Abstract
We describe a rare case of a 77-year-old woman with radiation-induced breast angiosarcoma (RIAS) in whom radical surgery with negative margins determined that at 14-month of follow-up there is no evidence of either local or systemic recurrence without having to resort to adjuvant chemotherapy.
1. Introduction
Radiation-induced breast angiosarcoma occurs as a rare, severe and late complication in patients that underwent breast conservation surgeries associated with radiotherapy in less than 0,3% [Citation1]. Literature reported for secondary angiosarcoma 5-year survival rate lower than 22.5% [Citation2].
The initial management of patients affected by radiation-induced breast angiosarcoma (RIAS) is complex due to the fact that it usually presents in the form of multifocal reddish-purple papular skin lesions underestimated by clinicians because of its benign presentation and skin changes are easily attributed to radiation even if radiation-induced breast angiosarcoma RIAS are frequently associated with a poor prognosis [Citation3–5]. An incisional biopsy of the skin and underlying mass is necessary and the treatment is surgical resection, however the role of chemotherapy has not yet been clearly defined. Angiosarcomas are aggressive, malignant and poor prognosis associated blood vessel cancers which originate from endothelial cells, they can arise spontaneously or in association with many factors [Citation6].
Sporadic angiosarcoma of the breast is extremely rare, in contrast, radiogenic angiosarcoma of the breast is much more common among women with a history of breast irradiation with an incidence about 1% [Citation7].
Spontaneous (primary) and radiogenic (secondary) angiosarcomas are morphologically undistinguishable, but there are notable pathogenetic differences. For example, Lae et al. [Citation8] compared the c-myc amplification on chromosome 8q24.21 in 32 radiogenic angiosarcoma specimens and 15 sporadic angiosarcoma specimens amplification (5- to 20-fold) of the c-myc oncogene was found in all radiogenic angiosarcomas cases but only in one sporadic angiosarcoma demonstrating a specific oncogenic pathway for radiogenic angiosarcoma. Secondary angiosarcoma can also occur in the event of chronic lymphedema after breast surgery and lymphadenectomy (Stewart–Treves syndrome) even if this condition has significantly decreased due to improved surgical techniques [Citation9,Citation10].
This study aims to offer details on the management in a multidisciplinary context of the rare tumor in question in a particular case in which adjuvant treatment was not proposed to the patient.
2. Case report
A 77-year-old patient was diagnosed with a left breast carcinoma in 2008.There was a family history of breast cancer: his mother was diagnosed left invasive ductal breast carcinoma at 80 years old, both her sisters were diagnosed right in situ ductal carcinoma. The patient medical history described: pharmacological treated arterial hypertension, senile arthrosis and hiatal hernia.The patient had undergone several surgeries: cholecystectomy, hysteroannessiectomy, bladder plastic and in 2008 received a quadrantectomy of the upper-outer left breast quadrant with radical lymphadenectomy of the left armpit due to invasive ductal carcinoma pT2N2G3, Estr. 40%, Pr. Neg, Ki67 12%, HER2 triple positive as a final diagnosis. She underwent 3 cycles of adjuvant chemotherapy with Docetaxel associated with Trastuzumab and adjuvant radiotherapy (50 Gy) followed by regular oncological follow-up with clinical examinations every 6 months, a chest X-ray, a bilateral ultrasound check of the breast and axilla, an abdominal ultrasound check, a bone scintigraphy and dosing of tumor markers (☐FP, CEA, Ca125, Ca 15.3, TPA). There were no signs of recurrence both local and systemic until today. From October 2021 the patient reported the presence of a singular periareolar purple cutaneous dyschromia in inner quadrant transition (IQT) of the left breast about 2 centimeters in diameter and physical examination described sclerotic breast skin due to results of RT, retracted nipple, an ecchymotic lesion of about 2 cm in IQT in the periareolar area under which a nodular ligneous lesion of about 2 cm in diameter adhering to the underlying planes can be appreciated. Patient underwent in December 2021 mammographic exam in which was described predominantly fibroadipose mammary structure (BI-RADS: B), neither accumulations of a suspicious nature nor microcalcifications with evolutionary characteristics were appreciated. The finding appears superimposable on the previous exam of 2020. The patient was proposed at the end of January 2022 a bilateral ultrasound check of the breast and axilla that reported the absence of solid lesions and absence of axillary involvement. There was no ultrasound correspondence with clinical findings. All that considered the team decided then to perform a punch biopsy of the lesions which histological exams reported the absence of epidermal involvement but the presence in the dermis of fissures and anastomosed vascular channels lined by endothelium with atypical, enlarged and hyperchromatic nuclei positive for ERG, negative for GATA 3, smooth muscle actin and e-Cadherin. The morphological and immunohistochemical finding was compatible with angiosarcoma. The patient at the same time underwent an MRI test of both breasts with and without contrast where no significant signal alterations to the right breast were detected. No evident adenopathies are described in the axillary and bilateral internal mammary chains. The Breast Unit team also decided to perform a chest and abdomen CT exam with and without contrast. CT abdomen exam revealed the absence of suspicious lesions, no detectable metastatic disease was found. In the left breast there is a slight inhomogeneous fixation, especially in the lower quadrants and external, without evidence of clear hyperaccumulation of a focal nature. The Breast Unit multidisciplinary team after evaluating patient specifications and considering the outcomes presented by literature proposed to the patient a left mastectomy with a primary surgical wound closure that was performed in February 2022. No complications occurred. The patient’s general conditions were good and five days later the patient was discharged. Radical lymphadenectomy of the left armpit was performed in 2008. Pathological specimen reported 3-cm-large cutaneous breast angiosarcoma with negative surgical margins. Considering the complete surgical excision of the lesion, the histological characterization, no systemic involvement and patient’s older age, adjuvant treatment was not proposed. Actually there is no evidence of recurrence in follow-up 14 months after surgery. Patient is still continuing follow-up each 3 months with CT total body, ultrasound check of soft tissues and abdomen, and an oncological medical examination for the next two years.
3. Discussion
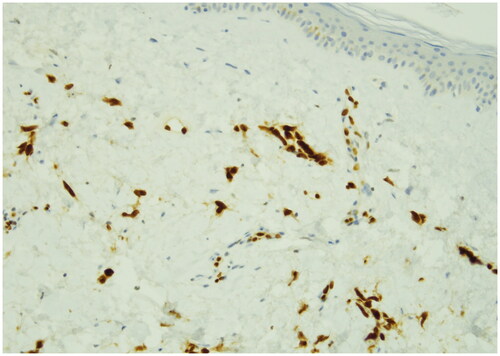
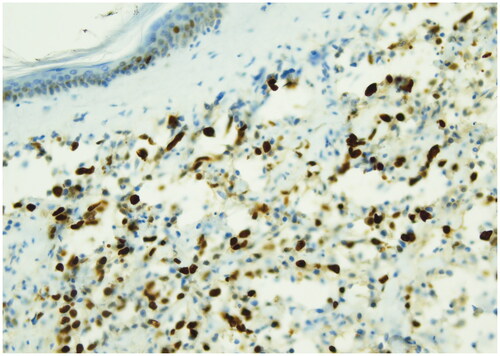
Radiation-induced angiosarcomas (RIAS) represents a diagnostic challenge because of its initial presentation and cutaneous changes are frequently attributed to radiation [Citation11]. For these reasons it’s important to consider RIAS as differential diagnosis in any cutaneous changes of the breast in patients who underwent radiotherapy [Citation12]. In literature RIAS is usually described to occur about 10 years after breast irradiation [Citation13,Citation14]. Early diagnosis and radical surgical treatment are potentially curative but due to the rarity of breast sarcomas, there are no prospective randomized trials to guide therapy [Citation15]. Radical surgery of the tumor either by local resection or mastectomy is the most commonly cited treatment [Citation16,Citation17] and complete tumor resection is associated with an improved prognosis. In contrast to the well established role of surgery, the value of re-irradiation and systemic chemotherapy is less clear [Citation18,Citation19]. It is then important to increase knowledge regarding the presenting symptoms and underline the importance of following up patients who have undergone breast conserving cancer therapy for many years after. RIAS frequently have a nonspecific mammographic and sonographic appearance [Citation20], 33% of patients with angiosarcoma have normal mammography pattern [Citation21], for these reasons MRI plays an important role in the assessment of extent of RIAS [Citation22]. An incisional biopsy of the skin and underlying mass is the most accurate and fastest way to obtain a diagnosis [Citation23]. RIAS arises in differential diagnosis primarily with radiotherapy-associated atypical vascular lesions present as clinically harmless flesh-colored erythematous papules or plaques. Atypical vascular lesions within an irradiation site are suggested to be in a state of morphologic continuum, which may progress to more aggressive malignant angiosarcoma. An important criterion for the differential diagnosis is represented by the evaluation of the proliferation index with Ki-67 which is greater than 10% in the forms of angiosarcoma. Histologically, angiosarcoma shows multilayered nuclei, atypia and mitosis. For these reasons immunohistochemical staining for MYC can be used to aid categorization since MYC is usually amplified in RIAS but not in atypical vascular lesions [Citation24]. In our case, the macroscopic presentation of the tumor was a purple plaque in which the cutting surface showed the presence of multiple hemorrhagic areas extending in superficial dermis. ( and ) The microscopic exam reported the absence of epidermis involvement but the presence in the dermis of fissures and anastomosed vascular channels lined by endothelium with atypical, enlarged and hyperchromatic nuclei positive for ERG, negative for GATA 3, smooth muscle actin and e-Cadherin. Hematoxylin-eosin stain revealed irregular staghorn branched blood vessels in the tumor lined by atypical endothelial cells typically plump. () Ki-67 immunostain showed strong nuclear positivity of neoplastic cells >20% and positivity of epidermal basal cells. () ERG immunostain of angiosarcoma revealed strong nuclear marker expression. () The most cited treatment for breast angiosarcomas in literature is surgical resection (mastectomy) that aims to obtain negative margins [Citation25]. Prognosis has been historically poor especially for RIAS with a median survival of 25 months [Citation26] All that considered, RIAS still represents a challenge to the Breast Unit team in deciding the best therapeutic strategy. In our patient only the prompt and correct diagnosis, in a multidisciplinary context where the lesion has been quickly identified and investigated, associated with a radical surgical approach of the breast affected by the RIAS, as suggested by last evidences in literature [Citation27], allowed an improvement in the prognosis of our patient without having to resort to adjuvant chemotherapy and avoiding all the possible complications of this treatment which are more serious in elderly patients.
Figure 1. Gross examination shows a small purple skin lesion with irregular margins in periareolar region in breast left inner quadrant transition and no evidence of skin ulceration.
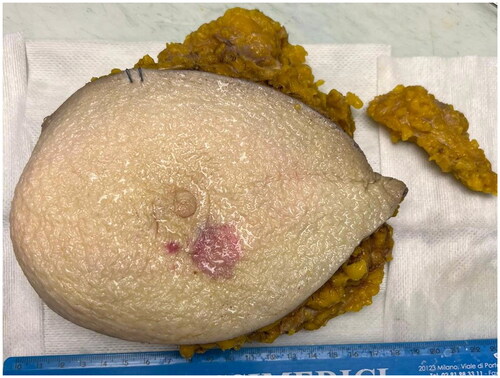
Figure 2. Gross examination shows purple plaque with hemorrhagic cut surface extending in superficial dermis. At the cut surface the lesion comes close to the epidermis with no evidence of epidermal invasion.

Figure 3. H&E stain (40x), histological findings tipically shows dermal basal proliferation of irregular staghorn branched blood vessel with atypical epithelioid cells with large pleomorphic vescicular eosinophilic nuclei and small micro papillary proliferations.
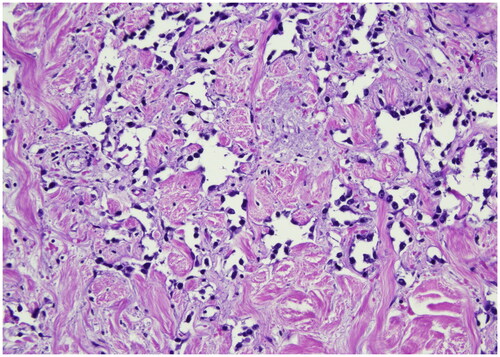
Figure 4. Ki-67 immunostain (40x), this picture shows strong nuclear positivity of neoplastic cells >20%. antigen KI-67 is a nuclear protein that is associated with cellular proliferation and ribosomal RNA transcription. The Ki-67 percentage score is defined as the percentage of positively stained tumor cells among the total number of malignant cells assessed.
4. Conclusion
This case report offer details on the management in a multidisciplinary context of the rare tumor in question in a particular case in which adjuvant treatment was not proposed to the patient. Radical surgery with negative margins determined in our patient that at 14 months of follow up there is no evidence of recurrence both local and systemic. Literature is still poor in guidelines to manage patients affected by this rare and severe tumor, for these reasons our contribute could represent a particular case for larger studies to develop more robust data.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Any patient, service user, or participant (or that person’s parent or legal guardian) in any type of qualitative or quantitative research, has given informed consent to participate in the research.
Authors confirm that they have obtained written informed consent to publish the details from the affected individual.
Disclosure statement
The authors report there are no competing interests to declare.
References
- Wang XY, Jakowski J, Tawfik OW, et al. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol. 2009;13(3):1–6. doi:10.1016/j.anndiagpath.2009.02.001.
- Yin M, Wang W, Drabick JJ, et al. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer. 2017;17(1):295. doi:10.1186/s12885-017-3292-7.
- Verdura V, Di Pace B, Concilio M, et al. A new case of radiation-induced breast angiosarcoma. Int J Surg Case Rep. 2019;60:152–155. doi:10.1016/j.ijscr.2019.06.006.
- Jayarajah U, Nagodavithane K, Basnayake O, et al. Unusual presentation of bilateral radiation-induced angiosarcoma of the breast. Case Rep Oncol Med. 2020;2020:5768438. doi:10.1155/2020/5768438.
- Vuille-dit-Bille RN, Sauter D, Pfofe D, et al. High-grade cutaneous angiosarcoma of the breast 8.5 years after radiotherapy. Breast J. 2013;19(4):435–436. doi:10.1111/tbj.12130.
- Dogan A, Kern P, Schultheis B, et al. Radiogenic angiosarcoma of the breast: case report and systematic review of the literature MC cancer. BMC Cancer. 2018;18:463.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113(3):616–627. doi:10.1002/cncr.23571.
- Laé M, Lebel A, Hamel-Viard F, et al. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. 2015;19(3):168–174. doi:10.1016/j.canrad.2015.01.001.
- Abdou Y, Elkhanany A, Attwood K, et al. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat. 2019;178(3):523–533. doi:10.1007/s10549-019-05432-4.
- Ginter PS, McIntire PJ, Shin SJ. Vascular tumours of the breast: a comprehensive review with focus on diagnostic challenges encountered in the core biopsy setting. Pathology. 2017;49(2):197–214.
- Abbott R, Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol. 2008;5(12):727–736. doi:10.1038/ncponc1242.
- Tahir M, Hendry P, Baird L, et al. Radiation induced angiosarcoma a sequel of radiotherapy for breast cancer following conservative surgery. Int Semin Surg Oncol. 2006;3(1):26. doi:10.1186/1477-7800-3-26.
- Bolin DJ, Lukas GM. Low-grade dermal angiosarcoma of the breast following radiotherapy. Am. Surg. 1996;62:668–672.
- Lyou Y, Barber e, Mehta R, et al. Radiation-associated angiosarcoma of the breast: a case report and literature review. Case Rep Oncol. 2018;11(1):216–220. doi:10.1159/000488314.
- Alves I, Marques J. Radiation-induced angiosarcoma of the breast: a retrospective analysis of 15 years’ experi- ence at an oncology center. Radiol Bras. 2018;51(5):281–286. doi:10.1590/0100-3984.2017.0129.
- Shah S, Rosa M. Radiation-associated angiosarcoma of the breast. Clinical and pathologic features. Arch Pathol Lab Med. 2016;140(5):477–481. doi:10.5858/arpa.2014-0581-RS.
- D’Angelo SP, Antonescu CR, Kuk D, et al. Highrisk features in radiation-associated breast angiosarcomas. Br J Cancer. 2013;109(9):2340–2346. doi:10.1038/bjc.2013.590.
- Fodor J, Orosz Z, Szabó E, et al. Angiosarcoma after conservation treatment for breast carcinoma. Our experience and a review of the literature. J Am Acad Dermatol. 2006;54(3):499–504. doi:10.1016/j.jaad.2005.10.017.
- Peterson CB, Beauregard S. Radiation-Induced breast angiosarcoma: case report and clinical approach. J Cutan Med Surg. 2016;20(4):304–307. doi:10.1177/1203475416631525.
- Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190(2):533–538. doi:10.2214/AJR.07.2909.
- Liberman L, Dershaw DD, Kaufman RJ, et al. Angiosarcoma of the breast. Radiology. 1992;183(3):649–654. doi:10.1148/radiology.183.3.1584913.
- Chikarmane SA, Gombos EC, Jagadeesan J, et al. MRI findings of radiation-associated angiosarcoma of the breast (RAS). J Magn Reson Imaging. 2015;42(3):763–770. doi:10.1002/jmri.24822.
- Yang WT, Hennessy BTJ, Dryden MJ, et al. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007;242(3):725–734. doi:10.1148/radiol.2423060163.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25(1):75–85. doi:10.1038/modpathol.2011.134.
- Lindford A, Böhling T, Vaalavirta L, et al. Surgical management of radiation-associated cutaneous breast angiosarcoma. J Plast Reconstr Aesthet Surg. 2011;64(8):1036–1042. doi:10.1016/j.bjps.2011.02.014.
- Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28(12):2064–2069. doi:10.1200/JCO.2009.25.1728.
- Salminen SH, Wiklund T, Sampo MM, et al. Treatment and prognosis of radiation-associated breast angiosarcoma in a nationwide population. Ann Surg Oncol. 2020;27(4):1002–1010. doi:10.1245/s10434-019-08085-1.