weight loss, dysphagia, or facial swelling. He has no significant medical or surgical history. He chewed betel quid for twenty years but quit seven years ago.
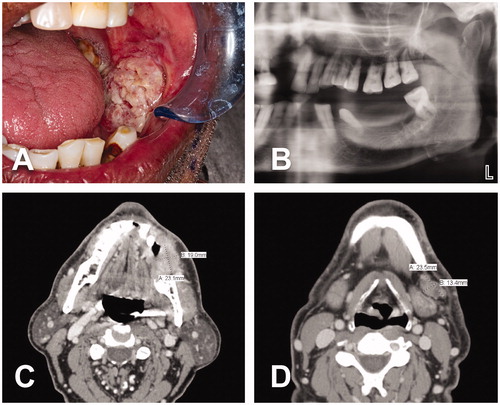
On physical examination, his neck was soft and non-tender with a mobile 2-cm node at the left submandibular region. Intraoral assessment confirmed a tongue with full range of motion and a 3 × 3 cm exophytic ulcerative lesion of the left buccal mucosa with no bony invasion (). The CT neck demonstrated hyperdensity along the left buccal mucosa ridge measuring 2.3 × 1.9 × 2.3 cm as well as a centrally necrotic ipsilateral lymph node at level 1B (). The CT chest was unremarkable and the CTA of lower extremities showed severe peripheral vascular disease. The histological study of the incisional biopsy revealed invasive keratinizing moderately differentiated squamous cell carcinoma. Finally, the case was examined by the institutional tumor board and classified as a cT4aN1M0. Based on findings, the tumor board recommended surgery as primary treatment. Accordingly, three-dimensional (3D) printed models, a pre-bent reconstruction plate and surgical cutting guides for the mandible resection were obtained prior to the procedure.
Figure 1. A 62-year-old Indian male with a cT4aN1M0 intraoral lesion. (A) Exophytic ulcerative squamous cell carcinoma of the left buccal mucosa. (B) Left side of panoramic dental x-ray demonstrating absence of bony invasion. This finding was corroborated by CT scan. (C–D) CT neck revealed a 2.3 × 1.9 × 2.3 cm tumor at the left buccal mucosa and a 2.3 × 1.3 cm centrally necrotic ipsilateral lymph node at level 1B.
The surgery began with a standardized tracheostomy. Because an 8-cm skin defect at the left cheek was planned as part of the oncological resection, the surgery continued with the elevation of a cervicothoracic rotation flap. We utilized a medially based skin flap. The incision was made from the margin of the cheek skin resection (near to the left commissure) toward the tragus. Then, the incision continued a preauricular trajectory followed by a course around the ear lobe. Subsequently, the incision extended downward just anterior to the hairline and then along the anterior edge of the trapezius muscle and the deltopectoral groove. At the anterior border of the axilla, the incision turned inward in 90 degrees (back-cut) and continued in a medial direction, above the areola, until the lateral border of the sternum (). The flap was elevated in a sub-SMAS, subplatysmal and subfascial approach, respectively (). The left modified radical neck dissection was then performed including the levels 3–5 of lymphoareolar tissues. The external jugular vein was identified and ligated. Next, the anterior mandibular osteotomy was performed using reciprocating saw and cutting guide. Afterward, the mandible was displaced laterally to allow the dissection of the tumor and preserve 1.5-cm safety margins from surrounding mucosa. The left portion of the floor of the mouth, the exophytic lesion, the nearby buccal mucosa and muscular plane as well as the overlying cheek skin were all excised and incorporated to the specimen (). Subsequently, the distal mandibular osteotomy was carried out with reciprocating saw from the mandibular notch (to include the coronoid process), along the ramus, up to the inferior border of the mandible. The insertion of the temporal muscle was stripped off from the coronoid process to complete the release of the specimen (). Finally, extraction of all remaining teeth was performed.
Figure 2. Intraoperative views. (A-B) Marking of the oncological skin excision on the left cheek and design of the large medially-based left cervicothoracic rotation flap. (C–D) Cervicothoracic flap already harvested and reflected medially. Completion of tumor resection (including surrounding tissues and cheek skin), segmental mandibulectomy and left modified radical neck dissection.
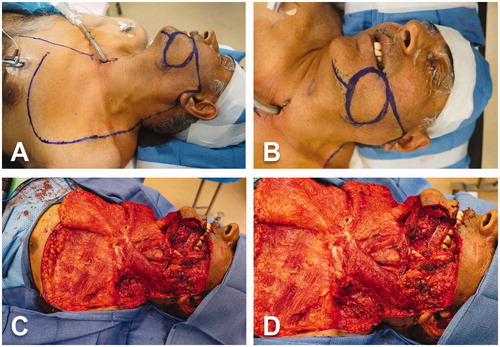
Figure 3. Intraoperative views (cont’d). (A) Surgical specimen including tumor and surrounding tissues, cheek skin, floor of mouth, segment of left mandible and lymphoareolar tissues of the left modified radical neck dissection. (B) Advancement and rotation of the cervicothoracic flap to cover the cheek defect. (C–D) Inset of the cervicothoracic flap.
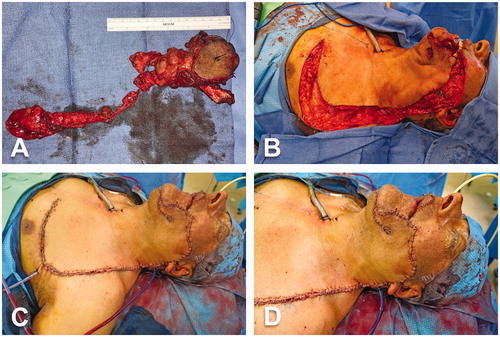
Simultaneously, a second surgical team harvested a left osteocutaneous radial forearm free flap. Briefly, centered on the radial artery trajectory, a 5 × 8 cm skin paddle was elevated in standard fashion from the medial edge up to the radial side of the flexor carpi radialis (FCR) tendon. The dissection continued downward through the muscle bellies of flexor digitorum superficialis (FDS) and flexor pollicis longus (FPL) until the radius was encountered behind the radial vessels. Thereafter, the lateral edge of the flap was dissected medially to the brachioradialis tendon and protecting the lateral antebrachial cutaneous nerve. Osteotomy of the anterolateral half of the radius with oscillating saw. This step was accomplished without cutting guides. A 9-cm bony segment was incorporated to the flap. The radial artery and its venae comitantes were released up to the antecubital fossa. Once the flap was brought to the left aspect of the face, microsurgical anastomoses were performed under the microscope: end-to-end arterial anastomosis with left facial artery using 9-0 nylon sutures and end-to-end venous anastomosis with facial vein using 2.5-mm coupler. The vascularized segment of radius was subsequently used to reconstruct the mandible with a pre-bent 2.4-mm reconstruction plate and 6-mm screws. The plate was then fixed to the remaining mandible with bicortical screws. Afterward, the intraoral lining was re-established by suturing the skin paddle to the surrounding mucosa with multiple 2-0 vicryl stitches. Finally, the cervicothoracic flap was advanced and rotated to close the cheek defect and sutured in layers with multiple stitches of 2-0 vicryl and 5-0 nylon ().
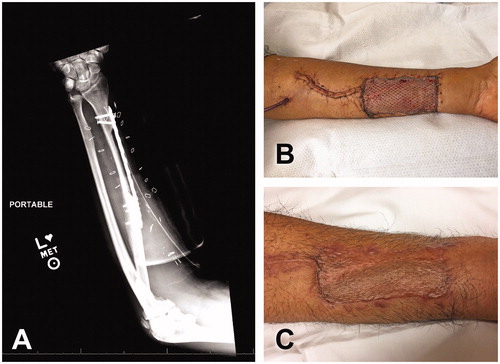
At the left forearm, to prevent stress fractures, a 14-cm reconstruction plate was placed over the bony defect. More specifically, the plate was partially placed over the bony defect and partially over the more lateral aspect of the intact radius. This practice facilitates the coverage of a thick and wide reconstruction plate with the nearby skin flap while maintaining the protection over the radius defect. Following our protocol, the plate length allowed two bicortical screws distally and three bicortical screws proximally to the radius bony defect (). The incision leading to the antecubital fossa was closed over a Blake drain with 3-0 vicryl stitches at the deep dermal layer followed by 4-0 nylon sutures at the skin. Even though peritendons were preserved, we buried exposed tendons inside the surrounding muscles or cover them by advancing the skin edges. The remaining open area (skin paddle harvest) was covered with 0.012-inch split thickness skin graft, meshed at 1:1.5 ratio. We squeezed the meshed skin graft in to almost occlude the holes of the 1:1.5 expansion. This maneuver secured the full coverage of the open area, allowed the interstitial fluid leakage and minimized the resulting scar (). The donor site was protected during the immediate postoperative period with a dorsal splint in 30 degrees of wrist flexion that was reversed with progressive splint adjustments and physical therapy.
Figure 4. Donor site of osteocutaneous radial forearm free flap. (A) Use of reconstruction plate to protect radius bony defect after flap harvest. Two distal bicortical and three proximal bicortical screws were placed. (B) Intraoperative view of donor site of left forearm. (C) Long-term follow-up.
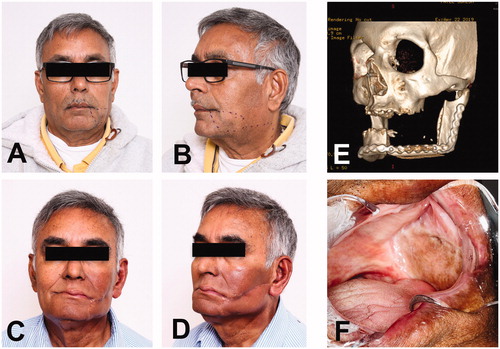
The postoperative period was satisfactory without complications. Patient was discharged on postoperative day 8 without tracheostomy. Following tumor board’s recommendation, patient underwent external beam radiation therapy. New PET/CT scan was obtained 3 months after completing radiation without evidence of residual or recurrent disease. Fourteen months after surgery, the patient continues an uneventful course, keeps regular cancer screening follow ups, and has healed well. He has adequate mandibular motion, no local pain, no trismus or hardware exposure ().
Figure 5. Long-term follow-up. (A-B) Preoperative pictures for evaluation of the surgical outcome. (C-D) Frontal and oblique views fourteen months after surgery. Patient has completed radiation therapy. (E) Image of CT facial bones 3D reconstruction with satisfactory osseous alignment between the bony segment of radius and remaining mandible with no blast or lytic process. (F) Intraoral image showing the left buccal vestibule and its lining with the skin paddle of radial forearm free flap.
Discussion
Free tissue transfer is one of the most frequently utilized surgical option to repair large through-and-through cheek defects. Thus, microsurgical flaps can be folded for simultaneous coverage of cheek skin and intraoral lining and even provide bony support between these two surfaces. They can also be harvested as chimeric free flaps to replace multiple components of the defect either in different planes or separated anatomical location [Citation7,Citation8]. However, when they replace different tissues or multiple planes, folded and chimeric free flaps may develop unexpected tissue fibrosis and scar contracture that can potentially affect the cheek flexibility and dental rehabilitation. In addition, free flaps usually provide a no ideal skin quality in term of color, texture and thickness.
Among reconstructive procedures for defects following head and neck cancer resection [Citation1,Citation2], loco-regional rotation flaps are still a widely accepted solution due to their excellent skin color and texture match, decreased morbidity at the donor site and reduced surgical risks [Citation3,Citation4]. In this regard, cervicofacial and cervicothoracic flaps have all advantages of smaller local flaps, but they are also suitable option after excision of advanced malignancies [Citation10–13].
Conley described cervical and thoracic rotation flaps to reconstruct wounds of the lateral neck and face [Citation14]. Since this initial report, cervicofacial and cervicothoracic flaps have demonstrated significant versatility for the coverage of defects following the removal of head and neck cancers [Citation13,Citation15]. They do not need delay procedures, relatively rapid to harvest and associated with excellent functional and cosmetic outcomes [Citation15]. However, they may develop distal ischemia or necrosis when harvested without the blood supply provided by deep planes [Citation15]. Cervicofacial and cervicothoracic flaps are flat and thin, and therefore, they are not primarily indicated for the treatment of composite full-thickness defects of the cheek. Nevertheless, they can be used in combination with other flaps to resurface simultaneously cheek skin and intraoral lining [Citation13]. Accordingly, in difficult clinical settings such as patients with multiple comorbidities and locally advanced head and neck cancer, cervicofacial and cervicothoracic flaps should be seen as valid partners of free flaps or other regional flaps to minimize surgical time and complexity as well as reduce intra- and post-operative risks [Citation13].
In our case, the osteocutaneous radial forearm free flap provided an adequate mandibular bony structure and a thin and supple intraoral lining. The quality of the skin paddle played a pivotal role to obtain a clear definition of the buccal vestibule.
Other flaps to be considered for similar cases include deep circumflex iliac artery (DCIA) flap, scapular territory flaps and pectoralis major flap, among others. Based on several perforators, the DCIA flap can include iliac crest bone, a segment of internal oblique muscle and even skin island. Thus, DCIA flap is suitable for reconstruction of mandibular defects of up to 10 cm in length as well as defects of floor of mouth and gingiva [Citation16]. Due to no reliable anatomical distribution of perforators, the skin paddle offers limited options for external coverage [Citation16,Citation17]. Unfortunately, this flap is not only technically demanding to harvest with a short pedicle (4–7 cm in length), but also it may cause significant morbidities such as chronic pain, paresthesia, gait difficulties and weakness or even hernia of the abdominal wall [Citation16,Citation17].
The scapular territory offers numerous options for flap harvest. It has a chimeric nature based on multiple anatomically consistent arteries that branch off from a long pedicle. Thus, the scapular system can provide customized flaps with multiple components (i.e. bone segments, several skin islands, fascia, muscles) for the reconstruction of intraoral lining, bony defects and external coverage along with sealing dead spaces [Citation17]. To maintain the position during the entire surgical procedure, the patient is placed in a modified lateral decubitus (30 degrees hip elevation and 45 degrees chest rotation). This position allows a two-team approach and decreases the surgical time, but it can cause shoulder morbidities from transitory local pain up to permanent reduction of its range of motion [Citation17]. By definition, chimeric flaps have multiple tissue components that could be utilized in spatially distant locations. Since they have multiple raw surfaces to heal, we believe chimeric flaps have the potential risk to develop undesirable scar contracture with numerous vectors of tension that may negatively impact the cheek flexibility and oral rehabilitation. As seen in our case, the reduction of raw surfaces between the intraoral lining and the skin cheek may substantially help the healing process and scar remodeling for a better cosmetic and functional outcome.
Finally, the pectoralis major myocutaneous flap is a very reliable and easy to harvest flap for the reconstruction of head and neck defects [Citation18]. However, due to high incidence of functional and cosmetic adverse outcomes, we reserve its use as rescue flap in case of significant complications with our primary reconstruction (i.e. flap necrosis, oral fistulas, etc.).
In general, cervicofacial and cervicothoracic flaps are technically easy to dissect and reliable. They can reach numerous defects within well-known boundaries namely supraorbital margin (superiorly), midline (medially) and retroauricular region (posteriorly). They are also indicated in anterolateral defects of the neck [Citation19]. Inside this area, they also cover anatomical structures such as major blood vessels, facial nerve and exposed bones, among others [Citation13]. The use of cervicofacial and cervicothoracic flaps for cheek reconstruction results in excellent color and texture match, adequate thickness, good scar location and concealment and acceptable sensory recovery and morbidity at the donor site [Citation11].
Even though we had no postoperative complications in our case, superficial epidermolysis (10–14.3% of cases), and full-thickness tip necrosis (6–9.5% of cases) have been reported [Citation13,Citation19,Citation20]. To reduce ischemia events, it is crucial to incorporate SMAS, platysma and fascia planes to the flap [Citation11,Citation20,Citation21]. However, it is important to take into account that this deeper approach is time consuming and imply higher risk of facial nerve injury [Citation13].
Finally, after more than fourteen months since the surgery, our patient has completed the radiation treatment and remains without evidence of residual or recurrent malignancy. Locally, patient reached excellent functional and cosmetic outcome with no local pain and normal sensation except a small retroauricular area of numbness. He exhibits appropriate cheek thickness and contour, predominantly inconspicuous scars, normal mouth opening and lip competence as well as acceptable intraoral vestibular space and support to wear dentures.
Conclusion
Cervicofacial and cervicothoracic flaps are important tools to be considered in the reconstruction of large wounds caused by resection of head and neck cancer. As shown in this report, in combination with microsurgical or other regional flaps, cervicofacial and cervicothoracic flaps can be used in through-and-through cheek defects to reduce operative time and complications without compromising the functional and cosmetic outcomes.
Disclosure statement
The authors have no conflict of interest with any people or organizations that could potentially influence the results and conclusions of this manuscript.
References
- Mathes SJ, Nahai F. Reconstructive surgery: principles, anatomy and techniques. Vol 2. New York; St. Louis: Churchill Livingstone; Quality Medical; 1997.
- Gottlieb LJ, Krieger LM. From the reconstructive ladder to the reconstructive elevator. Plast Reconstr Surg. 1994;93(7):1503–1504.
- Stark RB, Kaplan J. Rotation flaps, neck to cheek. Plast Reconstr Surg. 1972;50(3):230–233.
- Crow ML, Crow J. Resurfacing large cheek defects with rotation flaps from the neck. Plast Reconstr Surg. 1976;58(2):196–200.
- Ariyan S. The pectoralis major myocutaneous flap: a versatile flap for head and neck reconstruction. Plast Reconstr Surg. 1979;63(1):73–81.
- Granzow JW, Suliman A, Roostaeian J, et al. The Supraclavicular Artery Island Flap (SCAIF) for head and neck reconstruction: surgical technique and refinements. Otolaryngol Head Neck Surg. 2013;148(6):933–940.
- Wong CH, Wei FC. Microsurgical free flap in head and neck reconstruction. Head Neck. 2010;32(9):1236–1245.
- Hanasono MM, Matros E, Disa JJ. Important aspects of head and neck reconstruction. Plast Reconstr Surg. 2014;134(6):968e–980e.
- Mustardé JC. The use of flaps in the orbital region. Plast Reconstr Surg. 1970;45(2):146–150.
- Boyette JR, Vural E. Cervicofacial advancement-rotation flap in midface reconstruction: forward or reverse? Otolaryngol Head Neck Surg. 2011;144(2):196–200.
- Sakellariou A, Salama A. The use of cervicofacial flap in maxillofacial reconstruction. Oral Maxillofac Surg Clin North Am. 2014;26(3):389–400.
- Shetawi AH, Quimby A, Fernandes R. The cervicofacial flap in cheek reconstruction: a guide for flap design. J Oral Maxillofac Surg. 2017;75(12):2708.e1–e6.
- Liu FY, Xu ZF, Li P, et al. The versatile application of cervicofacial and cervicothoracic rotation flaps in head and neck surgery. World J Surg Oncol. 2011;9:135.
- Conley JJ. The use of regional flaps in head and neck surgery. Ann Otol Rhinol Laryngol. 1960;69:1223–1234.
- Kaplan I, Goldwyn RM. The versatility of the laterally based cervicofacial flap for cheek repairs. Plast Reconstr Surg. 1978;61(3):390–393.
- Lou C, Yang X, Hu L, et al. Oromandibular reconstruction using microvascularized bone flap: report of 1038 cases from a single institution. Int J Oral Maxillofac Surg. 2019;48(8):1001–1008.
- Wilkman T, Tornwall J, Vuola J, et al. The free scapular flap with latissimus muscle reduces fistulas in mandibular reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(6):802–808.
- Ariyan S. The pectoralis major myocutaneous flap: a versatile flap for reconstruction in the head and neckPlast Reconstr Surg. 1979;63(1):73–81.
- Moore BA, Wine T, Netterville JL. Cervicofacial and cervicothoracic rotation flaps in head and neck reconstruction. Head Neck. 2005;27(12):1092–1101.
- Tan ST, Mackinnon CA. Deep plane cervicofacial flap: a useful and versatile technique in head and neck surgery. Head Neck. 2006;28(1):46–55.
- Kroll SS, Reece GP, Robb G, et al. Deep-plane cervicofacial rotation-advancement flap for reconstruction of large cheek defects. Plast Reconstr Surg. 1994;94(1):88–93.
