Abstract
Idiopathic avascular necrosis of the scaphoid bone, Preiser’s disease, was originally described as a deteriorative pathology whereby the osseous structure necroses due to loss of blood supply. It may present with multifactorial etiology, which is still largely not well understood. We describe a case of Preiser’s disease in a 70-year-old female, with worsening pain and loss of range of motion in her right wrist over a two-year period. Past medical history was significant for Sjogren’s disease, fibromyalgia, and dystonia. Pain began several months following traumatic right dorsal wrist injury. Diagnosis of traumatic scaphoid fracture was originally suspected. Conservative treatment was unsuccessful. Radiographs did not demonstrate evidence of primary fracture. CT scan and MRI demonstrated osteonecrosis of the proximal pole of the scaphoid, but no evidence of fracture, either residual or healing, was found. Proximal row carpectomy was performed for avascular necrosis of the scaphoid. Histology confirmed diagnosis and verified absence of fracture. Postoperatively, the patient’s pain and range of motion improved. This report combines histological findings of Preiser’s disease with radiographic images which may ameliorate understanding of the clinical pathophysiology. We describe an unusual manifestation of Preiser’s disease whereby a single traumatic event, in the absence of fracture, led to idiopathic scaphoid avascular necrosis, which may have been associated with Sjogren’s syndrome and fibromyalgia. These conditions may have negatively impacted microvasculature and decreased bone mineral density, inversely correlated with the production of fatty marrow, facilitating the onset of osteonecrosis in the scaphoid.
Introduction
Idiopathic avascular necrosis (AVN) of the scaphoid, known as Preiser’s disease, is a rare condition that first presents with progressive unilateral dorsoradial wrist pain in the anatomical snuffbox [Citation1]. Pain limits wrist range of motion (ROM), grip strength of the hand, and culminates with loss of function due to joint deterioration [Citation1,Citation2]. It is a debilitating pathology whereby ischemia and subsequent necrosis of the scaphoid bone occur in the absence of fracture. Previous case studies have suggested that collagen vascular disease, repetitive microtrauma, steroid therapies, alcohol abuse, and idiopathic conditions are risk factors associated with its etiology, ultimately disrupting blood supply to the scaphoid with resultant ischemia and necrosis [Citation3–5]. The scaphoid, lunate, and capitate are known to have tenuous blood supply and are considered ‘bones at risk’ for avascular necrosis [Citation2,Citation6]. The scaphoid blood supply is derived from the palmar and dorsal branches of the radial artery in a retrograde manner [Citation1]. While AVN of the scaphoid proximal pole following fracture is a common, well-documented occurrence, idiopathic necrosis of the entire scaphoid is rare [Citation7].
Scaphoid fractures and subsequent AVN of the scaphoid may present with similar clinical symptomatology as Preiser’s disease. Thus, in the acute setting, the diagnosis of idiopathic scaphoid AVN, due to its rarity, may be deferred [Citation8]. Snuffbox pain, decreased ROM, grip strength, and loss of function are characteristics of both [Citation2]. Often, a patient’s presenting history is the most important diagnostic tool to differentiate the two pathologies, with trauma from a fall from an outstretched hand being a common but not exclusive mechanism of scaphoid fractures. Nonetheless, repetitive trauma, which may exacerbate Preiser’s disease, can create complexities in these clinical timelines, necessitating an intricate examination to diagnose the pathology accurately. Moreover, the collapse of carpal bones and the development of osteoarthritis may be mistaken for traumatic arthritis from a previously suspected fracture. Due to the lack of case reports documenting Preiser’s disease, its clinical pathogenesis and progression are difficult to differentiate from other traumatic injuries, creating diagnostic dilemmas that may be present when idiopathic AVN of the scaphoid presents atypically. We describe a case of Preiser’s disease, which was initially interpreted to be a scaphoid fracture following traumatic injury. The patient’s previous medical history, notable for Sjogren’s syndrome and fibromyalgia, may have been associated with the development of the disease.
Case report
A 70-year-old female patient with a medical history of fibromyalgia, dystonia, and Sjogren’s disease, presented to our outpatient department complaining of unilateral right wrist joint pain with limited ROM, which worsened over several months. She reported tenderness over the scaphoid. Her active ROM was limited to 20° of dorsal flexion and 10° of palmar flexion, ROM stiffness was observed upon passive movement. A contralateral examination of the left wrist was unremarkable. Her symptoms began two years prior after sustaining a forceful dorsal impact to the wrist. She reported no pain and an intact range of motion before that injury. Initial radiographs did not demonstrate any acute fractures or other abnormalities. The patient was treated nonoperatively with non-steroidal anti-inflammatories (NSAIDs) and physiotherapy.
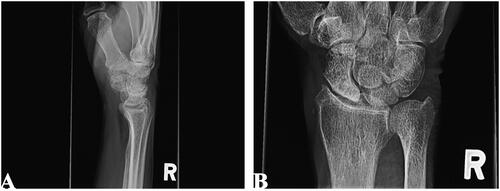
At follow-up one year later, the patient had mild improvement in symptoms, but pain persisted. Interval radiographs and computerized tomography (CT) were suggestive of arthritis and sclerosis at the proximal pole of the scaphoid (). The scapholunate (SL) and lunotriquetral (LT) joint spaces were not altered (). No acute or residual fractures were identified.
Figure 1. Lateral (A) and posteroanterior (B) radiographs of right wrist joint demonstrating proximal pole sclerosis of scaphoid.
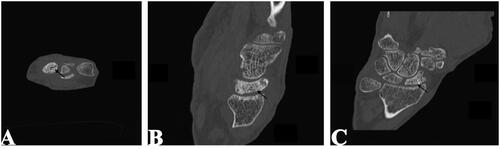
In retrospect, a CT scan revealed a subchondral crescent sign on the scaphoid, suggesting impending articular collapse due to osteonecrosis (). The patient continued with non-operative management.
Figure 2. Axial (a), sagittal (B), and coronal (C) CT of right wrist. Subchondral crescent sign on scaphoid (black arrows) present.
At two-years of follow-up, wrist pain had not improved. Magnetic resonance imaging (MRI) and repeat radiographs demonstrated diffused altered marrow signals in the scaphoid, which were suggestive of AVN ( and ). There was marked flattening of the scaphoid with collapse of the cancellous bone. Once again, no evidence of a fracture was appreciated. The presence of AVN, associated pain, and worsening symptoms were indications for surgical management.
Figure 3. MRI of right wrist joint showing diffused altered marrow signal (white arrows) of the proximal scaphoid.
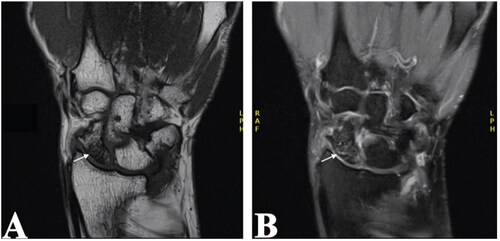
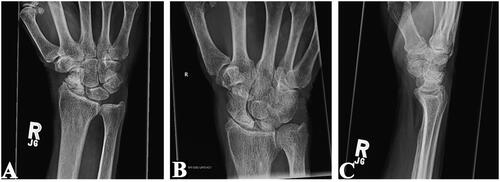
Figure 4. Plain radiographs of the right wrist joint demonstrating progressive wrist degeneration and scaphoid sclerosis/fragmentation with narrowing of the radiocarpal joint. A & B: Posteroanterior view. C: Lateral view.
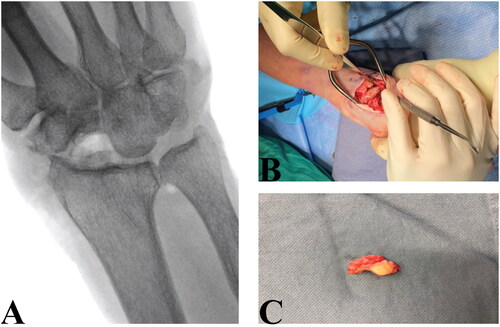
The patient was consented for proximal row carpectomy. The wrist joint was accessed by a minimally invasive dorsal approach over the fourth extensor compartment with preservation of the retinaculum. The scaphoid, lunate, and triquetrum bones were excised. A neo-articulation between the capitate and lunate fossa of the radius was created. The scaphoid was excised en-bloc and was collapsed and flattened proximally with evidence of fragmentation, but no fracture ().
Figure 5. A: Postoperative radiograph of right wrist joint. B: Intraoperative image showing excision of proximal carpal bones. C: Scaphoid with flattened and collapsed proximal pole.
Cross-section histological analysis of the scaphoid demonstrated diffuse AVN, suggestive of Preiser’s disease ( and ). These findings were supported by intramedullary fat necrosis of the bone with resulting acellular bone trabeculae (). Histology further examined the cross sections of the scaphoid bone and discovered the presence of marrow fibrosis ().
Figure 6. Regions of avascular necrosis underlying cancellous bone of right scaphoid. Reference of 5 mm.
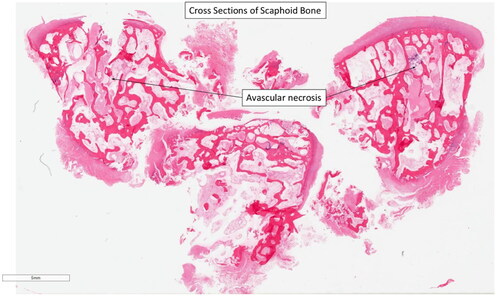
Figure 7. Avascular necrosis of the trabeculae. The articular cartilage of the scaphoid is intact, dismissing osteoarthritic processes as a contributor to bony degeneration. Reference of 1 mm.
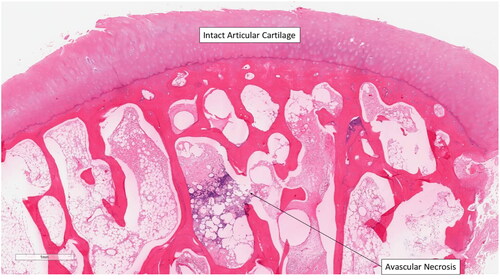
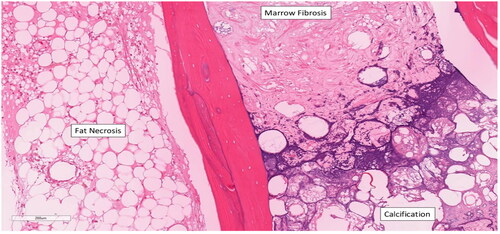
Figure 8. Right resected scaphoid bone with area of fat necrosis with and without calcification, acellular cancellous bone, and marrow fibrosis at the proximal end of the scaphoid. Reference of 200 µm.
For histological analysis, the specimen was placed in 10% buffered formalin for a minimum of 24 h after the operation. Following fixation, the samples were placed in an adequate volume of demineralization solution (Cal-ExTM Decalcifying Fluid, Fisher ChemicalTM) and subsequently checked daily by a histotechnologist until deemed adequately demineralized for routine tissue processing, paraffin embedding, microtomy, and hematoxylin and eosin staining.
Postoperatively, the patient experienced pain relief and improved wrist function. Wrist range of motion was still limited, but improved to injury baseline, and hand therapy was recommended.
The Herbert and Lanzetta grading scale [Citation9] is used to assess the four stages of Preiser’s disease, beginning with density (sclerotic) changes to the proximal pole of the scaphoid, to complete collapse of the carpal bones with osteoarthritis: Stage I – Normal radiograph and CT, may show MRI signal change; Stage II – Increased sclerotic density of proximal pole of scaphoid; Stage III – Fragmentation of proximal pole of scaphoid without pathologic fracture; Stage IV – Carpal collapse with osteoarthritis. MRI signal changes can be further graded using the Kalainov criteria, whereby Type I and II are differentiated by complete or incomplete necrosis of the scaphoid bone, respectively [Citation10]. In line with these grading scales, our patient had Stage III Herbert and Lanzetta, with Type II Kalainov idiopathic AVN of the scaphoid.
Discussion
This case introduces an intriguing clinical timeline that, to our knowledge, has not been described in the literature. Our patient presented with a two-year history of unilateral right wrist joint pain with limited ROM. History of trauma to the wrist led to investigations to assess for scaphoid fracture and scapholunate injury, despite negative imaging and atypical injury mechanism. Throughout this patient’s treatment, it was difficult to appreciate whether arthritic changes were resultant from traumatic arthritis or osteoarthritic changes from subcortical osteonecrosis. Ultimately, this delayed the diagnosis of Preiser’s disease, allowing for osseous deterioration to progress. Previous reports propose AVN in Preiser’s disease occurs in the absence of prior history of traumatic injury [Citation2–4,Citation6]. Others suggest that repetitive microtrauma may predispose the carpal bones to the eventual development of osteonecrosis due to hindered blood supply and pressure differentials in vascular structures [Citation2,Citation4]. In the presence of a single previous traumatic injury, the diagnosis of Preiser’s disease would normally be excluded, as was initially accepted in our patient [Citation2,Citation4–7]. With continued radiographic progression of AVN in the absence of fracture and worsening symptomatology, necessitating proximal row carpectomy, it was determined that histological analysis of the scaphoid may confer further diagnostic clarity.
The presence of marrow fibrosis, intramedullary fat necrosis, and acellular bone trabeculae at the scaphoid supported a diagnosis of idiopathic AVN, in line with previous histologic literature regarding Preiser’s disease [Citation5,Citation8]. Contrary to previous reports documenting AVN fragmentation and collapse of the scaphoid [Citation2,Citation4], which occur in end-stage Preiser’s disease, histology confirmed that the cortical bone and articular cartilage remained undisturbed for the duration of our patient’s clinical timeline. Osteonecrosis typically involves convex articular bone surfaces due to decreased diameter of terminal vessels and lack of collateral vascularization [Citation8]. This effect is magnified in retrograde blood-supplied structures [Citation6], such that exist in the scaphoid and capitate [Citation11]. Moreover, osteonecrosis has been correlated with reduced vascularity of fat marrow in comparison to hematopoietic marrow, as seen in our patient’s histological analysis [Citation6]. Literature has reported the presence of frequent trabecular microcracks in aseptic bone necrosis, this microdamage may lead to fatigue fractures [Citation5,Citation6]. Regardless of fracture occurrence, the weakened bone contributes to vascular defects at the capillary level [Citation8], either from fat cell pressure or vessel ruptures, further necrosing the osseous structure [Citation5,Citation6]. Based on the histology findings of Preiser’s disease, it appears the idiopathic AVN of the scaphoid presented in our report was, at least in part, precipitated by a single traumatic event.
Our patient had a known history of Sjogren’s, fibromyalgia, and dystonia. Sjogren’s disease and fibromyalgia have been associated with systemic manifestations that impact the musculoskeletal system, resulting in osteopenia and osteomalacia with additional vascular compromise, including calcification, providing further inference to the histological findings discovered in our report [Citation12]. Moreover, Sjogren’s has been documented as a rare clinicopathologic entity of bone marrow fibrosis [Citation13]. In the present case, we strongly suspect that AVN of the scaphoid was likely an interplay of mechanical injury with longstanding Sjogren’s and fibromyalgia, which may have increased this patient’s susceptibility to lower bone mineral density, inversely associated with fatty marrow production, promoting necrosis [Citation6,Citation12,Citation13]. While it is difficult to confirm this observation based on the findings of a single report, it may offer valuable insights exploring histological and clinical features supporting the association of inflammatory processes in the pathogenesis of Preiser’s disease.
Our patient was initially treated non-surgically, attributable to atypical presentation. Despite the ineffectiveness of non-surgical management, early radiographic signs of hand and wrist AVN are overlooked or undetectable, and with no clear indication for surgery, conservative treatment is often initiated [Citation7,Citation8]. Non-surgical treatment of Preiser’s disease includes NSAIDs, corticosteroid or hyaluronic acid injections, and immobilization. They have not shown efficacy in preventing osseous deterioration nor improvements in ROM, grip strength, or wrist pain [Citation7,Citation14,Citation15]. Neither have they demonstrated improvements in radiographic progression of the disease [Citation7,Citation14]. Other reports suggest that corticosteroid injections may further disrupt the blood supply to the scaphoid, providing contraindication to their use in cases of suspected Preiser’s disease [Citation7].
Surgical treatment consists of revascularization surgery, salvage surgery, and joint leveling surgery, depending on stage criteria [Citation7,Citation14,Citation15]. A recent meta-analysis examining 138 cases of Preiser’s disease determined that while evidence for surgical management of stage I is inconclusive, stage II is managed most appropriately via vascularized bone grafting and closing radial wedge osteotomy, in the absence of radiocarpal osteoarthritis [Citation15]. These approaches may be effective in decompressing venous hyper pressure, redistributing contact surfaces, and promoting revascularization [Citation14,Citation15], albeit they are still unable to halt long-term radiographic progression of the disease [Citation14]. Arthroscopy may provide diagnostic and therapeutic modalities in the early stages of the disease where the chondral surfaces are intact [Citation8]. Probe ballottement of subchondral integrity and presence of ligamentous injury, synovitis, fracture, or fragmentation can be evaluated and treated concurrently [Citation8]. Stages III and IV are managed most appropriately by proximal row carpectomy (PRC) [Citation15]. PRC has evidence supporting its utility for individuals greater than 35 years of age with low physical demand [Citation14]. Further, it permits functional wrist ROM with a flexion-extension arc of 73.5° and radial/ulnar deviation of 31.5°, as well as improved grip strength and improvement with pain [Citation15]. The development of radiocapitate arthritis typically occurs 10 years following surgery [Citation14]. PRC’s utility over four-corner fusion acknowledges similar outcomes between the two but also remarks increased risk of hardware infection, osseous non-union, and training time with the latter [Citation14]. Limited evidence supports the use of scaphoid replacement with silicone implants due to carpal collapse, subluxation and dislocation, increased pain, and increased likelihood of secondary surgery [Citation14,Citation15]. In the case presented, PRC appears to have been an acceptable treatment modality. The patient was satisfied with results, requiring no further operative interventions.
Conclusion
It is inherently difficult to identify Preiser’s disease, as its diagnosis is typically reliant on clinical progression and radiographic sequence of osteosclerosis and subsequent fragmentation of the scaphoid, which may not be evident until end stages [Citation5,Citation6,Citation8]. Our case report is unique in that it demonstrated that its diagnosis is often complicated and may be masked by other degenerative problems. Additionally, Preiser’s may be confounded by other musculoskeletal diseases with subtle changes leading to bone collapse. Based on our patient report, in the setting of previous trauma to the hand, regardless of mechanism and etiology, routine follow-up for residual symptoms may be indicated to monitor for progression of idiopathic scaphoid AVN, which cannot be readily ruled out. Moreover, further studies are required to ameliorate the understanding of the association between inflammatory conditions and the development of Preiser’s disease.
Patient consent
Patient data including radiographs and intra-operative images used in the case report were obtained via written informed consent from the patient. Declaration of patient consent/patient consent form can be shared with the journal editorial office by the corresponding author, Topham, JJ upon request.
Author contributions
All authors contributed equally to the preparation, writing, and editing of the case report.
Acknowledgments
The authors do not have any financial acknowledgements relating to the current case report. We thank the Western University Department of Pathology and Laboratory Medicine with assisting with histological analysis of our scaphoid cross sections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, Topham, JJ. The data are not publicly available due to information that could compromise the privacy of research participants.
References
- Patterson RM, Moritomo H, Yamaguchi S, et al. Scaphoid anatomy and mechanics: update and review. Oper Tech Orthop. 2003;13(1):2–10. doi:10.1053/otor.2003.36316.
- Panagis JS, Gelberman RH, Taleisnik J, et al. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375–382. doi:10.1016/s0363-5023(83)80195-6.
- Kocaoglu M, Landsleitner B, Kotas J, et al. Preiser’s disease a case report. Acta Orthop Traum Turc. 1990;24(3):179–182. doi:10.3944/aott.v24i3.1147.
- Lauder AJ, Trumble TE. Idiopathic avascular necrosis of the scaphoid: Preiser’s disease. Hand Clin. 2006;22(4):475–484; abstract vi. doi:10.1016/j.hcl.2006.07.005.
- Trumble TE. Avascular necrosis after scaphoid fracture: a correlation of magnetic resonance imaging and histology. J Hand Surg Am. 1990;15(4):557–564. doi:10.1016/s0363-5023(09)90015-6.
- Fondi C, Franchi A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab. 2007;4(1):21–26.
- Tomori Y, Nanno M, Takai S. Clinical outcomes of nonsurgical treatment for Preiser disease. Medicine. 2020;99(4):e18883. doi:10.1097/MD.0000000000018883.
- Bellringer SF, MacLean SBM, Bain GI. Preiser’s disease – current concepts of etiology and management. Hand Clin. 2022;38(4):469–477. doi:10.1016/j.hcl.2022.03.013.
- Herbert TJ, Lanzetta M. Idiopathic avascular necrosis of the scaphoid. J Hand Surg Br. 1994;19(2):174–182. doi:10.1016/0266-7681(94)90159-7.
- Kalainov DM, Cohen MS, Hendrix RW, et al. Preiser’s disease: identification of two patterns. J Hand Surg Am. 2003;28(5):767–778. doi:10.1016/s0363-5023(03)00260-0.
- Kadar A, Morsy M, Sur YJ, et al. The vascular anatomy of the capitate: new discoveries using micro-computed tomography imaging. J Hand Surg Am. 2017;42(2):78–86. doi:10.1016/j.jhsa.2016.12.002.
- Rozis M, Vlamis J, Vasiliadis E, et al. Musculoskeletal manifestations in Sjogren’s syndrome: an orthopedic point of view. J Clin Med. 2021;10(8):1574. doi:10.3390/jcm10081574.
- Kakiuchi S, Takagi I, Akiyama H, et al. Autoimmune Myelofibrosis in Sjögren’s syndrome: report of a case. Am J Case Rep. 2020;21:e924983-1–e924983-4. doi:10.12659/AJCR.924983.
- Chim H, Moran SL. Long-term outcome of proximal row carpectomy: a systematic review of the literature. J Wrist Surg. 2012;1(2):141–148. doi:10.1055/s-0032-1329547.
- Kazemi M, Daliri M, Moradi A. A systematic review on the management of idiopathic avascular necrosis of the scaphoid (Preiser’s disease). Orthop Traumatol Surg Res. 2023;109(3):103480. doi:10.1016/j.otsr.2022.103480.
