Abstract
There has been great progress in the 30 y since the reporting in 1984 of the cDNA for interleukin1 (IL1) β in the human and IL1α in the mouse. However, the history of IL1 begins in the early 1940s with investigations into the nature of an endogenous fever-producing protein released rabbit peritoneal neutrophils. Most researchers in immunology today are unaware that the field of cytokines, particularly the field of inflammatory cytokines. Toll-like receptors and innate immunity traces back to studies on fever. Researchers in infectious diseases wanted to know about an endogenous protein that caused fever, independent of infection. The endogenous fever-producing protein was called by various names: granulocyte, endogenous or leukocytic pyrogen. It is a fascinating and sometimes controversial story for biology and medicine and for the treatment of inflammatory diseases. Few imagined that this fever-producing protein would play such a major role in nearly every cell and in most diseases. This paper reviews the true background and milestones of interleukin1 from the purification of leukocytic pyrogen to the first cDNA of IL1β and the validation of cytokine biology from ill-defined factors to its present day importance.
Abbreviations
| IL | = | interleukin |
| IL-1R | = | interleukin-1 receptor |
| LAF | = | lymphocyte-activating factor |
| TIR | = | Toll-interleukin-1 receptor |
| TLR | = | Toll-like receptor(s) |
| TNF | = | tumor necrosis factor |
Introduction
The interleukin (IL)1 story is also an example of how we did biology. First there was the biological question, in this case, what was the cause of fever in the absence of infection? Next, the need to develop a relevant bioassay; for a pyrogen, it was fever in rabbits. With a reliable and relevant assay, the next step was the purification of the molecule with the objective of obtaining the amino acid sequence of the purified protein. With the advent of molecular biology, came the requirement to isolate the cDNA, produce the recombinant protein and confirm its biological activity. For leukocytic pyrogen, we wanted to demonstrate that the recombinant protein produced fever in animals and humans. It took nearly 40 y (1948 to 1986) to reach that endpoint.Citation1,2 Nowadays, the steps are reversed. First, a gene is isolated but with no known function; the recombinant protein is produced in order to find a function(s). To confirm the importance of the function, the gene is deleted in cells or in mice using an expanding number of techniques. An example of this latter discovery process is the molecule IL32. The gene was first isolated without a function.Citation3 Twelve years later, the recombinant protein was produced, purified and a function was established.Citation4 Silencing of endogenous IL32 in cells confirmed its function, as reviewed in reference.Citation5
The IL1 story is also a history of “soluble factors” from leukocytes. Not all soluble factors would attain the importance of IL1s role in human disease and not all soluble factors would be validated by the clinical benefits of anti-IL-1-based therapeutics for inflammatory diseases.Citation6 Tumor necrosis factor (TNF) α has a similar history, although the initial biological activity of killing tumors is nearly absent compared to the role of TNFα in inflammation. Indeed, it was the lone voice of Anthony Cerami who had related a TNFα-linked biological assay to a clinical question.Citation7 Indeed, few imagined that blocking IL1 or TNFα would be so effective in the treatment of human diseases. A recent study on the beneficial effect of a neutralizing monoclonal antibody to IL1α in endstage cancer patients;Citation8 or that 93% of CD4 positive T-cells in HIV1 infection die by an IL1β-related mechanism of cell death called pyroptosisCitation9 tells us that the IL1 story is hardly finished.
Early Years of Research
As a medical student at Yale I was required to perform original research and write a thesis in order to receive the MD degree. Selecting a thesis supervisor took place at the mid-point of the first year and I selected Elisha Atkins. Elisha had given our class a lecture on fever and the existence of an endogenous protein that produced fever. It was called “endogenous pyrogen.” He explained that endogenous pyrogen was produced by white blood cells and had been described in the early 1940s by Paul Beeson.Citation1 I became interested in this “endogenous pyrogen” and asked to join the Atkins laboratory. I learned to train rabbits in their restrainers, how to insert a flexible rectal thermometer and how to give a bolus injection into the narrow lateral ear vein of trained rabbits. Phyllis Bodel was a young investigator in the Atkins laboratory and I learned a great deal from her guidance. The project started in the summer of 1966. In 1968, I published my first paper on an endogenous pyrogen.Citation10 In 1993, I was still training rabbits, inserting rectal thermometers and testing new cytokines for the production of fever.Citation11 But as laborious as fever studies are, nothing is more impressive than to see the rapid rise in core temperature within 10 minutes after the bolus injection.
I was interested in pyrogenic tolerance, which takes place with daily injections of small doses of endotoxins. All studies pointed to the Kupffer cells and so my project was to isolate these cells from rabbit livers and study the production of the endogenous pyrogen. But I became increasingly interested in the “endogenous pyrogen” itself and how within minutes following an intravenous bolus injection of unfractionated cell supernatants, rectal temperature would rise, reach a peak level in less than the hour and then rapidly subside during the next 2 hours. Once rectal temperature reached baseline, one could inject the same supernatant in the same rabbit and obtain nearly the same monophasic fever.
Although my thesis was on the production of endogenous pyrogen from the liver, I wanted to return to “endogenous pyrogen” once I finished my clinical training. I left Yale in June of 1969 to begin internship and residency in the Children's Service of the Massachusetts General Hospital in Boston. In July of 1971, I began my obligatory military service in the United States Public Health Service, which during wartime was a branch of the United States Navy. Indeed, the National Naval Hospital is across the road from the National Institutes of Health where I began as a Clinical Associate. My research was in the laboratory of Sheldon M. Wolff and I explained to him that my sole objective was to purify human endogenous pyrogen. Those working in the Wolff laboratory such as Richard Root used the term “leukocytic pyrogen” for “endogenous pyrogen.” Much of what is written below would never have taken place without the guidance and support of Shelly Wolff. Shelly was a giant in the field of fever and inflammation and I owe much of my career to him. Shelly died in 1994, a great loss to me and to the IL1 story.
Historical Perspective on Fever
For centuries, fever has been associated with leukocytic infiltrates. Roman military physicians had made the observation that draining “pus” from wounds would reduce fever. The first studies on substances released from “pus” that induced fever were published in 1943, the year I was born. The Russian émigré Eli Menkin injected rabbits with supernatants from neutrophils taken from sterile peritonitis induced in rabbits (reviewed inCitation12 and called the fever-inducing property “pyrexin;” others used the term “granulocytic pyrogen,” as neutrophils were the prominent cell in the peritoneal exudate. Regardless of the name, the concept was that white blood cells released a substance that produced fever when injected into rabbits. Menkin's studies were suspect in that his preparations were likely contaminated with endotoxins. But he had the correct concept. The ability of bacterial products, but particularly endotoxins, to produce fever was known in the 1940s. In 1948, however, Paul Beeson confirmed Menkin's observation and reported that a protein material, released from rabbit peritoneal leukocytes but free of endotoxin, caused a rapid onset fever upon an intravenous bolus injection into rabbits.Citation1 This was a milestone because there was an explanation for fever in the absence of infection. The supernatants from leukocytes of human cells when injected intravenously into rabbits also caused this rapid onset of fever. Plasma from patients with fevers were also injected into rabbits. The field of fever research expanded after Beeson's paper and many forgot the work of Menkin.
To increase core temperature, the rabbit uses vasoconstriction of the vessels in the ears to conserve heat; heat loss takes place with vasodilation. The response is dramatic and one can feel the ears rapidly becoming cold. The rabbit also becomes quiet and motionless, and this observation resulted in the discovery that leukocytic pyrogen was a sleep factor.Citation13 But as cytokine biology expanded into in vitro assays, the fever assay remained relevant and reliable for purifying the endogenous fever-inducing protein, leukocytic pyrogen; later, in 1979, this same molecule was shown to augment Tcells responses to antigensCitation14 and its name changed to IL1.
The battle with Lipid A
Few delved into the area of purifying a protein from crude supernatants of human blood leukocytes using the “fever assay.” Since Menkin's work in 1943 and for the next 30 years, many believed that leukocytic pyrogen did not exist. Lipid A, the active component from Gram-negative bacterial endotoxins, could bind to any protein, such as albumin, and produce a monophasic fever indistinguishable from the fever produced by leukocyte supernatants. In fact, in the early 1970s several papers were published showing this to be the case. Battle lines were drawn: the camp proposing that “endogenous pyrogen” was a complex of Lipid A bound to any protein had its base from the biochemical studies on Lipid A by 2 of Germany's outstanding scientists: Otto Westphal and Otto Luederitz.Citation15 The purification of Lipid A, its structure and the concept that the biological properties of endotoxins were due to the Lipid A moiety was a milestone in infectious disease research. The other camp was in North America and England, where scientists supported the existence of a leukocyte-derived protein causing fever, which was not due to Lipid A. Prominent in providing data for the existence of leukocytic pyrogen were our laboratory at Yale and the laboratory of Barry Wood and Patrick Murphy at Johns Hopkins. At the National Institutes of Health in Bethesda, Maryland, Sheldon Wolff was making significant contributions to fever research. In Britain and Canada, studies were also focusing on leukocytes and fever.Citation16 In 1973, there was an international meeting in Virginia attended by Otto Westphal and the monograph published from this meeting reveals how the issue of the existence of endogenous pyrogen was challenged by the Lipid A hypothesis.
In 1960, Elisha Atkins wrote a classic paper on fever and made the argument that endotoxins and endogenous pyrogen were distinct fever-producing substances.Citation17 In this review, Atkins summarized the data, which supported the concept that leukocytic (endogenous) pyrogen was induced by microbial products called “exogenous pyrogens.” Thus, the pathogenesis of fever was based on “exogenous” pyrogens such as microbial products and Lipid A inducing leukocytes to release the “endogenous” pyrogen. One can appreciate that this concept is called many years later the “non-specific innate immune response.” The concept of exogenous pyrogens inducing an endogenous pyrogen, however, did not directly address the issue of fever in the absence of infection. Fever occurs in many diseases in the absence of infection such as rheumatoid arthritis, lupus and sterile inflammation such as strokes and tumors. Today, we know that IL1 is induced by “damage associated molecular patterns” or “alarmins,” of which fever is likely due to the release of IL1α from dying cells.Citation18,19
Characterization of Biologically Active Leukocyte Supernatants
In 1967, Phyllis Bodel and Elisha Akins changed the field when they reported that human blood monocytes produced a pyrogen. Published in the New England Journal of Medicine, they did not use endotoxin to stimulate the monocytes but rather heat-killed Staphylococcus epidermidis and in doing so, challenged the concept that fever was due to Lipid A bound to a leukocyte protein because Staphylococcus epidermidis do not have Lipid A.Citation20 It was paradigm change in that attention now turned away from the granulocyte to the monocyte. The data revealed that the human blood monocyte was the dominant source of leukocytic pyrogen; thus the study explained why patients with severe neutropenia often developed fever. At the time, I was a second year medical student in the Atkins-Bodel laboratory and I can remember the excitement that surrounded the paper. Little did I know then that when I began the purification of human leukocytic pyrogen in 1971, we would stimulate fresh human blood monocytes with heat-killed Staphylococcus epidermidis.
Early attempts at purifying leukocytic pyrogen from human blood cells
The 1970s were dominated by reports subjecting the supernatants from activated cells to various biochemical characterizations. The first step was to show that the biological activity in the supernatants was non-dialyzable and therefore had a molecular weight greater that 10000 Daltons. The next step was to provide a molecular weight using gel-filtration. In 1971, we started our own attempt to characterize and purify human monocyte pyrogen to homogeneity; it is an understatement that this goal was a daunting task. First, obtaining blood monocytes in sufficient quantities was a limiting step. Second, protein purification from cell supernatants was in its infancy. Coommassie Blue staining was used to assess the level of purity but this method was useless for sub-microgram levels of proteins and there was no silver staining. Third, and most challenging, was the loss in the activity. At any given step using even the most simple separation procedure, one would face the sobering fact that there was less than 10% of the starting activity remaining. To reduce the low concentrations of proteins from “sticking” to glass, we used silicon coated glass tubes. We added various compounds to “protect” the low concentrations of proteins from sticking but any “stabilizer” could not contain microbial products, had to be removable and did not interfere with the bioassay. However, we did have the advantage that since the rabbit assay was rapid, we could move from one separation step to the next within days. This was an advantage over scientists using 3-day in vitro cell proliferation assays.
How did we protect ourselves against introducing microbial products during the various separation methods? All gel-filtration media (Sephadex) and buffers were autoclaved. Dialysis tubings were boiled for 30 minutes. We made long glass columns ourselves that were too long to fit into autoclaves so these were sterilized with formaldehyde. All glassware had to be baked for 4 hours at 100°C. We use clinical grade intravenous tubing for connecting columns to fraction collectors. It was an endless battle not to introduce contamination and gel-filtration buffers contained 0.02% sodium azide to prevent bacterial growth. To reduce losses, most procedures were at 4°C. However, cold-growing Pseudomonas species were a constant concern. Effluents were cultured routinely at 37°C but also a 4°C to monitor bacterial contamination. Although we controlled microbial contamination, we could not control fraction collectors that “jammed” or buffers reservoirs that ran dry. We tolerated procedure-related losses but not accidental losses.
Six Years, Two Molecules, Two Molecular Weights and Many Failures
Starting in 1971, we used supernatants from human blood monocytes present in peripheral blood mononuclear cells stimulated with heat-killed Staphylococcus epidermidis as the starting material. We established a “unit of activity” as a peak fever at least 0.6 above baseline in a 3 kg rabbit. We tested several but never found a cell line as a source of supernatant that was comparable to the activity of Staphylococcus epidermidis stimulated human monocytes. Although the fever assay was rapid in that data were available within one hour, placing the rabbits in their restrainers, inserting the rectal thermometer and waiting for a stable baseline temperature took about 90 minutes. We made our own glass columns of increasing length to improve separation (initially 105 cm but later 181 x 3.5 cm with a bed volume of 3300 mL of Sephadex G-50). In the early years, we used alcohol precipitation to concentrate the supernatants but this method and other precipitation methods resulted in high losses. The best recovery of activity was using large dialysis tubing filled with the crude supernatants and placed in front of high speed fans. The temperature inside the bag remained at 10–12°C and as the supernatants concentrated, the high salt prevented losses. Dialysis was carried out in the same bags.
Bringing the purification to completion
From 1975 to 1977, several different methods were used to increase the specific activity of leukocytic pyrogen (protein per unit of biological activity). A typical “purification run” began with 4 Ls of pooled supernatants, concentration in the large dialysis bags, gel-filtration on Sephadex G-50 and isoelectric focusing. An important advance was the production of rabbit neutralizing antibodies to human leukocytic pyrogen.Citation21 Anti-human leukocytic pyrogen was likely one of the first if not the first anti-cytokine. The immunogens were at various levels of purity of leukocytic pyrogen and certainly contained some other proteins found in monocyte supernatants. We found that the unfractionated anti-human leukocytic pyrogen contained antibodies to human serum proteins such as albumin and α1antitrypsin. To remove these, we co-valently bound human serum proteins as well as unstimulated monocyte supernatants without leukocytic pyrogen activity to Sepharose. The crude antiserum was then passed several times until these were removed. Next, we made an IgG fraction of the “clean” antiserum, which was bound covalently to Sepharose. Poured into a glass column, we named this the “immunoaffinity” column. As shown in , when we added the immunoaffinity purification step, we greatly improved the specific activity of leukocytic pyrogen; however but we no longer could determine the level of protein by standard methods. illustrates an autoradiograph of intrinsically 35S-labeled methionine proteins from human blood monocytes stimulated in vitro.Citation22 These data were published several years after the 1977 report in which we calculated the specific activity using extrinsic labeling of proteins with 125I.Citation23
Figure 1. Two-dimensional PAGE of purification steps of 35S-labeled proteins in supernatants from heat-killed Staphylococcus epidermidis-stimulated human blood monocytes. The first-dimension pH measurements are derived from direct measurement of a parallel standard gel. Each panel indicates the purification step. Fractions eluting from the immunoaffinity step were chromatographed on Sephadex G-50 and the pyrogenic activity of fractions less than 20000 Daltons were pooled and subjected to chromatofocusing. The fractions eluting from the chromatofocusing step in the neutral pH range were then pooled and subjected to 2-dimensional PAGE. A single protein is visible by autoradiography with a molecular weight near 18000 Daltons and a pI between 6 and 7. Previous chromatofocusing in flat-beds revealed that the pI of leukocytic pyrogen was at 6.8.Citation21 The figure is reprinted from.Citation22
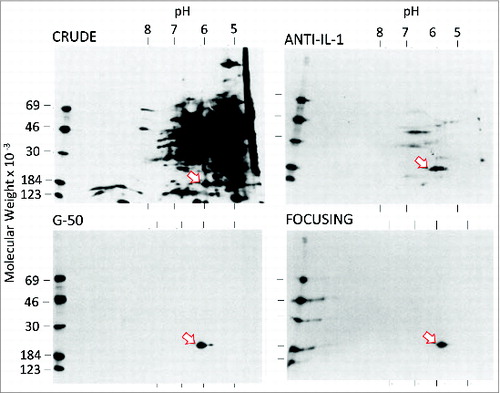
We cannot see any band!
Before the production of the anti-leukocytic pyrogen antibody, we summarized our studies to date and published a report on the partial purification of leukocytic pyrogen.Citation24 In that study, we showed a gel with the pyrogenic activity that had migrated to the area of proteins that are 15000 Daltons, but there was no stainable protein. Facing this problem, we decided that the only way to see leukocytic pyrogen was to use extrinsic radioactive 125I labeling. illustrates the 4 major activities that co-elute with a single radiolabeled protein. However, labeling proteins and maintaining biological activity was and still is challenging. The standard labeling methods of chloramine T oxidized leukocytic pyrogen and destroyed its activity. At the time a new reagent called 125I Bolton-Hunter was available. This reagent forms covalent peptide bond with lysines on proteins. Radiolabeling of preparations during the late stages of purification revealed several bands in the 10–20000 Dalton range but fortunately the preparation was active after radiolabeling with Bolton-Hunter.Citation23 Two more steps were required, gel-filtration and ion-exchange gradient. Losses were enormous but the gel revealed a single band (). Using 125iradiolabeling of these preparations of leukocytic pyrogen, we wrote in the 1977 paper:Citation23 “at this stage, a rabbit pyrogen dose is less than 50 ng”.Citation23 A rabbit pyrogen dose is the amount of leukocytic pyrogen that produced a mean temperature maximum (peak) of 0.6°C above baseline temperature within 60 minutes following bolus intravenous injection into 3 kg trained rabbits. Within 5–10 minutes of “peak fever,” the ears become warm as the vessels dilate, rectal temperature begins to fall and within the hour, rectal temperature returns to with 0.2°C of baseline. Thus, in a 3 kg rabbit, we calculated that 10–20 ng/kg of purified leukocytic pyrogen was the specific activity of the molecule.
Figure 2. Co-elution of IL1 activities using radiolabeled proteins. A. SDS-PAGE of trichloracetic acid-precipitated fractions 24–36 of 35S-methionine-labeled monocyte supernatants during chromatofocusing. The pH of the chromatofocusing gradient is shown. Before chromatofocusing, 4 Ls of pooled monocyte supernatants were concentrated and subjected to sequential immunoadsorption and gel filtration.Citation23 B. Top. The same fractions shown in A were assayed for induction of PGE2 from dermal fibroblasts. Fever was assessed in trained rabbits and LAF activity was measured using in D10.G4.1 cells. Serum SAA was determined following intraperitoneal injection into mice.Citation30 Adapted from.Citation22
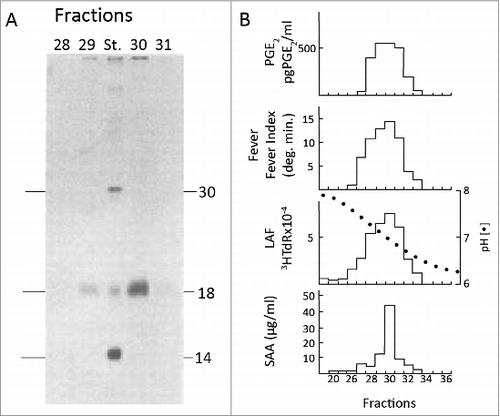
As can be viewed in the 1977 paper,Citation23 ion-exchange yielded yet more labeled proteins; however, the large peak eluting early during the salt gradient migrated as a single band. When these fractions were pooled and injected into rabbits, the peak was active and produced the expected monophasic fever. The other fractions of radioactive proteins were not active. Thus, purification could be accomplished with immunoaffinity, 2 steps of gel-filtration and ion-exchange using a shallow salt gradient. Using radiolabeled leukocytic pyrogen, we subjected preparations to HPLC and observed a single spike. In the subsequent years, to protect against losses, each time we purified leukocytic pyrogen from 4 Ls of monocyte supernatants, we added a small amount of clinical grade 5% human serum albumin to the collection tubes from the ion-exchange step.
The purification was published in the Proceedings of the National Academy of SciencesCitation23 and communicated by Christian Anderson, who had received the Nobel Prize for his work on his discovery that the primary sequence of proteins determined their tertiary structure. Chris had been working on the purification of fibroblast interferon and was encountering the same issues, that is, biological activity in the absence of measureable protein. Chris, Dahlia Rotman, who was Chris's Israeli post-doctoral fellow, and I often discussed our respective problems with purification of biologically active proteins in the nanomolar range. Chris read our manuscript and offered to communicate our paper to the Proceedings.
The end of the search but not the field
Ten years later, humans as well as rabbits were injected with recombinant IL1β resulting in fever following the intravenous injections of 10 ng/kg (reviewed in referenceCitation25). In some human studies, a dose of only 1 ng/kg produced fever. Humans were also injected with recombinant IL1α, which we had first identified in 1974,Citation26 and similarly developed chills and fever.Citation27 In many ways, the specific pyrogenic activity of recombinant human IL1β (or IL1α) brought the decades-long search for the endogenous fever-producing molecule to a close. We have now come full-circle from the concept that there is an endogenous mediator of fever, independent of infection, and dating back to 1943 and 1948 (Menkin and Beeson), and possibly to the physicians of the Roman Military, to the reality of knowing the molecule's amino acid sequence, the specific activity of the recombinant pyrogen and that leukocytic pyrogen, now renamed IL1, causes fever without any lipid moiety. Although the search for the endogenous fever-producing protein was completed, we did not know how this molecule induced fever. It was the discovery of the IL1 receptor (IL1R) 1 by SimsCitation28 and the IL1 receptor accessory protein (IL1R3) by GreenfederCitation29 that completed the story.
Leukocytic Pyrogen is the same molecule as Lymphocyte Activating Factor
During the purification process to homogeneity, we lost at least 95% of the amount of fever-producing activity of the starting material. We compensated for these losses by increasing the number of the monocytes we obtained from platelet-pheresis by products, improved the rapidity of each separation step and collected the fractions from the ion-exchange step into human serum albumin. We thus could perform more testing of purified leukocytic pyrogen on various bioassays including in vivo models. At the time, I believed that characterizing other biological activities of leukocytic pyrogen was more productive than purifying large amounts for amino acid sequencing. For example, in 1980 we published data that purified leukocytic pyrogen induced serum amyloid A.Citation30 There was considerable interest at the time on another activity found in stimulated mouse macrophage supernatants, which augmented lymphocyte responses to antigens or mitogens. Indeed, at the Dental Institute across the road from Building 10 at the NIH, Joost Oppenheim and Steve Mizel were purifying lymphocyte-activating factor (LAF) from mouse cell lines. The history of LAF goes back also to Yale where Igal Gery and Byron Waksman had reported macrophage supernatants that non-specifically “helped” lymphocyte responses to specific antigens but also to mitogens. Lawrence Lachman was also at Yale working on purifying LAF. At the NIH, Lanny Rosenwasser worked in the laboratory of Alan Rosenthal and used an assay that also added macrophage supernatants for their ability to augment lymphocyte responses to specific antigens.
Simply because LAF was induced by the same stimulants as leukocytic pyrogen from macrophagic cells, I gave Lanny a preparation of semi-purified leukocytic pyrogen in 1977 and he added increasing dilutions of the material to antigen-sensitized mouse Tcells in the presence of the antigen and after 3 d of incubation, measured proliferation by uptake of3H thymidine. I will never forget that Saturday morning when we both were gazing at the β-counter. What we saw indicated that upon diluting the preparation of leukocytic pyrogen over 10000 fold, the material was still able to act as a “lymphocyte activating factor.” The concentration of leukocytic pyrogen in the assay that doubled the proliferation of cells compared to antigen alone was calculated to be 3–30 pg/mL based on the rabbit pyrogen dose was 10 ng/kg (30 ng). Subsequent to that first experiment, we tested several preparations of highly purified leukocytic pyrogen in which we assayed fever production and lymphocyte activation at each step in the purification from starting material of the unfractionated monocyte supernatants to the most pure material. After two trying years, we submitted the data to the Journal of Experimental Medicine with some trepidation as we wrote that leukocytic pyrogen and lymphocyte activating factor are the same molecule. The paper was published in 1979.Citation14 Actually, these data from 1979 are the basis of what Janeway would later call “innate immunity.” In my opinion, “innate immunity” is a name change, and not a new concept. Shelly Wolff, Lanny Rosenwasser, Jos van der Meer, myself and several other scientists working in this field called the concept “non-specific resistance to infection.” The concept wasd hardly new and most relevant property of “innate immunity” is not its “innateness” but its non-specificity.
The “discovery” of IL-1
No researcher working on lymphocyte activating factorCitation31-33 purified the molecule sufficiently to provide a specific activity, that is, weight per unit of bioactivity, which was later confirmed by the activity of the recombinant molecule. Many immunologists believe that IL1 was discovered as “lymphocyte activating factor” despite the fact that we published in 1979 that purified leukocytic pyrogen was the same molecule that immunologists called lymphocyte activating factor.Citation14 There are many “lymphocyte activating factors” but only one pyrogenic cytokine that produces fever at 110ng/kg. TNFα is pyrogenicCitation34 but one needs at least 20–50 times more TNFα to produce the same fever as 10 ng/kg of IL1β. IL6 and the IL6 family are also pyrogenic cytokines but one needs 1000 times more IL6 to produce fever.Citation35 In fact, IL6 is under the control of IL1, as IL6 serum levels fall with IL1 blockade in human inflammatory diseases. Therefore, the discovery of IL1 is first for its property as a pyrogenic cytokine (termed leukocytic or endogenous pyrogen) and second as a “lymphocyte activating factor.” From what we know today about the many cytokines that increase Tcell responses to antigens, it is best to characterize IL1 as “the cytokine that includes activation of lymphocytes as one of its many properties.”
The Interleukin Nomenclature and the Expanding Field
During the late 1970s and early 1980s, other laboratories were purifying supernatants from immunocompetent cells and using various bioassays to define novel properties. Nearly all these studies were based on in vitro cell assays, the effects from which were often due to a mixture of molecules. In contrast, the pyrogen assay in a rabbit is a direct assay on the hypothalamic thermostat of leukocytic pyrogen whereas non-pyrogenic products present in the supernatants did not affect the febrile response. In contrast, it would be difficult to identify the property of a single factor in these bioassays performed in vitro until a purified protein was available. The in vitro assays also affected the estimation of specific activity, for example, the in vitro assays for IL2 are affected by the presence of IL1.Citation2 From all of the many names of “factors” the interleukin nomenclature emerged. With no amino acid sequence data, IL1 was the name assigned to the monocyte product and IL2 the name assigned to the lymphocyte product.Citation36
Other cytokines that produce fever
No other cytokine is as potent as IL-1 in producing fever in humans; either IL1β or IL1α induce chills and fever in humans between 1 and 10 ng/kgCitation37,38 and reviewed in.Citation25 Although TNFα and IL6 are pyrogenic in humans, TNFα induces IL1Citation34 and fever induced by IL6 requires micrograms/kg. Moreover, IL6 appears to be induces by IL1 since circulating IL6 levels fall upon IL1 blockade in autoinflammatory diseases.Citation39 Interestingly, IL18, a member of the IL1 family does not induce fever in rabbits and mice and in humans, IL18 produces fever only at doses of 100 µg/kgCitation40 The evidence reveals that IL1β is the “endogenous pyrogen” that was contained in crude supernatants from white blood cell cultures of early investigators.
Barry Wood, Elisha Atkins, Phyllis Bodel, Ralph Kampschmidt and Patrick Murphy
The search for the endogenous fever-producing molecule encompasses many dedicated researchers. A partial list includes Barry Wood, Elisha Atkins, Phyllis Bodel, Ralph Kampschmidt and Patrick Murphy. I list these only because I worked with them These pioneers in fever research were the first to shown that the rabbit granulocyte pyrogen had a molecular weight of 15000 Da and a neutral isoelectric point (pI) of 7. Ralph Kampschmit focused on the hepatic acute-phase-protein-inducing properties of “leukocytic endogenous mediator” but also used published pyrogenic activity. He never could separate the pyrogenic activity of his preparations from the acute-phase-protein-inducing properties of “leukocytic endogenous mediator.” In 1980, we reported that the purified pI 7 human leukocytic pyrogen induced the production of serum amyloid A,30 a protein, which, like C-reactive protein, is a classic marker of the acute-phase response. Thus, by the late 1970s, IL-1 was well on its way to being a thoroughly defined molecule in terms of both chemical characterization and identification of non-pyrogenic biological activities. Patrick Murphy deserves a great deal of credit for his purification of rabbit leukocytic pyrogen, initially from peritoneal exudate cells attributed to granulocytes.Citation41 Murphy also showed that there are 2 rabbit leukocytic pyrogens, the neutral form (IL1β) and the acidic form (IL1α).Citation42
The need to isolate a cDNA for IL1
Criticism focused on the lack of an amino acid N-terminal sequence to prove that a single molecule could, in fact, possess such a wide and varied spectrum of biological activities. Detractors claimed that the multiple activities were due to a mixture of proteins with similar molecular weights or contaminating microbial products. A paper was published solely to prove that lymphocyte activating factor and leukocytic pyrogen are not the same molecule using bioassays.Citation43 Looking back, those experiments never addressed the issue directly. But a cDNA and recombinant IL1 would settle the issue that a single polypeptide (IL1) possessed diverse biological activities. In 1982, cDNA cloning was still in its infancy. In 1982, the cDNA of only 3 human genes had been reported. To protect mRNA from degradation, vanadium was used and there were no “kits.” We had no amino acid sequences to construct primers to isolate a cDNA. It was even doubtful that making a full-length cDNA was possible due to RNAses in the monocytes, the source of mRNA for the project; however, we had one advantage: the antibodies we had made to the pI 7 human leukocytic pyrogen.Citation44 Together with Alex Rich and his post-doctoral fellow Phil Auron, I started the cloning project at the Massachusetts Institute of Technology on February 1, 1982, and entered my first experiment into my notebook. The first experiments were performed using rabbit reticulocyte lysates that had transcribed mRNA isolated from human blood monocytes. We immunoprecipiated the lysates with anti-leukocytic pyrogen and identified the primary transcript as a 36000 Da protein on autoradiographs. This molecular weight was consistent with the existence of the previously identified large molecular weight leukocytic pyrogen.Citation21
Andrew Webb joined this ambitious, high-risk cloning project, and after 2 years, we succeeded in isolating a putative cDNA. This project itself deserves its own telling. Our putative IL1 cDNA was used to isolate a single species from a mixture of polyadenylated mRNA of monocytes. When the cDNA hybridized mRNA was translated in frog oocytes, we observed the presence of activity on lymphocytes and thus validated the activity of IL1.Citation45 The cDNA, in fact, translated the IL1β precursor, which we now know is biologically inactive; however, the frog oocyte most likely contains proteases, which cleaved the precursor at the serine protease site close to the caspase-1 site. Using intrinsic labeling of cultured human monocytes with 35S methionine, 3H-leucine and 14C-glutamic acid labeled amino acids, we cut out the radioactive single band from the SDS PAGE and subjected the band to Edman degradation. We identified the amino acid sequence by the type of emitted radioactivity. The labeled amino acid sequence match the cDNA-derived sequence at a trypsin cleavage site upstream from the caspase-1 site.Citation46 So the frog egg had processed the IL1 precursor.
Thus, the title of the paper was “Nucleotide sequence of human monocyte interleukin 1 precursor cDNA.” We submitted the paper to nature in May of 1984; the manuscript was rejected in July. But we managed to publish the sequence in the last issue of Proceedings of the National Academy of Sciences in 1984. Five months later, Nature published a paper from the Immunex Corporation on the cloning of IL1 with our same sequence.Citation47 Years later, we discovered that scientists at Immunex had reviewed our paper, rejected the manuscript but had submitted our sequence to the United States Patent Office as their own discovery. For many years, the citation for the first cloning of IL1β was the March paper and not ours. How did we discover that Immunex had copied our sequence for their patent application and not their own? We had 9 non-coding nucleotide errors in sequence and those same errors are present in the Immunex patent. We concluded that Immunex never cloned IL1β nor IL1α but had used our sequence and the mouse sequence for their paper. Nature has never apologized for the rejection.
Early studies had assumed that the pyrogenic activity of leukocyte supernatants was due to a single molecule but, in 1974, we reported 2 distinct pyrogenic proteins, both having molecular weights in the range of 15–20000 Daltons. One activity was at the expected isoelectric focusing point (pI 7) but the second activity was at pI 5.Citation21 Today, the issue has been resolved and the pI 5 form is identified as IL1α. The large molecular weight pyrogen, estimated by gel-filtration to be 38000 Da, was likely the IL1α precursor. The cDNA for mouse IL1α, reported by Peter Lomedico and Steven Mizel,Citation26 was isolated from a cell line, whereas the cDNA for human IL1β was isolated from blood monocytes. However, whereas immunologists purifying IL1α as “lymphocyte activating factor,” Jeremy Saklatvala was purifying IL1α as “catabolin,” which stimulated the break-down of cartilageCitation48
Confirmation and Much More
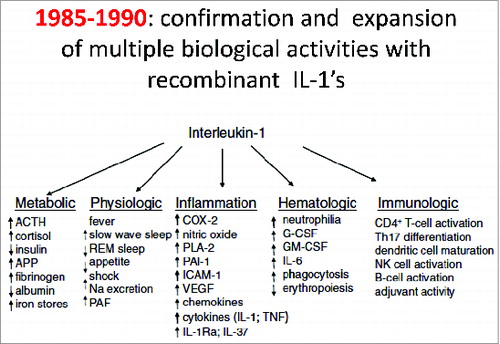
Subsequently, as depicted in , nearly all the various biological properties of the purified leukocytic pyrogen/IL1 were confirmed with recombinant IL1. Several additional activities were also discovered and the field of IL-1 biology rapidly broadened to include diabetes, hemodynamic shock, heart failure and bone marrow stimulation. The cDNA's of IL1 also provided essential data for the convergence of IL1 and Toll like receptor (TLR) activities:Citation49 the convergence being that the Toll-IL-1R (TIR) domain of the IL1 receptor and the TIR of TLR are nearly identical; deletion of TIR results in failure of IL1 and failure of TLR signaling.Citation50 Mihai Netea once stated, “Without knowledge of the TIR domain of IL1 receptors, Toll proteins would have remained of interest primarily for Drosophila embryology.” I find it rather revealing that it took until 1996, several years following the report by Gay in 1991,Citation49 for Drosophila embryologists to test the hypothesis that “non-specific resistance to infection” was a function of Toll.Citation51 Indeed, Jos van der Meer had shown in 1988Citation52 that a low dose of IL1β induced “non-specific resistance to infection.”
Figure 3. Multiple activities of human leukocytic pyrogen. A compilation of activities using recombinant human IL1β derived fromCitation2 and other published studies.Citation25,53
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Beeson PB. J Clin Invest 1948; 27:524 (abstract); PMID:18939147
- Dinarello CA, et al. J Clin Invest 1986; 77:1734–9; PMID:3519678; http://dx.doi.org/10.1172/JCI112495
- Dahl CA, et al. J Immunol 1992; 148:597–603; PMID:1729377
- Kim SH, et al. Immun 2005; 22:131–42; PMID:15664165
- Joosten LA, et al. Cell Mol Life Sci 2013; 70:3883–92; PMID:23463238; http://dx.doi.org/10.1007/s00018-013-1301-9
- Dinarello CA, et al. Nat Rev Drug Discov 2012; 11:633–52; PMID:22850787; http://dx.doi.org/10.1038/nrd3800
- Cerami A. The value of failure: the discovery of TNF and its natural inhibitor erythropoietin. J Int Med 2011; 269:8–15; PMID:21158973; http://dx.doi.org/10.1111/j.1365-2796.2010.02319.x
- Hong DS, et al. Lancet Oncol 2014; 15:656–66; PMID:24746841; http://dx.doi.org/10.1016/S1470-2045(14)70155-X
- Doitsh G, et al. Nature 2014; 505:509–14; PMID:24356306; http://dx.doi.org/10.1038/nature12940
- Dinarello CA, et al. Trans Assoc Am Phys 1968; 81:334–43; PMID:5721410
- Shapiro L, et al. Proc Natl Acad Sci USA 1993; 90:8614–8; PMID:8378338; http://dx.doi.org/10.1073/pnas.90.18.8614
- Atkins E. J Infect Dis 1984; 149:339–48; PMID:6232325; http://dx.doi.org/10.1093/infdis/149.3.339
- Krueger JM, et al. Am J Physiol 1984; 246(6 Pt 2):R994–999; PMID:6611091
- Rosenwasser LJ, et al. J Exp Med 1979; 150:709–14; PMID:314491; http://dx.doi.org/10.1084/jem.150.3.709
- Westphal O, et al. J Med Pharm Chem 1961; 4:497–504; PMID:14006366; http://dx.doi.org/10.1021/jm50019a008
- Cooper KE, et al. Israel J Med Sci 1976; 12:955–9; PMID:1002455
- Atkins E. Physiol Rev 1960; 40:580–646; PMID:13794961
- Kim B, et al. Front Immunol 2013; 4:391; PMID:24312098; http://dx.doi.org/10.3389/fimmu.2013.00391
- Chen CJ, et al. Nat Med 2007; 13:851–6; PMID:17572686; http://dx.doi.org/10.1038/nm1603
- Bodel P, et al. New Engl J Med 1967; 276:1002–8; PMID:5228572; http://dx.doi.org/10.1056/NEJM196705042761803
- Dinarello CA, et al. J Exp Med 1974; 139:1369–81; PMID:4829934; http://dx.doi.org/10.1084/jem.139.6.1369
- Auron PE, et al. J Immunol 1987; 138:1447–56; PMID:3492551
- Dinarello CA, et al. Proc Natl Acad Sci USA 1977; 74:4624–7; PMID:22079; http://dx.doi.org/10.1073/pnas.74.10.4624
- Dinarello CA, Wolff SM. Inflammation 1977; 2:179–89; PMID:617807; http://dx.doi.org/10.1007/BF00917594
- Dinarello CA. Blood 1996; 87:2095–147; PMID:8630372
- Lomedico PT, et al. Nature 1984; 312:458–62; PMID:6209582; http://dx.doi.org/10.1038/312458a0
- Smith JW, et al. New Engl J Med 1993; 328:756–61; PMID:8437596; http://dx.doi.org/10.1056/NEJM199303183281103
- Sims JE, et al. Science 1988; 241:585–9; PMID:2969618; http://dx.doi.org/10.1126/science.2969618
- Greenfeder SA, et al. J Biol Chem 1995; 270:13757–65; PMID:7775431; http://dx.doi.org/10.1074/jbc.270.23.13757
- McAdam KPWJ, Dinarello CA. Induction of serum amyloid A synthesis by human leukocytic pyrogen. Bacterial Endotoxins and Host Response., ed Agarwal MK. Elsevier/North-Holland and Biomedical Press: Amsterdam; 1980; 167–78.
- Gery I, Waksman BH. J Exp Med 1972; 136:143–55; PMID:5033418; http://dx.doi.org/10.1084/jem.136.1.143
- Auron PE, et al. J Mol Cell Immunol 1985; 2:169–77; PMID:3880507
- Mizel SB, et al. J Immunol 1978; 120:1504–8; PMID:307016
- Dinarello CA, et al. J Exp Med 1986; 163:1433–50; PMID:3486936; http://dx.doi.org/10.1084/jem.163.6.1433
- Dinarello CA, et al. Brain Res 1991; 562:199–206; PMID:1773338; http://dx.doi.org/10.1016/0006-8993(91)90622-3
- Watson J, et al. J Exp Med 1979; 150:849–61; PMID:315987; http://dx.doi.org/10.1084/jem.150.4.849
- Ogilvie AC, et al. J Immunol 1996; 156:389–94; PMID:8598489
- Tewari A, et al. Lancet 1990; 336:712–4; PMID:1975894; http://dx.doi.org/10.1016/0140-6736(90)92206-W
- Fitzgerald AA, et al. Arthritis Rheum 2005; 52:1794–803; PMID:15934079; http://dx.doi.org/10.1002/art.21061
- Robertson MJ, et al. Clin Cancer Res 2006; 12(14 Pt 1):4265–73; PMID:16857801; http://dx.doi.org/10.1158/1078-0432.CCR-06-0121
- Murphy PA, et al. Ciba Found Symp 1971; 59–79; PMID:5001484
- Murphy PA, et al. Infect Immun 1981; 34:177–83; PMID:7298180
- Damais C, et al. Int J Immunopharmacol 1982; 4:451–62; PMID:6982246; http://dx.doi.org/10.1016/0192-0561(82)90020-0
- Dinarello CA, et al. J Clin Invest 1977; 60:465–72; PMID:559692; http://dx.doi.org/10.1172/JCI108797
- Auron PE, et al. Proc Natl Acad Sci USA 1984; 81:7907–11; PMID:6083565; http://dx.doi.org/10.1073/pnas.81.24.7907
- Dinarello CA. Interleukin-1 and the pathogenesis of inflammatory diseases. Blood 2011; 117:3720-32..
- March CJ, et al. Nature 1985; 315:641–5; PMID:2989698; http://dx.doi.org/10.1038/315641a0
- Saklatvala J, Dingle JT. Biochem Biophys Res Commun 1980; 96:1225–31; PMID:7437067; http://dx.doi.org/10.1016/0006-291X(80)90082-0
- Gay NJ, Keith FJ. Nature 1991; 351:355–6; PMID:1851964; http://dx.doi.org/10.1038/351355b0
- Heguy A, et al. J Biol Chem 1992; 267:2605–9; PMID:1531143
- Lemaitre B, et al. Cell 1996; 86:973–83; PMID:8808632; http://dx.doi.org/10.1016/S0092-8674(00)80172-5
- van der Meer JWM, et al. Proc Natl Acad Sci USA 1988; 85:1620–3; PMID:3125553; http://dx.doi.org/10.1073/pnas.85.5.1620
- Dinarello CA. New Engl J Med 1984; 311:1413–8; PMID:6208485; http://dx.doi.org/10.1056/NEJM198411293112205
