Abstract
Acne keloidalis nuchae (AKN) is a progressive inflammatory condition that affects posterior neck and occiput. Treatment options include antibiotics, steroids, lasers, radiotherapy and surgery.
We present three patients with advanced ‘tumor-stage’ AKN that underwent radical local excision followed by either immediate or delayed skin resurfacing, and briefly review existing literature.
Introduction
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition characterized by the formation of fibrotic papules and plaques on the nape of the neck and occiput [Citation1]. AKN primarily occurs in young men of African descent. Even though dermoscopic exam is useful in early stages, the diagnosis is regularly based on clinical findings [Citation2]. Early AKN lesions exhibit small papule formation often accompanied by pustules due to secondary bacterial infection. Mild to moderate cases of AKN can be addressed with medical management featuring the use of antibiotics as well as corticosteroids and retinoids, individually or in combination with one another in both topical and intralesional formulations. In some individuals, papules coalesce to form large, keloid-like plaques often encompassing the entire back of the head and neck [Citation2]. Such lesions, termed ‘tumor-stage’ AKN, are usually managed with more aggressive methods and interventional procedures such as surgical excision, cryotherapy, radiation treatment and laser therapy [Citation1–3].
Millán-Cayetano et al. reported the use of radiation therapy in the treatment of refractory AKN [Citation3]. Even though the treatment was effective with no recurrence for 20 months, it remains controversial whether the benefits of radiation therapy outweigh its risks to treat benign lesions in young adults. Laser therapy includes a large variety of laser modalities including CO2 excision therapy, Nd:YAG laser and tUVB light treatments, among others. However, while laser therapies yield notable clinical improvement and minimal side effects in selected cases, recurrence can be common especially in more advanced cases [Citation2].
In this case report, we review three patients with large tumor-stage lesions requiring extensive surgical excision followed by skin grafting after failure of more conventional modalities of treatment.
Case reports
Case 1
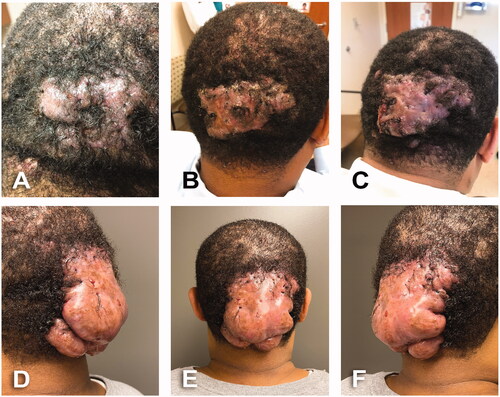
A 43-year-old male, BMI: 35, past-medical history of high blood pressure and high cholesterol. Patient consulted for 12 years history of dissecting scalp folliculitis that progressed into AKN. Initially managed by dermatologist with oral rifampin and clindamycin along with topical clindamycin lotion and triamcinolone cream (). Patient developed a large AKN lesion with firm thickened scarring plaques on occipital scalp with areas of crusting and no active drainage (). He received further treatment of oral doxycycline and local fluocinonide solution, clindamycin lotion and 0.05% tazarotene cream as well as intralesional injections of triamcinolone. Patient continued follow-up in dermatology clinic for 15 months where he was also treated with isotretinoin and additional intralesional injections of triamcinolone (). During the first assessment by plastic surgery team, patient had a 12 × 12 cm infected and fibrotic occipital mass with multiple ingrowing hairs and points of purulent drainage ().
Figure 1. A 43-year-old male with 12 years history of scalp folliculitis that progressed into AKN. BMI 35 kg/m2. Fitzpatrick skin type 5. (A) Initial dermatology assessment. Dissecting scalp folliculitis; recommended rifampin (300 mg oral BID), clindamycin (300 mg oral BID), clindamycin lotion daily and triamcinolone cream. (B) AKN with firm thickened scarring on occipital scalp, areas of crusting, no active drains. Recommended doxycycline (100 mg oral BID), rifampin (300 mg oral BID), lidex solution and clindamycin lotion BID and intralesional injections of triamcinolone. (C) Progression of AKN lesion. Pink, firm thickened scarring plaques and purulent drainage. Patient continued follow-up in Dermatology clinic for 15 months and treated with Isotretinoin (40 mg oral daily) and additional intralesional injections of triamcinolone. (D–F) Preoperative assessment by Plastic Surgery. A 12 × 12 cm occipital infected fibrotic mass with multiple ingrowing hairs and points of purulent drainage.
Case 2
A 65-year-old male, BMI: 27, past-medical history of high blood pressure and remote myocardial infarction. Patient had history of AKN for at least 9 years treated with oral doxycycline, 1% clindamycin lotion, 0.1% tretinoin cream and intralesional injections of triamcinolone. Preliminary assessment by plastic surgery team demonstrated an 18 × 8 cm AKN fluctuant lesion on the occipital area with multiple ingrowing hairs, hemorrhagic crusts, cysts and purulent drainage from several sites ().
Case 3
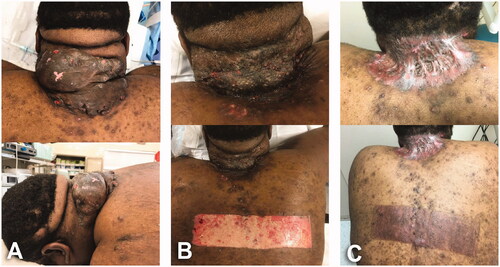
A 30-year-old male, BMI: 48.5, no past-medical history, who was initially managed by dermatologist with minocycline and intralesional injections of triamcinolone. Subsequently, the patient was referred to plastic surgery clinic due to the disabling condition generated by a giant 25.5 × 15 cm AKN lesion ().
Interestingly, all patients described in this case report had one or more clinical disorders associated with metabolic syndrome such as high blood pressure, obesity and hyperlipidemia. Patients 1 and 2 were under treatment for high blood pressure with amlodipine (both patients) and carvedilol and lisinopril (patient No. 2).
Operative technique
Patients underwent general anesthesia with endotracheal intubation in a prone position with padding of all pressure points. Sequential compression devices (SCD) were placed bilaterally and weight-based cefazolin was given intraoperatively.
In each case, posterior scalp and neck were shaved, prepped and draped in standard fashion. The AKN lesion was pre-marked with 1 cm margin to include all unhealthy hair follicles and scar tissues. The surrounding area was infiltrated with 1:1 ratio of 0.5% bupivacaine and 1% lidocaine containing epinephrine (1:100,000 final dilution). By using electrocautery, the incision was carried down to the level of subcutaneous tissue in the scalp, and then it continued under the lesion in a plane superficial to the galea. Of note, surgical dissection of these lesions was difficult and time consuming due to the presence of highly vascularized inflammatory tissue and significant perilesional fibrotic reaction. After the specimen was passed to the back table, special attention was focus on removing all remaining hair follicles still present in the wound bed. Under 3.5× magnification loupes, this step was meticulously done with forceps and Iris scissors (or similar). Hemostasis was achieved with electrocautery and 3-0 vicryl suture for major perforator vessels.
For patients No. 1 and No. 2, the post-excisional wounds were left widely open and packed with Dakin’s solution-soaked gauze and secured with ABD pads, kerlix and tubular elastic net ( and ). In case No. 3, a partial skin closure with 3-0 vicryl sutures followed by immediate split thickness skin graft (STSG) was performed due to the magnitude of the ablative wound (specimen weight: 815 g) and concerns of inappropriate wound management in outpatient setting ().
Figure 2. Post-excisional course. (A) Postoperative day 4; first postoperative assessment in outpatient clinic. (B) Postoperative day 39; open wound getting significantly smaller, more superficial and well vascularized. (C) Postoperative day 49; local condition during the second surgical procedure. Open area still reducing in size, smooth and well vascularized granulation tissue with small area of epidermal cells spreading out on surface. (D) Six days after second surgery with the bolster dressing in place. Skin graft was harvested from upper back. (E) Meshed split thickness skin graft after dressing removal the same day. (F) Long term follow-up 3 months after skin graft.
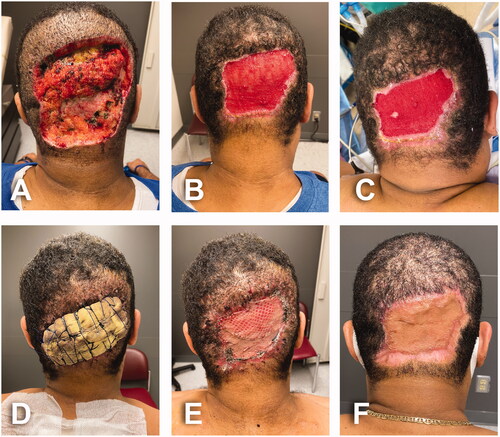
Figure 3. A 65-year-old male with history of AKN for at least 9 years. BMI 27 kg/m2. An 18 × 8 cm fluctuant AKN lesion on the occipital area with multiple ingrowing hairs, hemorrhagic crusts, cysts and purulent drainage from several sites. Fitzpatrick skin type 5. (A) Initial assessment in outpatient clinic. (B) Postoperative day 6 of 18 × 8 cm AKN excision. (C) Postoperative day 22; wound getting smaller, more superficial and well vascularized. Dark spot in the middle corresponds to overlying tissue of suture ligation of large arterial perforator. (D) Postoperative day 51; intraoperative view prior to skin graft procedure. (E) Initial dressing on day 6 after placement of split thickness skin graft. (F) Local condition one month after skin graft.
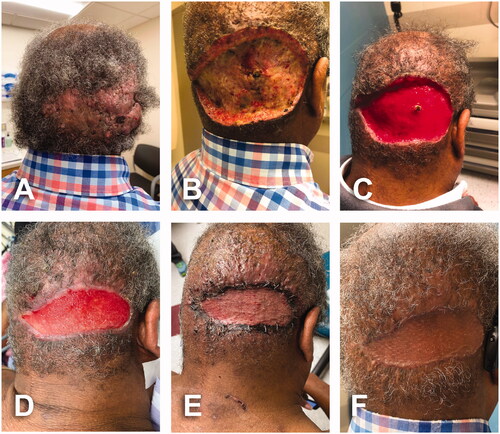
Figure 4. A 30-year-old male, BMI 48.5 kg/m2, Fitzpatrick skin type 5, giant AKN. (A) Intraoperative view of 25.5 × 15 cm ‘keloid-type’ lesion treated with wide local excision, complex partial wound closure and split thickness skin graft. Specimen weight: 815 g. (B) Initial dressing change. Donor site and STSG during first postoperative visit in outpatient clinic (postoperative day 6). Partial take of the skin graft treated with local care and systemic antibiotics. Of note, there are multiple skin openings on the area from underlying epidermal inclusion cysts; superficial biofilm also noticed. (C) Long-term follow-up (postoperative day 61). Local condition on grafted area and donor site.
After appropriate recovery from surgery and anesthesia, patients were discharged home with orders of cephalexin and analgesics (i.e. short course of hydrocodone/acetaminophen followed by acetaminophen alone). The initial dressing of patient No. 3 (immediate skin graft) was done on postoperative day 7. Despite partial graft take likely secondary to the presence of multiple epidermal inclusion cysts and bacterial contamination on the donor site, the skin coverage was reached satisfactorily with local care (daily dressings with Dakin’s solution as described above) and without additional surgical interventions (). The remaining two patients (delayed skin graft) were managed by one of their family members with daily dressing changes using Dakin’s solution and weekly follow-up in outpatient clinic to check the status of the wounds. Antibiotics were adjusted according to culture results which reported polymicrobial infection including Escherichia coli, Pseudomonas aeruginosa and predominantly Staphylococcus aureus. During the postoperative period, wounds exhibited significant improvement reducing the size and depth and developing well-vascularized granulation tissue on surface in preparation for skin graft ( and ).
Patients No. 1 and 2 were taken back to the operating room for skin resurfacing approximately 50 days after AKN removal. In both cases, the surgical area was prepped and draped in standard fashion and local infiltration was done as described above. Then, superficial granulation tissue and any biofilm present on the wound surface were sharply removed. The adjacent upper back was selected as donor site for skin graft. In this regard, tumescent solution (normal saline with epinephrine at 1:400,000 dilution) was injected under the skin to control bleeding and provide a more stable and turgid surface during the skin graft harvest. A 0.012-inch STSG was obtained with a 4-inch dermatome guard and subsequently meshed with a 1:1.5 expansion mesher. The meshed STSG was then fitted and secured to the wound edges using staples. The skin graft was covered with a tie-over bolster dressing made of xeroform and moistened cotton. Multiple interrupted 2-0 silk stitches were used as bolster ties. Afterward, the position of the bolster dressing was reinforced with a 2-0 Prolene suture that sequentially grabbed the skin in one side, passed through the xeroform at the top of the bolster dressing before it reached the other side of the skin (). This step was done in a running-suture fashion until the full extension of the bolster dressing was included. Finally, the dressing was protected with ABD pads and wrapped with kerlix and tubular elastic net. The donor site was dressed with xeroform and ABD pads. Patients returned to clinic 1-week post-operatively for bolster removal and examination of STSG. In both cases, we obtained nearly 100% skin graft take with a well vascularized graft ( and ). The grafted areas were then treated with xeroform daily dressing changes for 2 weeks. In long-term follow-ups, our patients were asymptomatic with stable coverage and areas of alopecia significantly smaller compared to that in the original AKN lesions ().
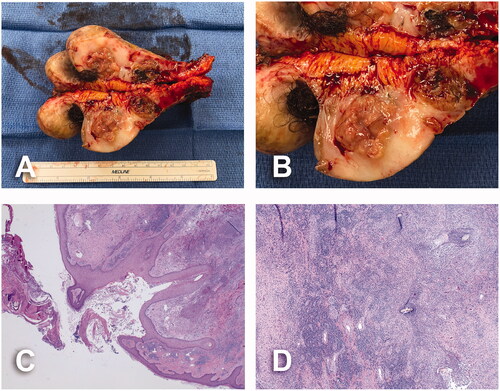
Surgical specimens revealed multiple, necrotic and hair-bearing intralesional cavities of variable sizes spanning the skin surface (). Their histological analyses established the presence of inflammatory infiltrate surrounding vessels and adnexal structures in the superficial and mid dermis which was composed of neutrophils, plasma cells, lymphocytes, histiocytes, and multinucleated giant cells. Features of suppurative folliculitis with neutrophils within and surrounding follicles were also present. There was background evidence of scarring within the dermis with dense collagenous tissue arranged parallel to the skin surface associated with numerous blood vessels (). No evidence of malignancy was found in our reported cases.
Figure 5. Pathology assessment of case No. 1. (A) Cross section of surgical specimen. Multiple, ragged, necrotic, hair-bearing lesions ranging from 0.1 × 0.1 × 0.1 cm to 6.5 × 5.2 × 4.3 cm, spanning the skin surface. (B) Details of necrotic, hair-bearing intralesional cavities. (C) Widened hair follicle surrounded by dense inflammatory process and dermal fibrosis, and overlying scale crust; 20× magnification. (D) Scattered hair shafts within a fibrotic dermis and dense acute and chronic inflammation; 40× magnification.
Discussion
Acne keloidalis nuchae also referred to as folliculitis keloidalis nuchae, is a condition associated with chronic inflammation and scarring of folliculitis [Citation1]. This name continues to be utilized today to describe the condition in spite of evidence that true keloid formation does not occur in AKN [Citation4]. AKN primarily occurs in young post-pubertal men of African descent, although instances of AKN affecting other ethnic groups have been described [Citation5,Citation6]. Even though the exact etiology remains to be elucidated, several authors have suggested androgen surges, inflammation, infection, trauma, genetic predisposition and ingrown hairs as inciting events in AKN development. Additionally, as seen in our patients, metabolic syndrome has previously been described as a predisposing factor [Citation6]. Some studies compare AKN to pseudofolliculitis barbae hypothesizing that curved growth of the hair shafts into skin results in irritation with subsequent inflammation [Citation7,Citation8].
Clinically, AKN lesions exhibit a large spectrum of presentations ranging from small papules and pustules to large nodules and plaques formed by the coalition of smaller refractory lesions. Their distinctive location, clinical manifestations with chronic and often progressive course and histological findings differentiate AKN from other entities such as hidradenitis suppurativa, acne vulgaris and keloid scars [Citation1,Citation7]. Surgery for AKN implicates numerous challenges including but not limited to complex post-operative wound management, risk of infection, and cosmetic concerns. Thus, surgery is reserved for advanced AKN that has been refractory to medical management and non-surgical interventions such as intralesional steroid injections and laser. Surgical excision may include immediate primary layered closure, delayed healing by secondary intention or skin grafting [Citation8–10]. Some authors have reported that primary closure usually develop wide scars as a result of stretching forces, and therefore, creating similar cosmetic outcomes compared to secondary closure [Citation10]. Conversely, other authors are in favor of primary closure over healing by secondary intention that require daily local care and often leave large defects. In giant AKN lesions, the depth of the excision is crucial for the success of their surgical management. By excising under the compromised tissues and tufted follicles and including uninvolved margins, the inflammatory cascade is quickly interrupted, and therefore, the likelihood of recurrence is greatly reduced as compared to other modalities of treatment [Citation8]. In this regard, when possible, radical excision of large AKN followed by delayed resurfacing can be employed to mitigate the formation of large areas of scarring alopecia in the posterior scalp. Thus, extending the time between the initial AKN excision and the second stage reduces the wounds’ sizes and facilitates their closure either primarily or with STSG ( and ). In our cases No. 1 and 2, staged approach was utilized yielding final scars significantly smaller than original defects ( and ). However, even though we did not experience surgical complications with our patients, this surgical modality implicates a prolonged healing time and required complex wound care that many patients are unable to accomplish in ambulatory setting. This was the main reason why our case No. 3 was managed with a single operation (). Due to generalized presence of epidermal inclusion cysts and heavy bacterial contamination of the skin, the surgical site developed an expected local infection that was treated with local care and systemic antibiotics (). On the other hand, the resulting scar contracture reduced the depth of natural folds on the occipital skin of this patient allowing easier hygiene and less episodes of folliculitis ().
The caveat to deep excision of tumor-stage AKN lesions is the creation of significant areas of cicatricial alopecia. In this regard, other therapeutic alternatives cannot reverse or at least significantly reduce the area of alopecia. Furthermore, other options are not able to efficiently treat well-established necrotic and hair-bearing lesions, fluid collections and tufted hairs. Thus, our surgical approach is in line with the main goals of AKN management not only eradicating any source of local inflammation but also reducing the long-term impact of this clinical condition with reasonable cosmetic outcomes. Of note, only patient No. 3 exhibited mild restriction of neck rotation and lateral deviation after surgery. However, these limitations were present during the initial encounter in clinic and it is not affecting his current activity of daily living and his performance at work.
Negative pressure wound therapy (NPWT) could be considered to shorten the time between the initial excision and the secondary resurfacing procedure as well as to improve the final scar contour. In our experience, the use of NPWT on scalp and posterior neck is more effective to flatten wounds rather than reduce their sizes. Furthermore, in this specific location, in part due to overnight changes of the sleep position, the NPWT sealing is challenging to maintain and the long-term patients’ compliance difficult to accomplish. Once skin coverage is achieved, periodical follow-ups are necessary to detect recurrences. The use of 1064-nm Nd Yag, 810-nm diode and other lasers could be helpful to manage early recurrences [Citation1,Citation2]. Because of the use of 1-cm safety margins, we have experienced no recurrences in our patients after 12-, 16- and 24- months of follow-ups. After completion of scar maturation process, the residual alopecia can be treated in several ways including tissue expander placement or with serial excisions followed by soft tissue advancement. Taking into account the level of skin damage and bacterial colonization of the skin in AKN patients, the use of tissue expander appears to have high risk of secondary infection and wound dehiscence [Citation11]. Thus, serial partial excisions and progressive approximation of normal hair-bearing scalp to eliminate the alopecia area seem to be a safer technique but require several interventions. This later solution was offered to our patients. However, they are enjoying a disease-free period, asymptomatic and with alopecia areas easy to conceal as well as cosmetically and socially more acceptable than their original AKN lesions. At the time of this report, they have no interest in any further reconstructive surgery.
Conclusion
Radical surgical excision is the only procedure that secures satisfactory results in giant ‘tumor-stage’ AKN lesions with cavities of necrotic tissue and ingrowing hairs and in those refractories to other less invasive modalities of treatment. In this setting, skin graft is required to cover the ablative defects as these wounds will not heal on their own in a timely manner. A common complaint with the use of skin grafts is the poor cosmesis due to depression of the resulting scars [Citation9,Citation10]. The use of staged-approach allows the wound not only to contract and get smaller but also to fill-in and create a smooth transition between the wound bed and surrounding skin.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483–489.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb)). 2016;6(3):363–378.
- Millán-Cayetano JF, Repiso-Jiménez JB, Del Boz J, et al. Refractory acne keloidalis nuchae treated with radiotherapy. Australas J Dermatol. 2017;58(1):e11–e13.
- Gloster HM. Jr. The surgical management of extensive cases of acne keloidalis nuchae. Arch Dermatol. 2000;136(11):1376–1379.
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in asian: a single institutional experience. PLoS One. 2017; 12(12):e0189790
- Verma SB, Wollina U. Acne keloidalis nuchae: another cutaneous symptom of metabolic syndrome, truncal obesity, and impending/overt diabetes mellitus? Am J Clin Dermatol. 2010;11(6):433–436.
- Kelly AP. Pseudofolliculitis barbae and acne keloidalis nuchae. Dermatol Clin. 2003;21(4):645–653.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33(2 Pt 1):243–246.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4(1):36–39.
- Bajaj V, Langtry JA. Surgical excision of acne keloidalis nuchae with secondary intention healing. Clin Exp Dermatol. 2008;33(1):53–55.
- Pestalardo CM, Cordero A, Jr, Ansorena JM, et al. Acne keloidalis nuchae. Tissue expansion treatment. Dermatol Surg. 1995;21(8):723–724.
