Abstract
We report a case of purulent flexor tenosynovitis caused by Mycobacterium haemophilum in an immunosuppressed patient who received renal transplantation. Three synovial debridements and multiple antimicrobial administrations with clarithromycin, rifampicin, and moxifloxacin have been performed. No apparent recurrence has been observed two years after the final operation.
1. Introduction
Nontuberculous mycobacteria should be kept in mind as the cause of the flexor tenosynovitis with poor inflammatory findings. Nontuberculous mycobacteria are present in soil, aerosols, and water and can be the causative agent of infections in immunosuppressed patients exposed to the bacteria. The lungs are the most commonly infected, followed by the extremities. Here, we report a surgical treatment for flexor tenosynovitis caused by Mycobacterium haemophilum, an extremely rare nontuberculous mycobacterium.
2. Case presentation
A 71-year-old woman underwent living donor kidney transplantation 20 years ago and continued to take immunosuppressants thereafter. She volunteered to engage in cleaning the river and hence had been exposed to soil. She noticed pain and swelling of her left middle finger and consulted her previous surgeon. Upon diagnosis of purulent flexor tenosynovitis, a synovectomy of the left middle finger was performed. Mycobacteria were detected; however, the species were not identified.
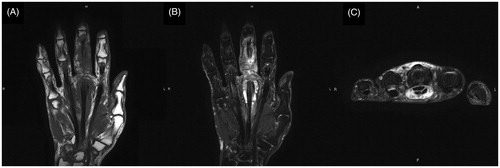
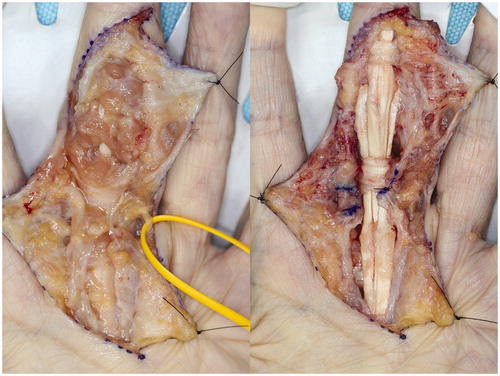
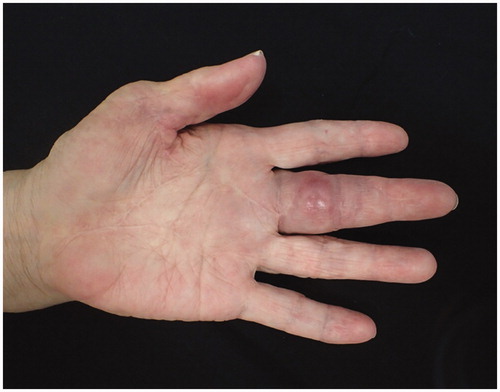
Although the course was good, she visited to our hospital 2 months after the first operation due to swelling, redness, and pain of the left thumb. Physical findings revealed pain, swelling, and warmth on the left thumb () and joint motion was restricted. Laboratory examinations yielded the following counts: 3700/μL for leukocytes, 0.17 mg/dL for CRP, and 3.8 pg/mL for β-D glucan, while rheumatoid factor and anti-CCP antibody were negative. Subsequently, after a diagnosis of purulent flexor tenosynovitis of the left thumb, a synovectomy of the left thumb was performed. Pathological examination of the resected synovium revealed numerous epithelioid cells and granuloma due to multinucleated giant cells. Microbiological examination revealed mycobacteria in the collected tissues ). In the culture at 37 °C and 30 °C for 8 weeks, no growth was observed in the Ogawa medium or in the mycobacteria growth indicator tube. However, growth was observed in the blood agar medium in the aerobic culture at 30 °C (). PCR reactions for detecting M. tuberculosis complex, M. avium, and M. intracellulare were negative. The gene sequence of the obtained PCR product was analyzed and the pathogenic bacterium was identified as M. haemophilum by homology search of the gene. Thereafter, antibacterial treatment with clarithromycin, rifampicin, and moxifloxacin was initiated. However, 10 months after the initial operation, the swelling and redness of the left middle finger recurred (). A plain radiograph showed no apparent osteolysis and plain magnetic resonance imaging showed synovial tissue relapse with low signal on T1-weighted image and high signal on T2-weighted image (). The patient was diagnosed with a recurrence of flexor tenosynovitis and synovectomy was repeated (). Mycobacterium haemophilum was detected again in the collected synovial tissue. Thereafter, the patient continued to receive antibiotics and no recurrence was observed 2 years after the last operation.
Figure 1. Photo of hands before the second synovectomy. Redness and swelling on the thumb are visible.
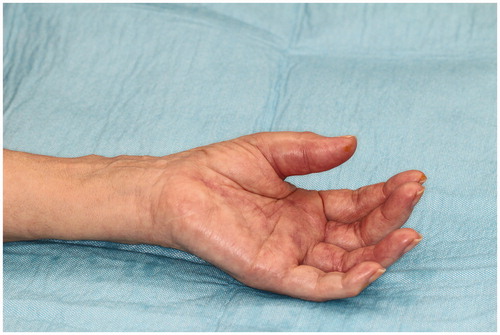
Figure 2. Histopathological and microbiological findings. (A) HE stain showing the granulomas due to numerous epithelial cells and multinucleated giant cells. (B) Chirnelsen stain showing anti-acid bacteria in large numbers. (C) Anti-acid bacteria stained after being cultured on blood agar.
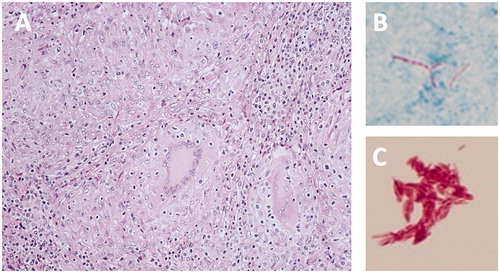
Figure 3. Photo of hands before the third synovectomy. Redness and swelling on the middle finger are visible.
3. Discussion
If tenosynovitis with a weak inflammatory response is observed, it is important to start treatment early, considering nontuberculous mycobacteria. Atypical mycobacteria inhabit the soil and water and their transmission to humans occurs from the natural environment. They do not transmit from person to person as M. tuberculosis. Nontuberculous mycobacteria are commonly transmitted to the lung; however, they are rarely reported in the hands. Nevertheless, one should be careful of its occurrence. Synovitis is the most common clinical presentation of nontuberculous mycobacterial infections in the hands; however, infection may spread further to deeper bone joints [Citation1] but are difficult to differentiate from other diseases. The diagnosis tends to be delayed, especially in immunocompromised patients who have been diagnosed for more than 4 months tend to exhibit treatment failure [Citation2]. Similar to other infectious diseases, it is advisable to make an appropriate diagnosis at the soonest and start treatment immediately. A review of non-tuberculous mycobacteriosis in the hands of 241 patients by Balagure suggested the presence of the following nontuberculous mycobacteria: M. marinum (82%), M. chelonae (5%), M. kansasii (2%), and M. intracellulare (2%) [Citation3]. In the review of 20 patients with insufficiency, M. chelonae (40%), M. marinum (30%), M. avium-intracellulare (10%), and M. kansasii (10%) were the most common [Citation2]. Recognition of these bacterial infections may be helpful in the selection of the appropriate treatment. However, there are several nontuberculous mycobacteria that cannot be identified with a PCR test kit. In cases similar to ours, we recommend genetic testing using PCR products to search for gene homology [Citation4] for the identification of the M. haemophilum. Although M. haemophilum infections are very rare, lung and skin lesions are occasionally reported in immunosuppressed patients [Citation4]. Mycobacterium haemophilum infections in the extremities are extremely rare, many of which have been reported as pathogens of osteomyelitis of the lower leg [Citation4,Citation5]. Mycobacterium haemophilum infections in fingers has been reported only in a German study by Schumacher and colleagues [Citation6]. Notably, in their report, it was difficult to identify the name of the bacterium. In addition to the antibiotics administered in combination with clarithromycin, ethambutol, and rifabutin, debridement was performed three times and one flap operation was required [Citation6]. Although there is no standard treatment for M. haemophilum infections, a combination mycobacterial therapy is commonly used. Lindeboom et al. recommend the administration of clarithromycin, rifabutin, ethambutol, or ciprofloxacin [Citation4]. Jacobs and colleagues have proposed the administration of clarithromycin, rifamycin, and ciprofloxacin for M. haemophilum infections in patients who have undergone kidney transplantation. Additionally, the duration of the administration depends on the severity of the infection and the state of immunosuppression. Long-term administration of up to 24 months is recommended [Citation7]. In this case, the oral administration of antibiotics was continued for 2 years after the final operation and no apparent recurrence has been observed since then. However, it is important to recognize that the discontinuation of antimicrobial therapy may result in a relapse.
4. Conclusion
Mycobacterium haemophilum can cause flexor tenosynovitis in immunosuppressed patients. Nontuberculous mycobacteria should be suspected in hand infections in immunosuppressed patients exposed to certain environments, and some species of bacteria may require genetic testing.
Statement of informed consent
The patients provided written consent for use of their images.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yano K, Kazuki K, Ikeda M, et al. Osteomyelitis and arthritis of the wrist caused by Mycobacterium intracellulare in an immunocompetent patient: a case report and literature review. Acta Reumatol Port. 2014;39(2):176–181.
- Sotello D, Garner HW, Heckman MG, et al. Nontuberculous mycobacterial infections of the upper extremity: 15-year experience at a tertiary care medical center. J Hand Surg Am. 2018;43(4):387. e1–e8.
- Balague N, Uckay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34(1):18–23.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24(4):701–717.
- Takeyari S, Hashii Y, Yoshida H, et al. Mycobacterium haemophilum osteomyelitis in the immunocompromised host. Pediatr Int. 2017;59(12):1279–1281.
- Schumacher O, Dabernig J, Nenadic I, et al. [Severe course of a rare non-tuberculous Mycobacteriosis (M. haemophilum) of the hand – case report and strategic comments]. Handchir Mikrochir Plast Chir. 2008;40(05):342–347.
- Jacobs SE, Zhong E, Hartono C, et al. The brief case: disseminated Mycobacterium haemophilum infection in a kidney transplant recipient. J Clin Microbiol. 2017;56(1):e00562. Epub 2017/12/28.