Abstract
Erosive lichen planus is an uncommon variant of lichen planus. We report a case of longstanding and refractory plantar ELPs causing disabling and opiate-resistant pain treated with ‘classic’ meshed skin graft combined with Rigenera® micrografts. After approximately 9 months follow-up, no clinical recurrence or pain were observed. Erosive lichen planus (ELP) is an uncommon variant of lichen planus, involving oral cavity and genitalia and, less often plantar areas, where it usually presents with chronic erosions of the soles, along with intense, disabling pain and progressive loss of toenails. An abnormal immune cellular response (CD8+ lymphocytes and macrophages) and the consequent altered production of multiple mediators (interleukin-12, interferon-γ, tumor necrosis factor-α, RANTES and MMP-9), seem to play a crucial role in the pathogenesis, although the etiology remains uncertain. From a histological point of view, ELP shows keratinocyte apoptosis, intense inflammatory response and basal epithelial keratinocytes TNF-α overexpression. Several therapies have been proposed, with variable and controversial results. While topical corticosteroids and topical calcineurin inhibitors are the treatments of choice for localized forms, short pulses of systemic glucocorticoids, phototherapy, and systemic immunosuppressants are recommended for generalized cases. Surgery has been reported as a possible therapeutic option in refractory and stable cases with localized lesions, either alone or with cyclosporine. Herein, we report a case of longstanding and refractory plantar ELPS causing disabling and opiate-resistant pain treated with ‘classic’ meshed skin graft combined with Rigenera® micrografts.
Case report
A 65-year-old Caucasian female presented with a 6-year history of a wide and extremely painful ulceration on the sole of her left foot, histologically diagnosed as erosive lichen planus. Over the years she had undergone several topical and systemic treatments, including steroids, antibiotics, retinoid and mycophenolate mophetil, with transient and incomplete results.Skin examination revealed an extensive, malodorous and irregular ulcer, having well-defined margins and a reddish and friable base ().
Figure 1. Extensive, malodorous and irregular ulcer, having well-defined margins and a reddish and friable base.
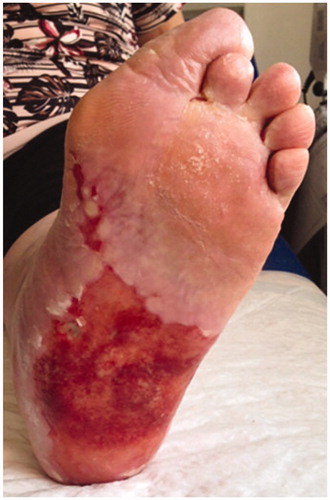
Patient reported strong pain and a burning sensation which impeded her to walk. Absence of toenails was also evident. Medical history included many comorbidities, viz. type-2 diabetes, hypertension, dislipidemia, obesity, chronic renal failure, IgG k gammopathy, multifactorial anemia, erosive gastritis, and esophagitis. Laboratory tests showed only some alterations related to the patient's comorbidity and an increase in the Erythrocyte Sedimentation Rate (38 mm/h) and the C-reactive protein level (1.56 mg/L). Anti-hepatitis C and B viruses’ antibodies were negative.
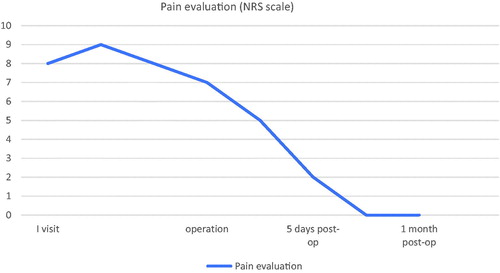
Steroid topical preparations were applied under occlusive wet dressings and systemic Methotrexate was started with a dosage of 15 mg once a week. The ulcer showed a moderate improvement with increase of granulation tissue at the base but without effect on the pain (steadily with NRS scale score >7), which turned out to be intense and resistant to any type of analgesic, including opiates, with consequent compromising of her life quality. For this reason, we proposed her a surgical treatment, consisting in the combination of a meshed split thickness autologous skin graft, and autologous skin micro-grafts (obtained by Rigenera®).
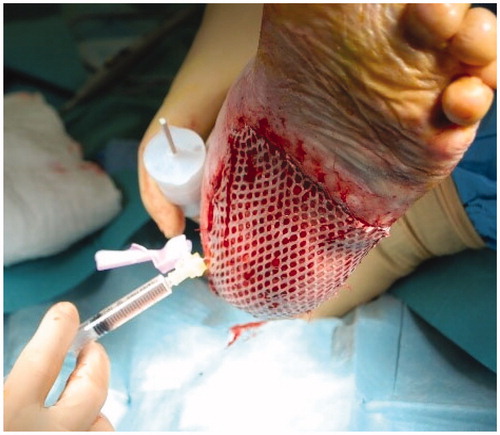
Surgery was performed under local anesthesia with sedation. After surgical debridement of the plantar lesion, two 3 mm2 skin punch biopsies where taken from the left thigh and processed through Rigenera® at 80 rpm for 2 min in 3 ml of 0.9% NaCl saline solution, each. The resulting solution was injected on margins and bottom of the lesion (). Then, the area was covered with a 1:2 meshed split thickness skin graft taken from her left thigh, and a nonadherent gauze and sterile gauze compression dressing.
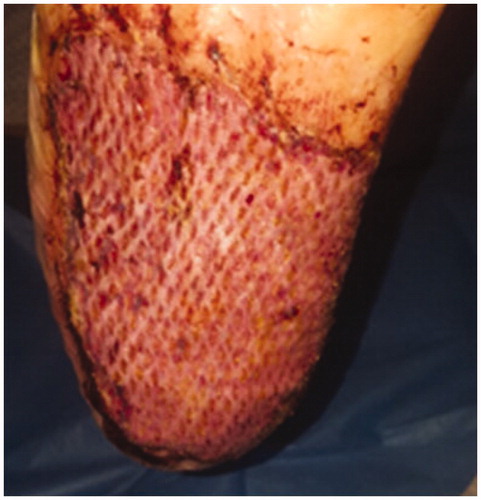
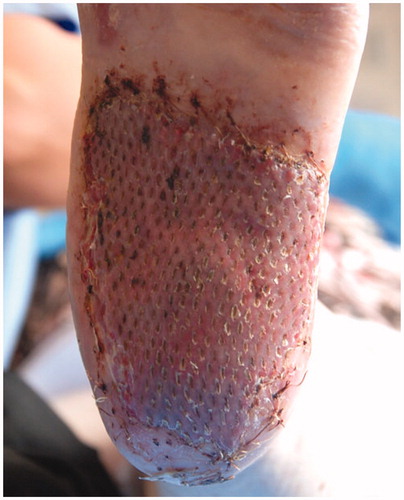
After 5 days, the compression dressing was removed, showing a gook skin engraftment, and complete re-epithelialization after 14 days ( and ).
The patient referred immediate, complete and stable regression of pain and local symptoms.
After hospital discharge, weekly medications in outpatient service followed, with progressive improvement of the skin engraftment and no reappearance of pain. Methotrexate was continued with a reduced dose of 10 mg once a week. The patient has been followed-up for approximately 9 months and neither clinical recurrence nor pain occurred.
Discussion
The repair and regeneration of human tissues is particularly difficult and given that regenerative ability declines with increasing age. The dramatic rise in the ageing population worldwide determined an increasing need for innovative approaches. Many approaches can be used when the normal process of tissue regeneration is impaired or simply insufficient, such as grafting which includes autografts, allografts, xenografts, and biomaterial substitutes [Citation1,Citation2].
Treatment of ELP is notoriously difficult, because of its frequent resistance to topical or systemic therapies and high rates of recurrence. Multiple treatment options have been proposed, but none of them has consistently demonstrated satisfactory remission of symptoms. Surgery could be an important alternative treatment for this disease [Citation3].
In a previous case of ELP, the patient demonstrated a notable clinical response using cyclosporine and skin grafting [Citation4]. In the present patient, the numerous comorbidities prevented the use of different immunosuppressants. As prescribed by Dermatologists, methotrexate was here used, albeit in the literature there was no previous data on its use in plantar ELP. Notably, even though we did not observe complete disease resolution, we obtained partial reduction of inflammation and increase of granulation tissue, that might have favored the surgical approach.
At the same time, it is known that immunosuppressive therapy causes an increased risk of delayed wound healing, infections and failure of the skin grafts engraftment [Citation5,Citation6]. Referring to all these variables, we had to consider an alternative solution, which would accelerate reepithelization, such as using micro-grafts. In our experience we have already tested their validity in other wound types [Citation7].
We choose autologous skin micro-grafts obtained by Rigenera® technology using a class I medical device called Rigeneracons (Human Brain Wave, Italy), already used in various fields of medicine and dentistry [Citation8–10].
We applied the same Rigenera® protocol reported in the literature [Citation8–13].
Micrograft technique in this patient had five functions:
Acceleration of skin wound healing: studies have shown a more rapid reepithelization when micro-grafts are associated with classic skin graft, with a better result also in terms of skin quality [Citation11,Citation12]. In vitro results show that the micrografts obtained with Rigenera® maintain a high cell viability, despite the disaggregation needed to obtain them. In particular, micrografts were described rich in mesenchymal stem cells (MSCs), with high CD90, CD73, and CD105 positivity [Citation13,Citation14]. Preclinical and clinical trials show that MSC therapy accelerates wound closure suggesting that it can be promising for treating wounds with delayed healing [Citation15,Citation16]. In addition, MSCs promote different stages of the wound repair process through modulation of inflammation, promotion of angiogenesis, and stimulation of cell movement during epithelial remodeling [Citation17]. On the basis of these evidences, we can suppose that the regenerative properties of micrografts can be in part determined by MSCs activity. This supports the capacity of these micro-grafts to improve wound healing [Citation18,Citation19], and having a more fast and complete reepithelialization respect to the other advanced dressings, with excellent results in terms of clinical efficacy and demonstration of the regenerative capacity of this method [Citation7].
Regenerative effect: in micrografts, the keratinocytes and fibroblasts are surrounded by a supportive microenvironment, increasing the regenerative capacity of the cells when transplanted [Citation12]. The goal of regeneration is implanting so small cells to ensure an optimal grafting, versus the critical points of a conventional autologous grafts: their limited regenerative capacity and the percentage of cellular death in the receiving site [Citation20]. Cell characterization by flow cytometry analysis shows a heterogeneous pool of cells [Citation18,Citation19]. The specific cell population of these micrografts includes progenitor cells [Citation19], which in association with the fragment of the Extracellular Matrix (ECM) and growth factors derived by starting tissue, initiate biological processes of cell proliferation and differentiation enhancing the wound healing process [Citation7,Citation11].
Anti-inflammatory effect: this micrograft technique allows to select factors such as TGF-β1 (which acts both as a promoter of tissue repair and as a suppressor of excessive inflammation) [Citation18,Citation21,Citation22,Citation23,Citation24] and alpha-smooth muscle actin (α-SMA), which plays a central role in the healing process (wound contraction) and which is produced from myofibroblasts, once they have differentiated from fibroblasts upon stimulation of TGF-β1 present in the ECM [Citation11].
Foot sole sensitivity: TGF-β1 appears to stimulate the de-differentiation of glial cells to neural crest stem cells. These cells, in turn, appear to be involved in the progression of healing and the restoration of peripheral sensitivity [Citation22].
Pain reduction: our patient developed immediate and permanent pain reduction (NRS 2 at first control at 5 days, 0 at 14 days, ). Although in literature the reduction of pain after treatment with micrograft has already been reported [Citation6], this result is to be ascribed to the aforementioned mediators obtained with this method [Citation11,Citation12]
The impressive clinical outcomes combined with the laboratory tests, demonstrate that this technology is really able to stimulate skin regeneration. On the basis of this evidence, Rigenera® can offer an efficient and promising strategy to improve symptoms and wound healing in patients who exhibit painful and nonhealing wounds.
Conclusion
In this case report, we presented an alternative use of autologous micro-grafts obtained with the Rigenera® protocol, i.e. treatment of chronic inflammatory condition as ELP. We believe that the addition of autologous micrografts to the meshed split thickness skin grafts not only promoted the skin engraftment and the healing of the erosive lesions, but also helped to reverse almost instantly the pain, thanks to the immunosuppressive and anti-inflammatory properties.
Finally, as showed in our instance, methotrexate could have a role in preparing the lesions to the surgical procedure and in ensuring prolonged post-surgery remission. Yet, future studies are needed to confirm such a speculation as we did not have data in the post-suspension period because we did not withdraw the medication to see the disease evolution due to ethical reason.
Declarations of interest: All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arragements), or non-financial interest (such as personal or professional relantionship, allilations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- De Francesco F, Busato A, Mannucci S, et al. Artificial dermal substitutes for tissue regeneration: comparison of the clinical outcomes and histological findings of two templates. J Int Med Res. 2020;48(8):300060520945508.
- Al-Hayder S, Gramkow C, Trojahn Kølle S-F. Use of autologous fat grafting for the correction of burn scar contracture in the hand: a case report. Case Reports Plast Surg Hand Surg. 2017;4(1):81–83.
- Romero W, Giesen L, Navajas-Galimany L, et al. Erosive lichen planus: a therapeutic challenge. An Bras Dermatol. 2016;91(1):84–86.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8(4):243–244.
- Gaucher S, Nicolas C, Piveteau O, et al. Sarcoidosis and wound healing after cellulitis of the lower limb: is methotrexate responsible for skin graft failure? Wounds. 2017;29(8):229–230.
- Svenning MIE, Bonde C. Adverse effect of tocilizumab treatment on split thickness skin graft—a case report. Eur J Plast Surg. 2019;42(1):101–104.
- Riccio M, Marchesini A, Zingaretti N, et al. A multicentre study: the use of micrografts in the reconstruction of full-thickness posttraumatic skin defects of the limbs-a whole innovative concept in regenerative surgery. Stem Cells Int. 2019;2019:5043518.
- Brunelli G, Motroni A, Graziano A, et al. Sinus lift tissue engineering using autologous pulp micro-grafts: a case report of bone density evaluation. J Indian Soc Periodontol. 2013;17(5):644–647.
- Miranda R, Farina E, Farina MA. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J Wound Care. 2018;27(2):60–65.
- Ceccarelli G, Gentile P, Marcarelli M, et al. In vitro and in vivo studies of alar-nasal cartilage using autologous micro-grafts: the use of the Rigenera ® Protocol in the treatment of an osteochondral lesion of the nose. Pharmaceuticals (Basel). 2017;10(4):53.
- Jimi S, Kimura M, De Francesco F, et al. Acceleration mechanisms of skin wound healing by autologous micrograft in mice. IJMS. 2017;18(8):1675–1679.
- Kiwanuka E, Hackl F, Philip J, et al. Comparison of healing parameters in porcine full-thickness wounds transplanted with skin micrografts, split-thickness skin grafts, and cultured keratinocytes. J Am Coll Surg. 2011;213(6):728–735.
- Marcarelli M, Trovato L, Novarese E, et al. Rigenera protocol in the treatment of surgical wound dehiscence. Int Wound J. 2017;14(1):277–281.
- De Francesco F, Mannucci S, Conti G, et al. Non-enzymatic method to obtain a fat tissue derivative highly enriched in adipose stem cells (ASCs) from human lipoaspirates: preliminary results. IJMS. 2018;19(7):2061.
- Garcia-Gomez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453–1468.
- Hocking AM. Mesenchymal stem cell therapy for cutaneous wounds. Adv Wound Care (New Rochelle). 2012;1(4):166–171.
- Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10(1):29–37.
- Purpura V, Bondioli E, Graziano A, et al. Tissue characterization after a new disaggregation method for skin micro-grafts generation. J Vis Exp. 2016;109:e53579.
- Trovato L, Monti M, Del Fante C, et al. A new medical device Rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol. 2015;230(10):2299–2303.
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408.
- Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293.
- Galliot B, Crescenzi M, Jacinto A, et al. Trends in tissue repair and regeneration. Development. 2017;144(3):357–364.
- Zhou G, Xia K, Du GF, et al. Activation of nuclear factor- kappa B correlates with tumor necrosis factor-alpha in oral lichen planus: a clinicopathologic study in atrophic-erosive and reticular form. J Oral Pathol Med. 2009;38(7):559–564.
- Porter SR, Kirby A, Olsen I, et al. Immunologic aspects of dermal and oral lichen planus: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(3):358–366.