Abstract
Breast enhancement by injectable materials is known to be used for decades. Possible complications can lead to complex management issues for treating physicians. This case demonstrates the challenges in managing giant siliconomas of the breasts, especially concerning breast reconstruction, in the quest for an aesthetically pleasing outcome with minimum risks.
Introduction
The injection of liquid substances has long been used for cosmetic breast augmentation, despite official prohibition [Citation1] and numerous reports describing severe and even lethal complications [Citation2]. Even though short term results can be satisfactory, several problems arise in the vast majority of cases, either in the early or late postoperative period [Citation3]. As a result, patients seek treatment for various symptoms and complications that arise, but also for catastrophic cosmetic problems [Citation4].
We describe a case of a female patient who had undergone major breast augmentation overseas via silicone injections, resulting in the formation of giant siliconomas bilaterally, seeking treatment years after for both cosmetic concerns and breast symptomatology.
Case presentation
A 54 year old woman presented to our Outpatient Department after breast augmentation she claimed to have had 25 years before, via bilateral liquid silicone injections. The patient had the procedure overseas by a medical professional (not specialized in plastic surgery) as an outpatient, having the injections with local anesthesia and sedation and leaving the facility on the same day. A short course of oral antibiotics was given. She could not provide any written documentation regarding the type and exact amount of silicone used. She claims her early postoperative course ran smoothly, with mild bruising, but through the next months she felt her breasts got subsequently larger in size, stabilizing at around three months postoperatively.
She complained that she was not satisfied with her current breast size anymore, but also that she often experienced diffuse breast pain and firmness. She denied having any other symptoms regarding her breasts.
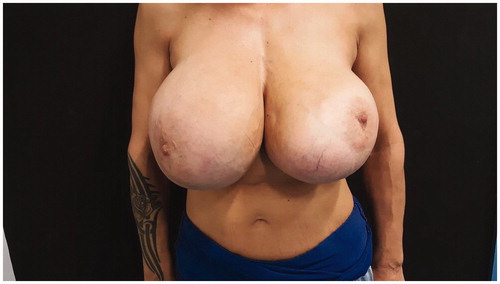
On clinical examination she had significantly large ptotic breasts (sternal notch to NAC: 30 cm right, 31 cm left) with palpable painless firm masses bilaterally, compatible according to her history with giant siliconomas (). She had no palpable axillary lymph nodes. Her medical history was significant for psoriasis, but she mentioned that she hadn’t been taking any medications for it. She was also an active smoker with a 30-year pack smoking history. She had one full term pregnancy and she did not breastfeed at the time. She denied any knowledge of hereditary risk for breast cancer.
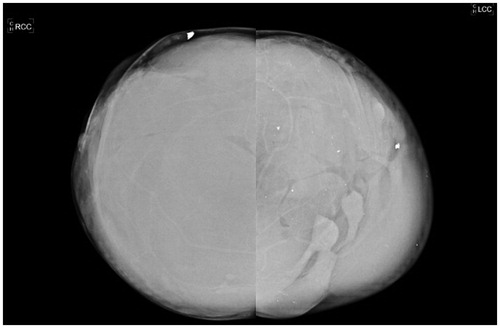
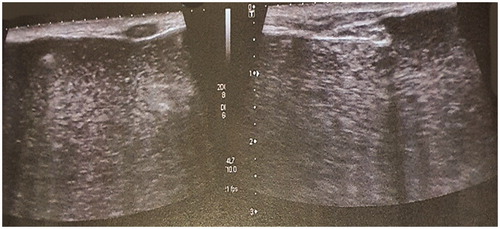
She provided documentation of recent mammography and ultrasound examinations. The mammogram showed diffuse infiltration of both breasts with the injected material, which obscured any normal breast parenchyma (). The ultrasound described a ‘snowstorm’ pattern of liquid material, with multiple hyperechogenic elements and sparse calcifications, which almost completely occupied the parenchyma. Above this layer, multiple complex cysts were observed, representing multiple globules of silicone ().
A preoperative MRI scan was conducted, which showed diffuse silicone deposits in both breasts, extending to the muscle fibers of pectoralis major muscles bilaterally. Also there was evidence of silicone intake by the axillary lymph nodes on both sites.
The possible treatment options were discussed between the medical team and the patient and smoking cessation was encouraged. A decision against breast conserving strategies was made, since all imaging studies showed almost completely obscured breast parenchyma and the patient did not accept the possibility of continuing symptomatology from granulomatous tissue. She also expressed worries over the interference the substance had caused the previous years in screening examinations for breast cancer. Also the long nipple to IMF distance of both breasts (13 cm right, 12 cm left) and significant ptosis of the breasts, served as an indication for free nipple grafting. She was then informed on the possibility of tissue expander use, if the skin quality of the mastectomy flaps was not sufficient intraoperatively.
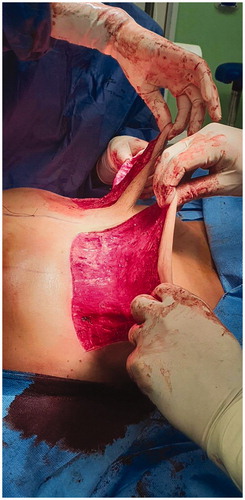
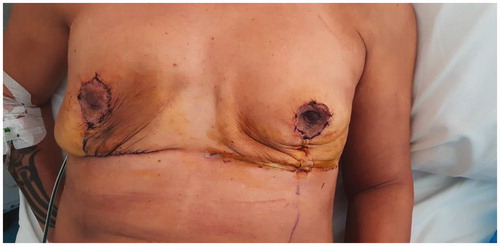
A skin reducing subcutaneous mastectomy with wise-pattern incision was performed, with removal of all granulomatous tissue. The injected substance had completely diffused in the breast parenchyma, which made the dissection of the mastectomy flaps extremely challenging. Due to the fairly thin resulting flaps on both breasts, direct to implant reconstruction was excluded and two-staged reconstruction with tissue expanders was decided. A subpectoral pocket was created on either site and the inferior skin pole was de-epithelialized and used as a dermal sling (). Tissue expanders with minimal fluid were inserted bilaterally, covered fully by the pectoralis major muscles and the inferior dermal sling. The nipple-areola complex was excised and was placed back as a full thickness skin graft at 22 cm from the clavicle bilaterally (). Two drainage tubes were inserted on each site (one in the subpectoral and another in the subcutaneous pockets) and postoperative antibiotics were administered (two doses of ciprofloxacin, cephalothin until the removal of the drains).
Histological examinations showed nonspecific chronic inflammatory reaction with excessive granulomatous tissue, as well as multiple large clusters of the amorphous substance injected. This layer was covered by dense fibrotic tissue. No signs of malignancy were observed.
The tissue expanders were gradually filled over a period of two months until a final volume of 660 cc. The second stage followed three months later, with the replacement of the tissue expanders with textured anatomical implants of 550 cc volume each, after capsulotomies on either site. The same antibiotic regimen was used.
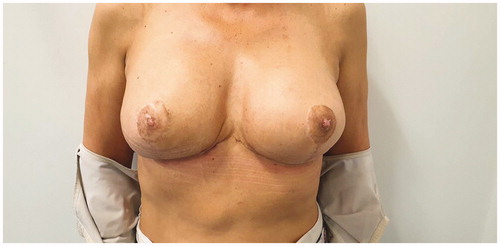
The postoperative course of both stages was uneventful and at 6 months followup the patient had a very satisfactory cosmetic result regarding breast volume, shape and NAC appearance (). She also stated that her breasts felt more natural, considerably less heavy and her previous symptoms were cleared.
Discussion
Breast augmentation with injection of free liquid silicone is being performed, often illicitly, despite multiple known associated complications [Citation5]. The procedure consists of injecting medical or, in many cases, non-medical grade liquid silicone into the retromammary space, between the pectoralis major muscle and the fibroglandular breast tissue.
Cosmetic appearance is initially satisfactory but may be significantly altered due to formation of granulomatous tissue in the breast, usually presenting with pain and firm irregular palpable masses, causing various symptomatology [Citation3,Citation6]. Besides the aesthetic problems, these lesions may also pose differential diagnostic problems in patients at risk for breast cancer [Citation7,Citation8]. Other complications include migration of the injectable substance, sometimes with severe results, fistula formation and gel leakage [Citation9–11].
There has been limited data on the literature regarding surgical options for the injected breast, with most advocating for surgical removal of all injected material by mastectomy and reconstruction with autologous tissue. In several cases, autologous reconstruction with the pedicled TRAM flap has been used, divided and transferred immediately after mastectomy [Citation12–14].
Implant based reconstruction after mastectomy has been reported in several case series with various complication rates and most authors advocating for immediate reconstruction [Citation15,Citation16]. Some authors have reported reconstruction after non total removal of breast tissue, either with implant insertion and mammaplasty after partial excisions of granulomas [Citation17] or with reduction mammaplasty reported in two asymptomatic patients [Citation18]. Bilateral breast siliconoma which was treated by total mastectomy with two stage tissue expander-prosthesis reconstruction has also been reported in one case [Citation19]. Also a case series with consecutive patients who were treated for complications after free injections to the breasts has been published, with the authors proposing a treatment algorithm [Citation20].
In the absence of official guidelines over the appropriate course of action in patients with foreign material injections, physicians are faced with challenging decisions. After consulting the patient about all the available options and probable risks, we felt it was best to follow through with mastectomies to ensure complete removal of the foreign material and immediate tissue expander insertion, since the specific case had very large and ptotic breasts and no previous reported cases of such extent have been reported before, in our knowledge. The patient had no complications postoperatively and was very satisfied with the final result.
Conclusions
Late symptomatology regarding breast augmentation by injectables ranges from benign inflammatory symptoms to more severe sequelae. As awareness is increasing, gradually more patients seek treatment, simultaneously requesting breast reconstruction. Treating physicians are faced with newly emerging surgical challenges. Consequently, ongoing investigation and reporting, with the eventual institution of formal recommendations, will be an important aid to the surgical community.
Ethical approval
This work was done with accordance to ethical standards and a written patient consent was given.
References
- Food and Drug Administration, USA. The FDA warns against injectable silicone for body contouring and enhancement; 2017. Available from: https://www.fda.gov/consumers/consumer-updates/fda-warns-against-injectable-silicone-body-contouring-and-enhancement.
- Peters W, Fornasier V. Complications from injectable materials used for breast augmentation. Can J Plast Surg. 2009;17(3):89–96.
- Mello DF, Gonçalves KC, Fraga MF, et al. Local complications after industrial liquid silicone injection: case series. Rev Col Bras Cir. 2013;40(1):37–42.
- Carson B, Cox S, Ismael H. Giant siliconoma mimicking locally advanced breast cancer: a case report and review of literature. Int J Surg Case Rep. 2018;48:54–60.
- Leonardi NR, Compoginis JM, Luce EA. Illicit cosmetic silicone injection: a recent reiteration of history. Ann Plast Surg. 2016;77(4):485–490.
- Majedah S, Alhabshi I, Salim S. Granulomatous reaction secondary to intramammary silicone injection. BMJ Case Reports. 2013;2013(1):bcr2012007961–bcr2012007961.
- Youk JH, Son EJ, Kim EK, et al. Diagnosis of breast cancer at dynamic MRI in patients with breast augmentation by paraffin or silicone injection. Clin Radiol. 2009;64(12):1175–1180.
- Cheung YC, Chen SC, Lo YF. Enhanced MRI and MRI-guided interventional procedures in women with asymptomatic silicone-injected breasts, 2012. Sci World J. 2012;2012:549801.
- Hilton JD, Steinke K. Extensive migration of injected free liquid silicone for breast augmentation with related major complications. BJR Case Rep. 2015;1(2):20150098.
- Soeroso NN, Rhinsilva E, Soeroso L. Acute pneumonitis following breast silicone liquid injection. Respirol Case Rep. 2018;6(6):e00335.
- Unukovych D, Khrapach V, Wickman M, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012;36(4):695–701.
- Lai YL, Weng CJ, Noordhoff MS. Breast reconstruction with TRAM flap after subcutaneous mastectomy for injected material (siliconoma). Br J Plast Surg. 2001;54(4):331–334.
- Aoki R, Mitsuhashi K, Hyakusoku H. Immediate reaugmentation of the breasts using bilaterally divided TRAM flaps after removing injected silicone gel and granulomas. Aesthetic Plast Surg. 1997;21(4):276–279.
- Chiu WK, Lee TP, Chen SY, et al. Bilateral breast reconstruction with a pedicled transverse rectus abdominis myocutaneous flap after subcutaneous mastectomy for symptomatic injected breasts. J Plast Surg Hand Surg. 2012;46(3-4):242–247.
- Cárdenas-Camarena L. Managing the mammary gland infiltrated with foreign substances: different surgical alternatives. Ann Plast Surg. 2009;62(6):621–626.
- Chen TH. Silicone injection granulomas of the breast: treatment by subcutaneous mastectomy and immediate subpectoral breast implant. Br J Plast Surg. 1995;48(2):71–76.
- Luo SK, Chen GP, Sun ZS, et al. Our strategy in complication management of augmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg. 2011;64(6):731–737.
- Prasetyono TO, Sadikin PM. Management of asymptomatic silicone-injected breast with reduction mammoplasty. Indian J Plast Surg. 2015;48(3):317–320.
- Chuangsuwanich A, Warnnissorn M, Lohsiriwat V. Siliconoma of the breasts. Gland Surg. 2013;2(1):46–49.
- Patlazhan G, Unukovych D, Pshenisnov K. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesth Plast Surg. 2013;37(2):312–320.