Abstract
The most used vessels for free flap breast reconstruction are the internal mammary, the thoracodorsal and the circumflex scapular. We present a case where those were inadequate. DIEP vessels were passed through a created sternal groove and anastomosed to the contralateral IM vessels, accessed by the breast symmetrisation incisions.
Introduction
The deep inferior epigastric perforator (DIEP) flap has become the gold standard for autologous breast reconstruction since its first report in 1994 [Citation1–4]. When there is surplus abdominal tissue and no compromising regional surgery has been done, it supplies enough skin paddle and volume to recreate a natural breast. This is especially useful in radiotherapy-injured thoraces, since implant-based reconstruction has high risk of failure [Citation5] and autologous techniques bring radiation-free tissues to the breast. Additionally, it allows to improve the abdominal contour in exchange for a new scar in an easily concealable location. Thus, it is a powerful body contouring reconstructive procedure with good level of patient satisfaction [Citation6].
The thoracodorsal (TD) vessels were used as recipient vessels in the first description of this technique [Citation1]. Even though both the axillary vessels and the internal mammary (IM) vessels can be used as recipient vessels, the latter are currently the preferred option for free flap breast reconstruction [Citation7–14]. Recipient vessel conversion rate ranges from 2 to 20%, mainly due to recipient-donor vessels size mismatch and radiotherapy induced scaring or frailty [Citation9,Citation12,Citation15].
Contralateral IM vessels have been used in bilateral breast reconstruction settings, unilateral breast reconstruction with contralateral autologous breast augmentation or bilateral breast augmentation [Citation16–22]. All these authors planned to use the contralateral IM vessels preoperatively. We present a case of contralateral IM vessels as last resource recipient vessels for DIEP flap breast reconstruction, performing the anastomosis through the contralateral breast reduction symmetrization incision.
Case report
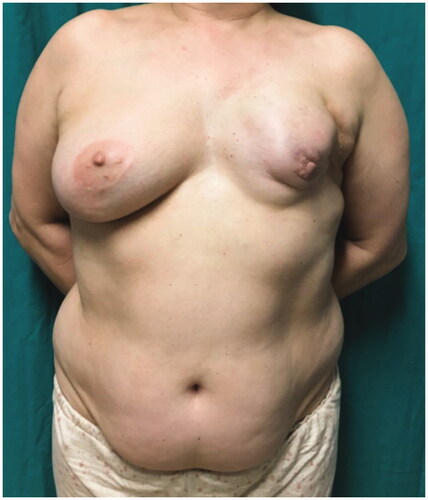
A 54-year-old woman, ex-smoker, presented with a dysmorphic left breast, after breast lumpectomy and radiotherapy, followed by implant-based reconstruction, 15 years ago (). Due to the severity of capsular contracture and breast deformity, we opted for total breast reconstruction with DIEP flap and immediate symmetrization with vertical scar breast reduction.
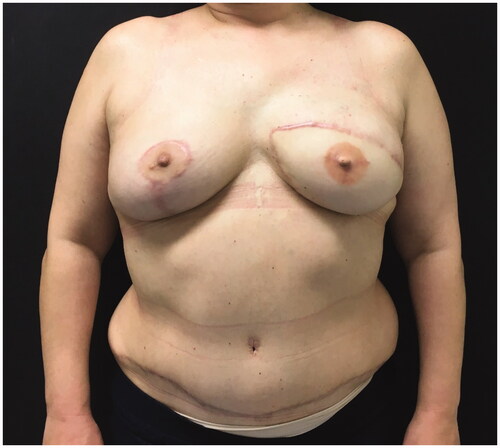
The ipsilateral IM artery was flowless and the IM vein had poor diameter. Both TD and circumflex scapular vessels diameters were negligible and with scarce blood flow. We opted for contralateral IM vessels as recipient vessels. To avoid vein collapse due to poor pliability of the presternal skin and sternal bone rigidity, a bony groove was scooped in the sternum to accommodate the pedicle. Anastomosis were performed through the symmetrization incision (). A postoperative CT scan shows the pedicle crossing the sternum ().
Figure 2. Left DIEP vessels anastomosed to the contralateral IM vessels. The pedicle is passed through an anterior sternal bony groove and the anastomosis were made possible by the symmestrization incision.
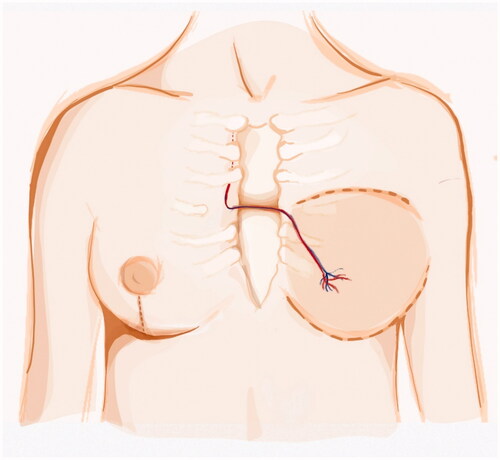
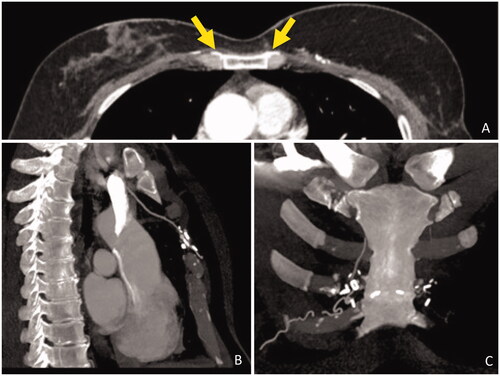
Figure 3. Postoperative CT scan. (A) Axial view, contrast enhancing shows DIEP vessels crossing the sternum (yellow arrows). (B) Sagittal view, IM vessels anastomosed to presternal vessels. (C) Coronal view, microvascular staples highlight the DIEP vessels path through the midline.
Despite midline thoracic pain during 6 weeks that were treated successfully with non-opioid analgesics the 2-year follow-up was uneventful ().
Discussion
The choice of recipient vessels is highly dependent on surgeon’s experience [Citation8]. The current trend seems to favor IM, which our group support. It allows for a better diameter match with deep inferior epigastric vessels than TD [Citation23]. IM artery blood flow rate is also higher than TD artery (mean 25 vs. 5 mL/min, respectively) [Citation8]. Moreover, TD vessels usage precludes the latissimus dorsi flap as a salvage option in case of DIEP flap failure.
Recipient vessels unavailability is a limiting factor for microsurgery. Arteriovenous loops, venous grafts or usage of cephalic vein may be part of the solution. However, they increase scar burden. This may be a limiting factor in an aesthetically demanding reconstructive procedure. In free flap breast reconstruction the conversion recipient vessels options include the IM, TD, circumflex scapular, subscapular, serratus and lateral thoracic [Citation9,Citation23]. Other options include IM perforators [Citation7] as well as distal end of IM vessels [Citation22], however the flowless homolateral IM artery rendered these options unavailable. The need to conversion may be due to vessel mismatch, scarring, short pedicle, poor flow, vessel friability and small recipient vein or retrosternal location (in case of IM) [Citation9]. Inadequate or absent IM veins is more commonly reported on the left side [Citation9,Citation24–26]. This relates with our case. Left side anastomosis is also more prone to venous thrombosis and overall complications [Citation13,Citation14].
There is conflicting data regarding radiotherapy and recipient vessel conversion rates [Citation9,Citation10,Citation12,Citation15]. However post-radiation sequelae include tissue fibrosis, edema, vasculitis, atherosclerosis and decreased IM artery diameter [Citation14,Citation27]. Intraoperative vascular complications seem to increase with radiotherapy [Citation28]. In the present cast, IM artery insufficiency may be related to previous radiotherapy. Complications might be offset by postponing breast reconstruction for one year [Citation14].
The literature so far presents anastomosis to contralateral IM vessel in cases where this option had been planned [Citation16–21]. We did not plan to use these vessels preoperatively, but it became possible in view of the simultaneous symmetrisation procedure. Our team supports immediate contralateral balancing procedures as a way of enhancing overall breast satisfaction and reducing the chance of a second breast procedure [Citation29–32]. This report further broadens the benefit of immediate breast balancing in free flap reconstruction in the rare cases of vessel insufficiency.
Other surgeons placed the pedicle in a subcutaneous plane [Citation16–22]. There is no evidence that it leads to increased venous thrombosis rates. However, we theorize that the tight space between the presternal inelastic skin and the sternum would increase the tendency to venous collapse and thrombosis. Therefore, scooping a bony groove in the anterior sternal wall might reduce that risk. The drawbacks may be increased post-operative pain and increased surgical time.
Contralateral IM vessels might be a safe rescue recipient vessel option, especially in the immediate contralateral balancing setting, since it will not lead to an increased scar burden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32–38.
- Atisha DM, Rushing CN, Samsa GP, et al. A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22(2):361–369.
- Murphy BD, Kerrebijn I, Farhadi J, et al. Indications and controversies for abdominally-based complete autologous tissue breast reconstruction. Clin Plast Surg. 2018;45(1):83–91.
- Opsomer D, Vyncke T, Depypere B, et al. Lumbar flap versus the gold standard: comparison to the DIEP flap. Plast Reconstr Surg. 2020;145(4):706E–714E.
- Sekiguchi K, Kawamori J, Yamauchi H. Breast reconstruction and postmastectomy radiotherapy: complications by type and timing and other problems in radiation oncology. Breast Cancer. 2017;24(4):511–520.
- Yueh JH, Slavin SA, Adesiyun T, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125(6):1585–1595.
- Hamdi M, Blondeel P, Van Landuyt K, et al. Algorithm in choosing recipient vessels for perforator free flap in breast reconstruction: The role of the internal mammary perforators. Br J Plast Surg. 2004;57(3):258–265.
- Nahabedian M. The internal mammary artery and vein as recipient vessels for microvascular breast reconstruction. Ann Plast Surg. 2012;68(5):537–538.
- Saint-Cyr M, Youssef A, Bae HW, et al. Changing trends in recipient vessel selection for microvascular autologous breast reconstruction: an analysis of 1483 consecutive cases. Plast Reconstr Surg. 2007;119(7):1993–2000.
- O'Neill AC, Hayward V, Zhong T, et al. Usability of the internal mammary recipient vessels in microvascular breast reconstruction. J Plast Reconstr Aesthetic Surg. 2016;69(7):907–911.
- Sultan SM, Rizzo AM, Erhard HA, et al. Revisiting the internal mammaries as recipient vessels in breast reconstruction: considerations in current practice. Breast Cancer Res Treat. 2020;184(2):255–264.
- Leppard W, Pomposelli T, Chang EI, et al. Internal mammary usability as recipient vessels in DIEP breast reconstruction in the setting of previous radiation. J Plast Reconstr Aesthetic Surg. 2018;71(8):1123–1128.
- Chang EI, Chang EI, Soto-Miranda MA, et al. Demystifying the use of internal mammary vessels as recipient vessels in free flap breast reconstruction. Plast Reconstr Surg. 2013;132(4):763–768.
- Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg. 2011;127(3):1100–1106.
- Temple CLF, Strom EA, Youssef A, et al. Choice of recipient vessels in delayed TRAM flap breast reconstruction after radiotherapy. Plast Reconstr Surg. 2005;115(1):105–113.
- Deramo P, Martinez CA, Boutros SG. Use of single-recipient vessels for cross-chest abdominal flap-based breast augmentation as an outpatient. Plast Reconstr Surg. 2020;8(7):1–5.
- Zeltzer AA, Andrades P, Hamdi M, Blondeel PN, et al. The use of a single set of internal mammary recipient vessels in bilateral free flap breast reconstruction. Plast Reconstr Surg. 2011;127(6):153–155.
- Kosutic D, Lambe GF. The use of single recipient internal mammary vessels for bilateral diep flap breast reconstruction in a recipient-vessel-depleted patient. Microsurgery. 2018;38(1):120–121.
- Huang JJ, Chao LF, Wu CW, et al. Simultaneous scarless contralateral breast augmentation during unilateral breast reconstruction using bilateral differentially split DIEP flaps. Plast Reconstr Surg. 2011;128(6):593–604.
- Lee JH, Varon DE, Halvorson EG. Unilateral internal mammary recipient vessels for bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2017;5(6):1–3.
- Satake T, Muto M, Kou S, et al. Contralateral unaffected breast augmentation using zone IV as a SIEA flap during unilateral DIEP flap breast reconstruction. J Plast Reconstr Aesthetic Surg. 2019;72(9):1537–1547.
- Opsomer D, D'Arpa S, Benmeridja L, et al. Bilateral DIEP flap breast reconstruction to a single set of internal mammary vessels: technique, safety, and outcomes after 250 flaps. Plast Reconstr Surg. 2019;144(4):554e–5564.
- Lhuaire M, Hivelin M, Dramé M, et al. Determining the best recipient vessel site for autologous microsurgical breast reconstruction with DIEP flaps: an anatomical study. J Plast Reconstr Aesthetic Surg. 2017;70(6):781–791.
- Moran SL, Nava G, Behnam AB, et al. An outcome analysis comparing the thoracodorsal and internal mammary vessels as recipient sites for microvascular breast reconstruction: a prospective study of 100 patients. Plast Reconstr Surg. 2003;111(6):1876–1882.
- Muto M, Satake T, Masuda Y, et al. Absent internal mammary recipient vein in autologous breast reconstruction. Plast Reconstr Surg. 2020;8(2):1–3.
- Pradas-Irun C, Azzawi K, Malata CM. A plea for recipient vascular pedicle versatility in microvascular breast reconstruction. Plast Reconstr Surg. 2012;129(2):383e–3385.
- Durhan G, Erdemir AG, Yuce Sari S, et al. Does internal mammary node irradiation for breast cancer make a significant difference to the diameter of the internal mammary artery? Correlation with computed tomography. Breast Care. 2020;15(6):635–641.
- Fosnot J, Fischer JP, Smartt JM, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg. 2011;127(2):496–504.
- Huang JJ, Wu CW, Leon Lam W, et al. Simultaneous contralateral breast reduction/mastopexy with unilateral breast reconstruction using free abdominal flaps. Ann Plast Surg. 2011;67(4):336–342.
- Chang EI, Selber JC, Chang EI, et al. Choosing the optimal timing for contralateral symmetry procedures after unilateral free flap breast reconstruction. Ann Plast Surg. 2015;74(1):12–16.
- Smith ML, Clarke-Pearson EM, Vornovitsky M, et al. The efficacy of simultaneous breast reconstruction and contralateral balancing procedures in reducing the need for second stage operations. Arch Plast Surg. 2014;41(5):535–541.
- Giordano S, Harkkila S, Oranges CM, et al. Immediate versus delayed contralateral breast symmetrisation in breast reconstruction with latissimus dorsi flap: a comparative study. Breast Care. 2019;14(5):272–276.