Abstract
Diverse transmitter systems (e.g. acetylcholine, dopamine, endocannabinoids, endorphins, glutamate, histamine, 5-hydroxytryptamine, substance P) have been implicated in the pathways by which nausea and vomiting are induced and are targets for anti-emetic drugs (e.g. 5-hydroxytryptamine3 and tachykinin NK1 antagonists). The involvement of TRPV1 in emesis was discovered in the early 1990s and may have been overlooked previously as TRPV1 pharmacology was studied in rodents (mice, rats) lacking an emetic reflex. Acute subcutaneous administration of resiniferatoxin in the ferret, dog and Suncus murinus revealed that it had “broad–spectrum” anti-emetic effects against stimuli acting via both central (vestibular system, area postrema) and peripheral (abdominal vagal afferents) inputs. One of several hypotheses discussed here is that the anti-emetic effect is due to acute depletion of substance P (or another peptide) at a critical site (e.g. nucleus tractus solitarius) in the central emetic pathway. Studies in Suncus murinus revealed a potential for a long lasting (one month) effect against the chemotherapeutic agent cisplatin. Subsequent studies using telemetry in the conscious ferret compared the anti-emetic, hypothermic and hypertensive effects of resiniferatoxin (pungent) and olvanil (non-pungent) and showed that the anti-emetic effect was present (but reduced) with olvanil which although inducing hypothermia it did not have the marked hypertensive effects of resiniferatoxin. The review concludes by discussing general insights into emetic pathways and their pharmacology revealed by these relatively overlooked studies with TRPV1 activators (pungent an non-pungent; high and low lipophilicity) and antagonists and the potential clinical utility of agents targeted at the TRPV1 system.
Abbreviations:
- AM404, N-arachidonoylaminophenol
- AMT, anandamide membrane transporter
- AP, area postrema
- BBB, blood brain barrier
- CB1, cannabinoid1
- CGRP, calcitonin gene-related peptide
- CINV, chemotherapy-induced nausea and vomiting
- CP 99,994
- CTA, conditioned taste aversion
- CVO's, circumventricular organs
- D2, dopamine2
- DRG, dorsal root ganglia
- FAAH, fatty acid amide hydrolase
- H1, histamine1
- 12-HPETE, 12-hydroperoxy-eicosatetraenoic acid
- 5-HT, 5-hydroxytryptamine
- 5-HT3, 5-hdroxytryptamine3
- i.v., intravenous
- LTB4, leukotriene B4
- NADA, N-arachidonoyl-dopamine
- NK1, neurokinin1
- 8-OH-DPAT, (±)-8-Hydroxy-2-dipropylaminotetralin
- POAH, preoptic anterior hypothalamus
- TRPV1, transient receptor potential vanilloid receptor1
Introduction to Nausea and Vomiting
Nausea and vomiting (emesis) are symptoms of many diseases and also present as side effects of drug therapy. Our understanding of the mechanisms involved has been slow to progress, and may partly relate to the fact that common laboratory animals (e.g., mouse, rat, guinea pig) are incapable of emesis,Citation1,2 and that nausea (assuming it occurs) is difficult to quantitate in animals, as it is a subjective self-reported experience.Citation3,4 One of the major leaps of understanding of emesis control came in the second half of the 20th century with the identification of central coordinating mechanisms for emesis (“vomiting center”) and, systematic exploration of afferent pathways to it from the gastrointestinal tract, and also from the area postrema located on the floor of the fourth ventricle; the area postrema became known as the ‘chemoreceptor trigger zone’ for emesis, as it mediated emesis to a number of systemically administered compounds that were thought to act via different receptors (seeCitation5 for review). From such foundations, knowledge on the connectivity of brainstem nuclei expanded to consider other afferent inputs and outputs, and information on receptor types and stimuli mediating emesis increased. Research into potential mechanisms of nausea and emesis intensified in the 1980s, to identify new drugs to reduce these side effects of radiation and cancer chemotherapy which are dose limiting and affect patient compliance with treatment as well as having a major negative impact on the quality of life.
Background
The precise anatomical pathways and common biochemical mediators involved in the control of nausea and emesis have been difficult to define. The study of the mechanisms involved requires animals possessing the capacity to vomit, and this is relatively expensive, not accessible to all research laboratories and raises a number of ethical concerns.Citation6 The study of nausea represents an even greater challenge since it is a subjective experience and is still relatively poorly understood.Citation3 The pioneers of emesis research studied classical neurotransmitters pathways including cholinergic, histaminergic, and dopaminergic and serotonergic (5-hydroxytryptamine; 5-HT) pathways, yielding information on the relative importance of these transmitters in several causes of emesis (for review and references see ref. Citation3). Thus, the anti-muscarinic receptor antagonist, scopolamine, and a range of anti-histamines blocking histamine H1 receptors (e.g. promethazine), were initially indicated for the treatment of motion sickness,Citation7 with dopaminergic agents (blocking dopamine D2 receptors) initially thought hopeful for a range of causes of emesis, but not motion sickness.Citation8,9 Control of radiation-induced emesis and chemotherapy-induced emesis appeared more problematic and the realization that serotonin may also be involved in emesis control was made from the study of metoclopramide in the clinic where its superiority to reduce emesis was distinct from other dopamine receptor antagonists and where it was later shown to additionally block 5-HT3 receptors.Citation10,11 The explosion of research to develop selective 5-HT3 receptor antagonists for the specific control of emesis (e.g., ondansetron and granisetron) also came at a time when new techniques to study pathways and transmitter systems had been developed. While muscarinic receptor antagonists and anti-histamines (many of which also have some potency at muscarinic receptors) were classically associated with predictable side effects of dry mouth, constipation and sedation,Citation12,13 the early dopamine receptor antagonists (many of which also weakly blocked histamine receptors) also caused sedation and in many patients caused Parkinson-like symptoms,Citation14 the selective 5-HT3 receptor antagonists were devoid of major side effects.Citation15
The 5-HT3 receptor antagonists proved highly effective to reduce the initial acute emesis induced by chemotherapy and radiotherapy in man.Citation16,17 However, their clinical introduction, and an increase in the quality of clinical trial design, revealed that delayed emesis was partially resistant, suggesting that different neurotransmitters or modulators were involved in the overall response.Citation17 This highlighted the need to further study the emetic reflex and to discover drugs that could be used alone, or in combination with the 5-HT3 receptor antagonists for the control of both the acute and delayed phases of emesis. As regards advances in the control of emesis afforded by 5-HT3 receptor antagonists, palonosetron, which is an order of magnitude more potent than the first generation antagonists, and also has a duration of action almost 3 times as long.Citation18 Palonosetron also has unique properties compared to the older generation of antagonists in that it may prevent 5-HT3 receptor recycling and through receptor cross-talk, may also prevent substance P mediated responses.Citation19
Substance P Tachykinin NK1 receptor antagonists are a relatively new class of anti-emetic first identified as capable of preventing emesis induced via diverse challenges in ferrets by blocking the action of substance P in the nucleus tractus solitarius (NTS) and/or closely associated brainstem structures.Citation20-23 Tachykinin NK1 receptor antagonists have been subsequently shown to be useful when combined with 5-hydroxytryptamine3 (5-HT3) receptor antagonists and glucocorticoids for the treatment of chemotherapy-induced acute and delayed emesis in man.Citation24 It is reasonable to assume that treatments depleting or reducing the release of substance P from emetic circuits could represent an alternative approach to the control of emesis. This hypothesis is explored in the present review in relation to the pivotal role of NK1 receptors in emesis control, and the location of substance P and transient receptor potential vanilloid receptors (TRPV1) in emetic circuits.
Most research on TRPV1 has focused on mechanisms of pain and inflammation given the high density of TRPV1 on primary sensory neurons originating from the dorsal root ganglia and also from the trigeminal and nodose ganglia.Citation25 The peripheral terminals of the dorsal root ganglia release substance P and calcitonin gene-related peptide (CGRP) during inflammation and contribute heavily to neurogenic inflammation. TRPV1 are noted as being ligand-gated ion channels, with a preference for calcium.Citation25,26 However, relevant to inflammatory mechanisms seen during tissue damage are the fact that noxious heat (>43°C) and low pH (<6 ) can activate the channelCitation27; protons and heat therefore occurring during pathological conditions are presumed to activated the channel, and may augment the effects of other inflammatory mediators (some known to cause emesis in their own right) to open the channels (e.g. bradykinin, 5-HT and prostaglandin E2 acting together can induce TRPV1 currents.Citation25,28
TRPV1 were later shown to be located within the brain at sites that may not be necessarily activated by classical inflammatory events because of the blood-brain barrier. This opened up the possibility that such channels serve other functions and may have an endogenous ligand for activation. Brain areas with high density of TRPV1 sites include the nucleus tractus solitarius, area postrema, locus ceruleus, preoptic area of the hypothalamus, many cortical regions, hippocampus, amygdala, substantia nigra, cerebellum, thalamic nuclei and the inferior oliveCitation29,30 N-arachcidonoylethanololamine (anandamide), N-arachidonoyl-dopamine (NADA), 12-hydroperoxy-eicosatetraenoic acid (12-HPETE) and leukotriene B4 (LTB4) are the proposed mediators to activate the channels.Citation31 However, anandamide is also widely identified as a cannabinoid CB1 receptor agonist;Citation32 it is produced by hydrolysis of phospholipids and inactivated by cellular reuptake by the anandamide membrane transporter (AMT) and/or fatty acid amide hydrolase (FAAH), which produces arachidonic acid.Citation32 Anandamide may also block 5-HT3 receptorsCitation33 and therefore has a complex role within emetic circuits. Arachidonic acid itself is released in its own right during inflammation and in the brain is a precursor of a range of eicosanoids with their own receptors and pharmacology (e.g., prostanoids, leukotriene, platelet activating factor).Citation34 Indeed, NADA and 12-HPETE are derived from arachidonic acid, with NADA also being an agonist at CB1 receptors, and also an inhibitor of AMT and FAHH.Citation35 Cannabis is known to reduce nausea and emesis, but is also associated with unwanted side effects.Citation36 Studies have attempted to identify which cannabinoid receptors are involved, or if inhibitors of metabolism of anandamide, could offer an advantage to inhibit emesis.Citation37,38 Clearly, great caution needs to be exerted during the interpretation of data involving endogenous candidates of TRPV1 activation, and should be delineated by their sensitivity to TRPV1 antagonists including capsazepine, ruthenium red, or iodo-RTX.Citation39 The same holds true for the interpretations of AMT and FAHH inhibitors, as tools to prolong the action of anandamide at CB1 receptors; effects that can also be delineated, in part, by the use of selective CB1 receptor antagonists.Citation40
It was proposed that there are subtypes of vanilloid/capsaicin receptors, and also species differences based in binding and physiological data (seeCitation25). Mammalian TRPV1 have been cloned and have 6 hydrophobic transmembrane domains and 3 intracellular ankrin repeats, with some areas of conservation between species.Citation41 In fact capsaicin and other ligands (including anandamide; effects that would be potentially reduced by AMT inhibitors designed to prolong its action at CB1) interact with the intracellular cytosolic sites of TRPV1, and not as originally assumed, with its extracellular domains.Citation42 However, there is also one extracellular binding site for vanilloids.Citation43 The location of the binding sites may have significant impact on interpretation of data: different rates of ligand uptake may go some way to explain differences in potency and also of ‘pungency’.Citation44
Why were TRPV1 activators investigated for involvement and nausea and vomiting?
To answer this question we need to consider aspects of research in emetic mechanisms in the early 1990s. A major challenge in anti-emetic research was the identification of drugs to block the nausea and vomiting induced by the drugs and radiation used to treat cancer. Of particular concern was cisplatin because it induced nausea and vomiting characterized by a high incidence (>95% of patients), a high intensity acute phase lasting 18–24 h, and a protracted delayed phase lasting a further 4–6 days; in some patients this was followed by further nausea and vomiting in anticipation of the next cycle of chemotherapy (i.e. anticipatory nausea and vomiting).Citation17 The identification in the ferret of the anti-emetic effect of selective 5-HT3 receptor antagonists such as granisetron and ondansetronCitation45,46 and the subsequent translation of these findings to the clinic (Kytril®, Zofran®) transformed the treatment of chemotherapy-induced nausea and vomiting (CINV). However, the primary efficacy of the first generation of 5-HT3 receptor antagonists was mainly confined to the acute phase (18–24h) of cisplatin–induced emesis as demonstrated in ferret (for meta-analysis of animal studies seeCitation47) and clinical studies (for review seeCitation48). Preclinical studies predominantly using the ferret revealed the early acute phase of emesis induced by high dose cisplatin and other chemotherapeutic agents (e.g. cyclophosphamide), and “low dose” total body X-radiation, was dependent upon an intact abdominal vagus with the mechanism proposed to be via activation of 5-HT3 receptors on gastrointestinal vagal afferents by 5-hydroxytryptamine (5-HT) locally released from enterochromaffin cells (reviewed inCitation49). In some respects, the involvement of the abdominal vagus in acute emesis induced by anti-cancer chemotherapeutic agents was surprising as it had often been assumed that systemic agents could only induce emesis via an action at the area postrema and its links to the NTS. The area postrema is a circumventricular organ located at the caudal part of the fourth ventricle where the blood-brain and blood–cerebrospinal fluid barriers are relatively permeable. The permeability of the area postrema provides a route via which small molecules can access dendrites of the NTS known to project into the area postrema and through which they could possibly gain access to the NTS itself or vagal afferent terminals in the NTS although there is disputeCitation50,51 about the extent of the diffusion barrier between the AP and the NTS (i.e., is the NTS “inside” or “outside” the BBB; there is also some evidence for the presence of fenestrated capillaries in the NTS itself (seeCitation4 for refs and detailed discussion). However, as both nausea and vomiting are components of the body's mechanism to defend against the effect of toxins accidentally ingested with the food, it is perhaps not surprising that the integrity of the abdominal vagus is required for the induction of emesis by a range of stimuli introduced into the gut lumen including copper sulfate,Citation5 plant toxins (e.g., emetineCitation5), and staphylococcal enterotoxin,Citation52 all of which were studied in animal models. While it is likely that the effect is due to activation of the afferent fibers comprising 80–90% of the nerve fibers in the abdominal vagusCitation4 surgical transection cuts both afferents and efferents making it difficult to draw firm conclusions about their relative roles, although the involvement of vagal afferents in detection of potentially emetic stimuli was supported by afferent recording studies (e.g.Citation53). In addition, at the time evidence emerged from studies in the ferret that surgical transection of the abdominal vagus induced a degree of plasticity in the emetic mechanisms.Citation54 Although separation of afferent and efferent vagal fibers by surgery at the nodose ganglion was a theoretical possibility in the ferret, this did not prove to be feasible.Citation55
Studies in the rat had shown that neonatal capsaicin treatment could selectively ablate a population of cutaneous and visceral afferents, but as rats and other rodents (e.g., mouse) do not have an emetic reflex,Citation1,56 they have limited utility for research in this area. The laboratory species commonly used in emesis research in the late 1980s were the dog, cat and ferret, with the latter used to identify the anti-emetic effect of the 5-HT3 receptor antagonists against the cytotoxic anti-cancer agents cisplatin and cyclophosphamide as well as radiation (seeCitation57). Ethical issues aside, at the time there was no protocol for the use of neonatal capsaicin in dogs, cats or ferrets, and in addition the cost and time for the animals to reach an age where study of emesis was feasible precluded consideration of this as an option.
Studies of resiniferatoxin (RTX), a naturally occurring ultrapotent analog of capsaicin, showed that in adult rats, a subcutaneous administration could induce acute desensitisation of afferent C-fibers (the main fiber type in the abdominal vagus).Citation25,58 In view of this observation, we investigated the use of RTX in the ferret as a potential tool for chemical vagal deafferentation, to allow a more precise insight into the involvement of the vagal afferents in emesis. We hypothesized that subcutaneous RTX given to adult ferrets would reduce or abolish the emetic effect stimuli such as intragastric copper sulfate and radiation, where a major involvement of the abdominal vagi had been demonstratedCitation54 and that the response to loperamide (an opioid receptor agonist) acting via the area postrema would be unaffected.Citation59 The next section describes the findings that showed our hypothesis was incorrect, but which led us to a more interesting conclusion.Citation59,60
Establishing the effect of RTX on emetic mechanisms
The dose of 100 μg/kg of RTX given subcutaneously was selected for study in the ferretCitation59,60 based upon the dose used in the rat for capsaicin-induced algesia, Evans Blue extravasation, and hypothermia.Citation61 Administration of RTX induced a transient (lasting a few minutes) increase in locomotor activity accompanied by an increase in respiratory rate. No indications of an algesic effect of RTX were observed as was also the case in subsequent dog and ferret studies in which a stimulation of respiration was also reported.Citation62 In the ferret studies, core body temperature also reduced from 38.5 ± 0.3°C (n = 6) to 36.4 ± 0.3°C within 30 min before reaching a nadir of 35.9 ± 0.6°C at 2 h; recovery to ∼37°C (∼1.5°C < normal core temperature) occurred at ∼3 h. Core temperature was not significantly different from control in animals left to recover for 24 hours and 8 d respectively following RTX treatment. Animals did not show any overt signs of hypothermia such a piloerection or shivering but did curl up in the corner of the cage, but as this is considered a normal behavior for the ferret, it was impossible to ascribe this to the effect of RTX. Animals remained responsive to external auditory and visual stimuli, periodically defaecated and urinated and would drink milk when offered. When tested 3 h after RTX, animals given intragastric copper sulfate (40 mg%, 30 ml) did not have emesis and the emetic responses to total body X-radiation (200rads, 250 kV, 15 mA) was blocked in 3 out of 4 animals tested and for loperamide (0.5 mg/kg, s.c.) there was a ∼95% reduction in emesis (60 % animals totally protected). The effect of RTX on the emetic reflex appeared selective as the gag reflex evoked by light mechanical stimulation of the pharynx was unaffected and in urethane anaesthetised ferrets RTX (100 μg/kg, s.c.) was without effect on cardiovascular (hypotension, bradycardia) or respiratory (rate and depth) components of the von Bezold–Jarisch reflex evoked by rapid intravenous bolus injection of 2-methyl-5-hydroxytryptamine (30 μg/kg). To investigate if RTX had a protracted effect on emesis, animals were tested either 3 d (radiation) or 8 d (loperamide, copper sulfate) after RTX (100 μg/kg, s.c.) administration, but in neither case was there any indication of a long-lasting effect.
The above studies provided the first demonstration that RTX had an anti-emetic action and indirectly implicated the subsequently identified TRPV1 in emesis. The ability to block loperamide–induced emesis was unexpected as it acts via the area postrema and not via the vagus (or other visceral nerves), as is the case for copper sulfate and low dose X-radiation in the ferret.Citation54 This observation suggested that RTX might have “broad spectrum” anti-emetic effects, as it affected emesis induced by more than one pathway (see above). Although the mechanism by which RTX exerted its effects were not investigated at the time, it was proposed that “the most likely mechanism to account for the anti-emetic effects is that RTX induces a depletion of a neurotransmitter, possibly substance P or CGRP, at a central site in the emetic pathway. The depletion may be followed by blockade of transmitter release mechanisms. The nucleus tractus solitarius would be a possible site of action as both substance P and CGRP–like immunoreactivity have been identified in this nucleus and both peptides have been proposed as transmitters in vagal afferents.”Citation60 Substance P applied to the dorsal brainstem of the anaesthetised ferret in the region of the area postrema had previously been shown to be capable of inducing emesis (Andrews and Wood, 1988, unpublished; see Figure 6 inCitation49), while mechanisms of CGRP in emesis control are still essentially unknown.
A series of studies in the late 1980s identified the house musk shrew, Suncus murinus, an insectivore as a small (body weight<100g) species sensitive to a range of emetic stimuli including motion.Citation63,64 In unrelated studies, Rudd and Naylor had been investigating the mechanism of action of broad inhibitory anti-emetics and had decided to compare the action of the mu-opioid receptor agonist, fentanyl,Citation65 and the 5-HT1A receptor agonist, 8-OH-DPAT,Citation66 with RTX in Suncus murinus. They showed that RTX (10 μg/kg. s.c.), fentanyl (40 μg/kg, s.c), and 8-OH-DPAT (250 μg/kg, s.c.), blocked the emetic response to nicotine (5 mg/kg, s.c) whereas the 5-HT3 receptor antagonist, ondansetron (1mg/kg, s.c.) had no effect.Citation67 As the emetic effect of nicotine was considered to be central this study provided additional evidence that RTX has broad spectrum anti-emetic effects. However, Rudd and Naylor (1995) also observed that RTX dose-dependently (1–100 μg/kg, s.c.) induced emesis, an effect not seen in the ferret.Citation60 The emetic effect of RTX was unexpected and together with the anti-emetic effect was pursued in subsequent studies in Suncus murinusCitation68-71 described in detail below.
Other groups had also begun to investigate the potentially useful anti-emetic effects of RTX. A study in the decerebrate dog showed that application of either capsaicin (33 mM) or RTX (160 mM) to the IV ventricle blocked retching induced by electrical stimulation of the abdominal vagal afferent within 10 min and 40 min respectively.Citation72 It was again concluded that the most likely (but not the only) explanation for the effect of RTX (and capsaicin) was depletion of substance P, although in this case the site of the depletion was proposed to be the vagal afferent terminals projecting to the nucleus tractus solitarius, rather than the nucleus tractus solitarius itself.Citation72 Additional studies in the ferret revealed that RTX (10 μg/kg, s.c.) given 30 min prior to cisplatin (10mg/kg, i.p., control latency to induced emesis 62.0 ± 5.6 min) completely blocked the acute emetic response monitored over 4 h.Citation62 When given 16 h before cisplatin, RTX caused a significant reduction in intensity of emesis (69.8 %), but was without significant effect when given 24 h prior to cisplatin.Citation62 In ferrets given RTX (10 μg/kg, s.c.) 36 h after cisplatin, it reduced the magnitude of the emetic response by ∼70% in the 36–72 h period (“delayed emesis”). The acute phase of cisplatin-induced emesis in dogs was also markedly (94%) reduced by RTX (10 μg/kg, s.c., 30 min prior to 3.2 mg/kg, i.v. cisplatin) and the same dose of RTX also significantly reduced the emetic response to apomorphine (a dopamine receptor agonist acting at the area postrema) and increased the latency.Citation62 These authors concluded that the anti-emetic effect of RTX resided in the central nervous system. These results further support the broad spectrum effect of RTX as although the “acute” phase of cisplatin-induced emesis in the ferret is predominantly or exclusively mediated by the abdominal vagi, the “delayed” phase requires an intact area postrema.Citation73
Most recently a study in the least shrew (Cryptotis parva) revealed that RTX (1–5 μg/kg) given subcutaneously or intraperitoneally reduced or blocked the acute emetic effect of cisplatin but as in the case of Suncus murinus emetic effects of RTX were observed when it was given subcutaneously (see below for details).Citation74 RTX and CB1/2 receptor agonists when given in combination at doses that were individually ineffective were shown to be capable of blocking cisplatin emesis.Citation74 An indication that TRPV1 activation may be implicated in cisplatin-induced emesis comes from the observation that ruthenium red reduced the response although curiously capsazepine did not. It is interesting to note that in the house musk shrew the emetic response to RTX can be blocked by ruthenium red but not by capsazepine raising a question about the selectivity of both compounds in shrews (family Soricidae) even though the 2 species concerned are from divergent subfamilies (Soricinae and Crocidurinae). Overall the above studies in 4 species (ferret, dog, Suncus murinus, Cryptotis parva; see for a summary) provide evidence that RTX when administered subcutaneously can reduce or abolish the emetic response to stimuli inducing emesis via pathways involving the vagus (intragastric copper sulfate, acute phase of cisplatin) and area postrema (apomorphine, loperamide, delayed phase of cisplatin). This range of anti-emetic effects can be unified by a central (brain stem) site of action of RTX. However, in addition to the area postrema and the abdominal vagal afferents the other major pathway capable of inducing emesis is the vestibular system implicated in motion sickness.Citation75 No protocol was available for the induction of motion sickness in the ferret, but Suncus murinus had become established motion sickness.Citation63,64,76,77 To investigate the potential for RTX to block motion sickness studies were undertaken in Suncus murinus, and as with the initial ferret studies these, revealed unexpected results.
Table 1. Spectrum of acute anti-emetic effects of resiniferatoxin given either subcutaneously (s.c.) or intracerebroventricularly (i.c.v.) in ferret, dog, Suncus murinus (house musk shrew) and Cryptotis parva (least shrew). Green = emetic response unaffected by RTX; Red = emetic response either completely blocked or significantly reduced by RTX. Note that studies in Cryptotis parva also investigated RTX in combination with other anti-emetics (see74 for details)
Emetic effects of RTX in Suncus murinus: species differences and pharmacology
The emetic response in Suncus murinus
The intention was to investigate whether RTX could block the emetic response to motion and nicotine in Suncus murinus, but when it was administered at the same dose as in the ferret studies (100 μg/kg, s.c.), it induced retching and vomiting.Citation67-69,78 In agreement with the initial Andrews and Bhandari (1993) study,Citation60 studies in the dog and did not observe any emesis in response to RTX (10 μg/kg.s.c.).Citation62 However, transient retching was reported in 2 out of 3 decerebrate dogs when capsaicin (33 mM) was applied to the floor of the IV ventricle.Citation72 In view of this marked species difference in the emetic potential of RTX between species, the dose response relationships and the pharmacology of the response was studied in detail in Suncus murinus. The threshold dose for emesis was ∼1 μg/kg, s.c. and while the number of emetic episodes and incidence of emesis reached a plateau at 10 μg/kg, s.c., the latency of the response decreased with dose and reached plateau value of 3.0 ± 0.8 min at 500 μg/kg, s.c. As with the ferret, no indications of an algesic effect manifested by continuous vocalisation, piloerection, urination, defaecation or scratching at the injection site was observed. However, intense ano-genital grooming was observed at doses of 500 and 1000 μg/kg, s.c. In view of this, the pharmacological mechanisms were investigated mainly by using a dose of 100 μg/kg, s.c. that reliably induced emesis with a latency of 6.7 ± 1.3 min and 11.6 ± 2 episodes over ∼10 min.
A recent study in the least shrew (Cryptotis parva) showed that subcutaneous RTX induced emesis emetic incidence and frequency showing a bell-shaped dose response curve (2–50 μg/kg, s.c.); the latency at the optimal dose (18 μg/kg) was ∼10min.Citation74 A surprising finding was that when given via the intraperitoneal route RTX did not induce emesis and this was also the case with E-capsaicin (up to 10mg/kg).Citation74
Pharmacology of the emetic response
Limited evidence that RTX is acting via TRPV1 to induce emesis derives from the ability of the non-selective TRPV1 channel blocker ruthenium red (0.03–3 μmol/kg, s.c. ) to reduce the emetic response to subcutaneous RTX (100 nmol/kg, s.c. ) in a dose related manner.Citation70 However, the classical TRPV1 receptor antagonist capsazepine was without effect.Citation70 Ruthenium red (1 or 10 mg/kg s.c. Thirty min before RTX 100 μg/kg.s.c.) blocked the emetic and genital grooming effects of RTX, but did not block the emetic effect of nicotine showing that ruthenium red does not have any broad spectrum anti-emetic effects itself (Toyoda, Suzuki, Otsuka, Woods, Andrews, Matsuki, 2000, unpublished observations). These observations support the findings of Cheng et al.Citation70 These data raise the possibility that RTX induces emesis and ano-genital grooming via different TRPV1 subtypes at which the agonist effects of RTX can be differentially antagonised by capsazepine and ruthenium red, or that the agonists/antagonists have different distributions to sites of action.
The emetic response to subcutaneous RTX was unaffected by 5-HT3 receptor antagonists (tropisetron, ondansetron and MDL72222), the 5-HT1B/1D agonists sumatriptan and dihydroergotamine, and the non-selective 5-HT2 receptor antagonist methysergide, but was markedly reduced/abolished by treatment with the 8-OH-DPAT, the non-selective opioid receptor agonist morphine, and 2 NK1 receptor antagonists, CP-99,994 and R116301.Citation68,70 A full list of substances investigated for their potential to antagonise RTX induced emesis is summarised in .
Table 2. Pharmacology of the emetic effect of vanilloids given either subcutaneously (s.c.) or intracerebroventricularly (i.c.v.) in Suncus murinus. *The effects of doses of the agents used to modify the emetic effects; + split boxes indicate that some of the animals in the group tested had the response abolished while other had the response reduced. The data for this column is taken from results using the highest reported dose of each compound. 5-HT = 5-Hydroxytryptamine; NK = neurokinin/tachykinin; D = dopamine; H = histamine; M = muscarinic; TRPV1 = transient receptor potential vanilloid type 1
A second dose of RTX given subcutaneously 60 min after emesis from the first dose had subsided failed to induce a response. This was not due to a refractory emetic system as consecutive doses of nicotine both induced responses and induction of emesis by motion failed to block the response to a subsequent dose of RTX.Citation68 Central administration of RTX (10 nmol, i.c.v.) blocked the emetic response to RTX (10nmol) and E-capsaicin (100 nmol) given 180 min later but interestingly E-capsaicin (100nmol) was without effect on the emetic response to subsequent RTX (10 nmol) or E-capsiacin (100 nmol).Citation69 Neonatal administration of capsaicin abolished the emetic response to RTX when the animals reached adulthood. The lack of effect of a 5-HT3 receptor antagonist while not conclusive argues against a vagal site of action of RTX. The efficacy of morphine, 8-OH-DPAT and CP-99,994 is consistent with their previously reported “broad spectrum” of emetic effects; how these anti-emetics reduce RTX-induced emesis therefore requires explanation. The blockade by the NK1 receptor antagonist CP-99, 994 is of particular interest, as the preferred endogenous ligand of the NK1 receptor is substance P.
Pathways and mechanisms involved in the emetic response
The emetic response to RTX (100 μg/kg, s.c.) was present in animals with an abdominal vagotomy. Additionally when given into the lateral ventricle, RTX induced a short latency (2.6 ± 0.5 min) emetic response and subcutaneous RTX (100 μg/kg, s.c.) resulted in intense fos-like immunoreactivity in the area postrema and nucleus tractus solitarius and to a lesser degree the dorsal motor vagal nucleus.Citation68 In addition RTX, E-capsaicin and Z-capsaicin when give i.c.v. all evoked a short latency (<5 min) emetic response in Suncus murinus (). The latency of the emetic response to RTX and other TRPV1 agonists is compared to a wide range of other emetic challenges in Suncus murinus in . Intense ano-genital grooming was induced by higher doses of subcutaneous RTX in Suncus murinus.Citation68 Although i.c.v. RTX had a similar emetic, neither E-capsaicin nor Z-capsaicin induced ano-genital grooming.Citation69
Figure 1. The latency (mean ± s.e.m, where available) of the emetic response to a variety of emetic stimuli in Suncus murinus. The TRPV1 ligands discussed in the text are highlighted in yellow. For references see: [1] Hu, D.L. et al., J Food Prot, 1999. 62: 1350–3.; [2] Ito, C. et al., Eur J Pharmacol, 1995. 285: 37–43.; [3] Chan, S.W. et al., Neuropharmacology, 2013. 70: 141–147.; [4] Mutoh, M. et al., Jpn J Pharmacol, 1992. 58: 321–4.; [5] Chen, Y. et al., Life Sci, 1997. 60: 253–61.; [6] Wan, C. et al., Unpublished observations, 2004.; [7] Yamahara, J. et al., J Ethnopharmacol, 1989. Twenty-seven: 353–5.; [8] Torii, Y. et al. J Radiat Res (Tokyo), 1993. 34: 164–70.; [9] Torii, Y. et al., Br J Pharmacol, 1994. 111: 431–4.; [10] Torii, Y. et al., Naunyn Schmiedebergs Arch Pharmacol, 1991. 344: 564–7.; [11] Cheng, F.H. et al., Eur J Pharmacol, 2005. 508: 231–8.; [12] Tashiro, N. et al., J Am Assoc Lab Anim Sci, 2007. 46: 81–5.; [13] Kan, K.K. et al., Eur J Pharmacol, 2003. 477: 247–51.; [14] Fujiwara-Sawada, M. et al., Pharmacometrics, 2000. 59: 39–46.; [15] Javid, F.A. et al., Eur J Pharmacol, 2013. 699: 48–54.; [16] Kan, K.K. et al. Eur J Pharmacol, 2003. 482: 297–304.; [17] Yamamoto, K. et al., Physiol Behav, 2004. 83: 151–6.; [18] Gardner, C. et al. Neuropharmacology, 1998. 37: 1643–4.; [19] Andrews, P.L.R. et al. Br J Pharmacol, 2000. 130: 1247–54.; [20] Rudd, J.A. et al. Eur J Pharmacol, 1999. 366: 243–52.; [21] Gardner, C.J. et al. Br J Pharmacol, 1995. 116: 3158–63.; [22] Ikegaya, Y. et al. Jpn J Pharmacol, 2002. 89: 324–6.; and [23] Smith, J.E. et al., Exp Physiol, 2002. 87: 563–74.
![Figure 1. The latency (mean ± s.e.m, where available) of the emetic response to a variety of emetic stimuli in Suncus murinus. The TRPV1 ligands discussed in the text are highlighted in yellow. For references see: [1] Hu, D.L. et al., J Food Prot, 1999. 62: 1350–3.; [2] Ito, C. et al., Eur J Pharmacol, 1995. 285: 37–43.; [3] Chan, S.W. et al., Neuropharmacology, 2013. 70: 141–147.; [4] Mutoh, M. et al., Jpn J Pharmacol, 1992. 58: 321–4.; [5] Chen, Y. et al., Life Sci, 1997. 60: 253–61.; [6] Wan, C. et al., Unpublished observations, 2004.; [7] Yamahara, J. et al., J Ethnopharmacol, 1989. Twenty-seven: 353–5.; [8] Torii, Y. et al. J Radiat Res (Tokyo), 1993. 34: 164–70.; [9] Torii, Y. et al., Br J Pharmacol, 1994. 111: 431–4.; [10] Torii, Y. et al., Naunyn Schmiedebergs Arch Pharmacol, 1991. 344: 564–7.; [11] Cheng, F.H. et al., Eur J Pharmacol, 2005. 508: 231–8.; [12] Tashiro, N. et al., J Am Assoc Lab Anim Sci, 2007. 46: 81–5.; [13] Kan, K.K. et al., Eur J Pharmacol, 2003. 477: 247–51.; [14] Fujiwara-Sawada, M. et al., Pharmacometrics, 2000. 59: 39–46.; [15] Javid, F.A. et al., Eur J Pharmacol, 2013. 699: 48–54.; [16] Kan, K.K. et al. Eur J Pharmacol, 2003. 482: 297–304.; [17] Yamamoto, K. et al., Physiol Behav, 2004. 83: 151–6.; [18] Gardner, C. et al. Neuropharmacology, 1998. 37: 1643–4.; [19] Andrews, P.L.R. et al. Br J Pharmacol, 2000. 130: 1247–54.; [20] Rudd, J.A. et al. Eur J Pharmacol, 1999. 366: 243–52.; [21] Gardner, C.J. et al. Br J Pharmacol, 1995. 116: 3158–63.; [22] Ikegaya, Y. et al. Jpn J Pharmacol, 2002. 89: 324–6.; and [23] Smith, J.E. et al., Exp Physiol, 2002. 87: 563–74.](/cms/asset/4d23ca6c-548e-435e-9087-0a4285e26e30/ktmp_a_1043042_f0001_c.jpg)
Table 3. The latency of the emetic response to vanilloids/capsiacinoids given subcutaneously (s.c.) or intracerebroventricularly (i.c.v.). Note that there is relatively little difference between the latency irrespective of substance or route
These observations are all consistent with a central, probably brainstem, site of action for the emetic effect of RTX. A brainstem site of action is supported more directly by studies in Suncus murinus using a decerebrated working-heart brainstem preparation, in which RTX in the perfusate evoked a short latency (∼1–2 min; ) “emetic-like” response.Citation79,1 Studies with slices of Suncus murinus brainstem including the area postrema and nucleus tractus solitarius showed that RTX (1 μM) stimulated substance P release (Toyoda, Suzuki, Otsuka, Woods, Andrews, Matsuki, 2000, unpublished observations). The molecular mechanism(s) of substance P release by RTX was not studied in these slice experiments.
Conclusion
RTX is one of the most potent emetic substances so far described in Suncus murinus. Taken together, the overall evidence supports the hypothesis that in Suncus murinus the emetic response to systemic RTX is mediated by TRPV1 located on neurones in the brainstem containing substance P which then acts on NK1 receptors to induce emesis (). Whist we consider the NTS to be the most likely site of action of RTX a recent study has demonstrated activation of TRPV1 on astrocytes located in the area postremaCitation80 giving rise to the possibility that activation of the NTS is secondary to AP activation via astrocytes. Additionally, we are unable to exclude effects for example on the abdominal vagi or hypothalamus which would also be expected to be blocked by an NK1 receptor antagonist and “broad spectrum” agonist anti-emetics such as morphine and 8-OH-DPAT. The emetic response to RTX is not present at birth but in common with other emetic stimuli (motion, pyrogallol) it develops about 2 weeks postnatally.Citation81 Studies of the pathways and transmitter systems which become functional at around 2 weeks, probably the nucleus tractus solitarius as it is the convergence point for the vestibular, area postrema and vagal afferent inputs capable of triggering emesis, and may provide insights into novel targets for anti-emesis
Figure 2. Diagram summarising potential brainstem sites at which resiniferatoxin (RTX ) given either subcutaneously (s.c.) or intracerebroventricularly (i.c.v.) in Suncus murinus can induce emesis. When given s.c. (1) RTX could access peripheral terminals of abdominal vagal afferents or the nodose ganglion (2) to cause activation of the brainstem nucleus tractus solitarius (NTS) via the release of substance P. The NTS could also be accessed by RTX from the circulation (3) as could the area postrema. The area postrema is the most likely site at which RTX given i.c.v. (4) acts but it is also possible that RTX could diffuse into the NTS via the area postrema where the blood-brain barrier is relatively permeable. At none of these locations are we able to distinguish between an action of RTX on TRPV1 receptors located on the cell bodies or presynaptically. Although it is likely that RTX induces substance P release at several sites in the dorsal brainstem it is known that substance P acting on NK1 receptors occupy a pivotal position in the emetic pathway at the point where the signals integrated in the NTS drive nausea and vomiting (5) so this may also be a likely site at which RTX could induce substance P release to induce emesis. It is speculated that the emetic effect of s.c. RTX is not seen in ferret or dog because the peak plasma concentration is lower resulting in a slower release of substance P so that the threshold (T) for induction of emesis at site 5 is not reached. This site (5) is also the most likely location at which RTX causes depletion of substance P (and possibly other transmitters) to have its broad spectrum anti-emetic effect observed in ferret, dog and Suncus murinus. See text for details and references.
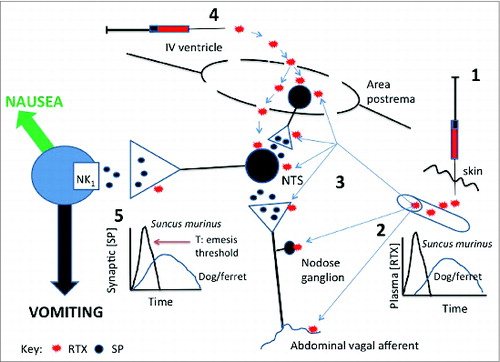
Why is systemically administered RTX emetic in Suncus murinus but not ferret or dog?
In Suncus murinus subcutaneous RTX evokes an emetic response with a dose-related reduction of latency: for example at 10 μg/kg latency 19.8 ± 4.1min; 100 μg/kg, latency 6.7 ± 1.3 min; 500 μg/kg, latency 3.0 ± 0.8 min; 1000 μg/kg, 2.7 ± 0.6 minCitation68 with values for the higher doses (>100 μg/kg) being comparable to Cheng et al.Citation70 using 100 nmol/kg that evoked a response with a latency of 8.2 ± 1.3 min.Citation70 These values are also similar to the latency of 5.7 ± 1.4 min reported for the highest dose (30 nmol) of RTX given i.c.v.Citation69 These observations imply that when given subcutaneously RTX must gain rapid access to the circulation and hence to the central nervous system either via the blood-brain barrier (BBB) and/or via one of the circumventricular organs (CVOs, e.g. area postrema, subfornical organ), where the BBB is relatively permeable; there may also be a simultaneous depolarization of the vagus by an action on the TRPV1 located particularly on the nodose ganglion or at the presynaptiC-terminals in the brainstem. With i.c.v. RTX, either the cerebrospinal fluid barrier provides little resistance to the passage of RTX and/or via one of the CVOs. In the arterially perfused, decerebrate preparation the latency to induction of emetic like-episodes was also short (1.7 ± 0.6 min). All these observations indicate that RTX has rapid access to the CNS, most likely the brainstem, and furthermore mobilises mechanisms that can bring the emetic reflex to threshold quickly (see ). Vanilloids can increase Na+ and Ca++ conductance in neurones and while this can account for the proposed release of substance P and resulting induction of emesis, it neither precludes the release of other neurotransmitters by a presynaptic action, nor a direct activation of neurones. It is worth noting that the area postrema has high and low voltage-activated calcium currents.Citation82 [3H] RTX binding sites are found in rat nucleus tractus solitarius extending into the area postremaCitation83 consistent with the proposed sites of emetic action of RTX in Suncus murinus where the dorsal vagal complex (nucleus tractus solitarius and area postrema) has relatively high levels of substance P (greater than the spinal trigeminal nucleus; Toyoda, Suzuki, Otsuka, Woods, Andrews, Matsuki, 2000, unpublished observations).
In contrast to Suncus murinus, in the dog and ferret,Citation62,68 s.c. RTX did not induce emesis, although capsaicin (33 mM) when applied directly to the IV ventricle in the decerebrate dog induced transient retching (in 2 of 3 animals) and increased neuronal firing in the NTS within 20 s of application.Citation72 Although the studies are not directly comparable, the Shiroshita et al.72 capsaicin study demonstrates that if a TRPV1 agonist reached the dorsal brainstem of the dog (or ferret) in a high enough concentration, neuronal activation and emesis could occur. In view of this we hypothesize that in Suncus murinus the BBB is more permeable to RTX than that in either the dog or ferret, so that the concentration in a given time accessing the neurones in the NTS is lower than in Suncus murinus and hence does not stimulate the central pathways sufficiently to reach threshold to induce emesis. This may also be contributed to by any transport or efflux mechanisms present in the blood-brain/blood cerebrospinal fluid barriers and a slower rate of absorption from the s.c. site in the dog and the ferret that would flatten the plasma concentration of RTX which could have an effect at sites such as the area postrema where the BBB is relatively permeable. In the ferret the highest dose of RTX given s.c. was 100 μg/kgCitation60 and in the dog the highest dose was10 μg/kg,Citation62 so we are unable to comment if higher doses would induce emesis in these species. It is also possible that the pharmacological characteristics of TRPV1 differ between Suncus murinus and dog/ferret. An additional possibility is that when given subcutaneously to the dog and ferret RTX activates an endogenous anti-emetic pathway so that an emetic effect of centrally released substance P is not observed; possibilities include activation of cannabinoid (CB1),Citation84 opioid,Citation85 or 5-HT1A pathwaysCitation86 and pulmonary vagal afferents that in contrast to abdominal vagal afferents inhibit the emetic reflex.Citation87
Anti-emetic effects of RTX in Suncus murinus: Acute and chronic effects
The spectrum of anti-emetic effects of RTX in the ferret, dog and least shrew are summarised in together with the studies on Suncus murinus discussed in this section. As RTX induced emesis in Suncus murinus, the effect of other treatments on the anti-emetic mechanism were tested at least 60 min later. RTX (100 μg/k, s.c.) blocked/markedly reduced the response to motion, i.p. cisplatin (acute phase), i.g. copper sulfate, s.c. nicotine, a second dose of RTX, and s.c. veratridine (Andrews et al., 2000; Toyoda, Suzuki, Otsuka, Woods, Andrews, Matsuki, 2000, unpublished observations). Intracerebroventricular administration of RTX markedly reduced/blocked the emetic response to i.c.v. RTX, E-capsaicin and i.g. copper sulfate, but was without effect on s.c. nicotine (cf. s.c. RTX).Citation69 The difference in the anti-emetic effect of s.c. and i.c.v. RTX against nicotine may be due to differing pretreatment times. Both E-capsaicin and Z-capsaicin given i.c.v. significantly reduced the response to i.g. copper sulfate.Citation69
Additional studies in Suncus murinus investigated the duration of the anti-emetic effect of RTX (100 μg/kg, s.c.) when given 1 h, 3, 10 and 30 d before emetic challenge. Animals responded normally to motion, nicotine (5 mg/kg, s.c.) and veratrine (0.5 mg/kg, s.c.) by 3 d after RTX administration but only one animal responded to copper sulfate and no animals responded to cisplatin. At 10 days, one out of 5 animals had a reduced response to cisplatin (20 mg/kg, i.p.) and 3 out of 5 responded to i.g. copper sulfate although with an increased latency. At 30 d the response to cisplatin was similar to that at 10 d and in response to copper sulfate, only 2 animals out of 5 tested responded (Toyoda, Suzuki, Otsuka, Woods, Andrews, Matsuki, 2000, unpublished observations).
The above series of observations in Suncus murinus both confirm and extend the original studies in ferret and dog and furthermore demonstrate the potential for a single dose of RTX to have a prolonged anti-emetic effects against copper sulfate and cisplatin where a predominantly abdominal vagal pathway is implicated. Although these unpublished observations require further investigation, the long lasting antagonism of cisplatin is particularly interesting as it demonstrated the potential for a single dose of RTX to cover the time period required for several individual cycles of chemotherapy. Although not directly comparable, a study by Geraghty and co-workers in the adult rat is of relevance as it showed that treatment with s.c. capsaicin reduced the density of [(125)I] Bolton-Hunter substance P and NK1 receptor immunoreactivity in the commissural nucleus of the NTS, further supporting the proposal that vanilloids can have long-term effects on brain stem function.Citation88 The long lasting desensitization produced by RTX has recently been drawn attention to in a review of its clinical potential for analgesia.Citation89
In the ferret, dog and Suncus murinus studies, no indications of an algesic response following administration of RTX were observed, but this potential liability would limit its potential development as an anti-emetic. In view of this studies investigating the anti-emetic potential of non-pungent vanilloids were undertaken. The ferret was used for these studies as development of radiotelemetric techniques in this species enables a range of physiological parameters to be monitored facilitating selection of compounds based on their overall profile of biological effects rather than anti-emetic effect alone.
Investigation of the anti-emetic potential of non-pungent vanilloids in the ferret: insights into the pharmacology of the anti-emetic effects of TRPV1 receptors
The discovery that certain ‘pungent’ culinary ingredients had potential anti-emetic effects, preceded the suggestion of capsaicin “pain” receptors.Citation90 In traditional Chinese medicine, ginger (Zingiber officinale), a well known pungent and aromatic spice, has been used since ancient times for the treatment of nausea and headaches; the active pungent constituents activating TRPV1 include gingerols, [6]-shogaol, and the degradation product, zingerone.Citation91 Ginger extracts were subsequently investigated for a capacity to antagonize cyclophosphamide-induced emesis in animal studies (using Suncus murinus) 25 y ago, but it was thought the mechanism was possibly via anti-serotonergic actions.Citation92 Similarly, extracts also antagonized cisplatin-induced, but not apomorphine-induced emesis in dogs, and it was assumed the mechanism involved a block of 5-hydroxytryptamine3 receptors (5-HT3)Citation93; there is also a report of ginger extracts reducing copper sulfate-induced emesis in frogs.Citation94 It is now known that several gingerols and [6]-shogaol also block 5-HT3 receptors at a modulatory site on the ion channel complex, and there may be weak actions to block at muscarinic receptors.Citation95 In mink (a close relative of the ferret), gingerols have also been shown to antagonize emesis and associated increases of 5-HT, dopamine and substance P in the area postrema.Citation96,97 Ginger has also been reported to reduce gastric dysrhythmia induced by hyperglycaemia in healthy volunteers,Citation98 but while some clinical evidence indicates the effectiveness of ginger for pregnancy-related nausea and vomiting,Citation99 meta-analysis could not demonstrate effectiveness against motion-induced emesis, or postoperative nausea and emesis,Citation100 or for the control of chemotherapy-induced acute or delayed emesis.Citation101
The major site of anti-emetic action of pungent vanilloids appears to be in the brainstem (see above). Pungent vanilloids have a number of undesirable actions and this has restricted their use as enteral or parenteral treatments for a number of disease modalities. Concerted efforts to circumvent the problems associated with pungency have been made particularly in the study of pain mechanisms. Several “non”- or “less”-pungent vanilloids were identified including SDZ 249–665, olvanil, and phorbol 12-phenylacetate 13-acetate 20-homovanillate (PPAHV).Citation102–104 These compounds appeared less irritating and it was initially presumed to relate to a slower gating of calcium that fails to reach the threshold for generating action potentials.Citation105 However, non-pungent compounds still inhibit transmitter releaseCitation106 and desensitize nociceptors.Citation107 These effects were proposed to explain the underlying mechanism of the analgesic action of non-pungent and pungent vanilloids. We reasoned that pungency and an ability to rapidly release substance P may explain the emesis seen with RTX, and that non-pungent compounds would not induce emesis. These studies were designed to follow up on the i.c.v. studies where we had observed tachyphylaxis to RTX.Citation69
To explore the above hypothesis, we decided to explore a number of vanilloids in Suncus murinus in an attempt to dissociate pungency from anti-emetic potential using animals with radio-telemetry implants. Specifically, could we dissociate emetic potential and a capacity to cause hypothermia (which we ascribed to pungency, a rapid release of substance P in emetic circuits and in the hypothalamus) from a capacity to antagonize emesis? In unpublished studies, we showed i.c.v. administered RTX (0.1–10 nmol), capsaicin (30–100 nmol), and PPAHV (10 nmol) induced transient emesis before subsequently antagonizing copper sulfate (120 mg/kg, i.g.)-induced emesis, which was given 3 h later (Wan and Rudd, 2004, unpublished observations). Hypothermia at doses below those inducing emesis was also seen in animals treated with RTX (0.3–10 nmol), capsaicin (10–100 nmol) and PPAHV (3–10 nmol); only RTX induced ano-genital grooming. Olvanil (10–600 nmol) did not cause significant emesis or hypothermia, but did antagonize copper sulfate-induced emesis (Wan and Rudd, 2004, unpublished observations). In peripheral administration studies, pungency was also assessed by an eye blink test where compounds were administered in a 10 μl volume to the eye surface and this yielded an order of potency to cause ∼10 blinks: RTX (0.01 μmol)>PPAHV (0.1 μmol)>olvanil (30 μmol)>capsaicin (3000 μmol). When administered subcutaneously, RTX, capsaicin and olvanil, but not PPAHV-induced emesis: (ED5 episodes) RTX (∼0.1 μmol/kg)>olvanil (∼30 μmol/kg)>capsaicin (∼300 μmol/kg). Subsequently, all compounds antagonized significantly acetic acid-induced writhing: (ID50) RTX (0.002 μmol/kg)>olvanil (0.8 μmolkg-1)>capsaicin (7.9 μmol/kg/)> PPAHV (25.1 μmol/kg) (Wan, Rudd, Chu, Ngan and Wai, 2008, unpublished observations). In other studies in the literature, olvanil was noted to have anti-nociceptive properties rodents and mice in the absence of causing hypothermia or affecting cardiovascular performanceCitation108-110; it was described as desensitizing, but not activating, sensory nerve terminals in the NTS of the rat.Citation111 Taken together, we decided that of the compounds studied, olvanil represented the most reasonable compound to explore as a ‘less pungent’ vanilloid to reduce emesis induced by diverse challenge.
At the time of deciding to work with olvanil, it was also emerging that the compound also inhibited the AMT, at concentrations 10 times higher than those that are required for TRPV1 activation.Citation112 Therefore, our studies were done in comparison with the AMT inhibitor/weak activator of TRPV1 receptors, AM404Citation112-113; there was also evidence of olvanil binding (in the micro-molar range) to CB1 receptors.Citation114 The studies were also done independently of work showing that the pungent TRPV1/cannabinoid CB1 receptor agonist, arvanil, antagonized morphine-6-glucuronide-induced emesis in ferrets.Citation115 We implanted ferrets with radiotelemetry devices, and found that subcutaneously administered RTX (0.1 mg/kg) caused hypertension, hypothermia (a fall of ∼4.6 ˚C was seen) and reduced food and water intake, but also significantly inhibited emesis induced by apomorphine (0.25 mg/kg, s.c.), copper sulfate (100 mg/kg, i.g.) and cisplatin (10 mg/kg, i.p.). In contrast, olvanil (0.05–5 mg/kg) did not have an adverse profile, and antagonized apomorphine- and cisplatin-induced emesis but not that induced by copper sulfate. AM404 10 mg/kg) reduced only emesis induced by cisplatin without affecting other parameters measured. The maximum inhibition of cisplatin-induced emesis by was very similar to that obtained by AM404, and it was not possible to know if anandamide had been elevated by both compounds to act at CB1 receptors in addition to TRPV1.Citation116 However, in the same studies, olvanil (30 μg) antagonized cisplatin-induced emesis when administered i.c.v. but RTX (0.3g, i.c.v.) and AM404 (60 μg, i.c.v.) were inactive. Our use of radio-telemetry clearly indicated that RTX and olvanil had been used at equipotent doses to olvanil, with both olvanil and RTX inducing a comparable reduction in temperature, which may also indicate that olvanil has some degree of pungency via this route; conversely, AM404 did not induce hypothermia administered peripherally.Citation116 Nevertheless, the studies indicate that olvanil may need to penetrate to the brain to inhibit cisplatin-induced emesis and that the spectrum of anti-emetic effects was less than observed with RTX.
Given the positive results against apomorphine and cisplatin, we investigated if administration of olvanil (0.05–5 mg/kg, s.c.) 3 times a day could antagonise the acute and delayed emesis induced by a lower dose of cisplatin (5 mg/kg, i.p.). The acute (day 1) and delayed (days 2–3) emetic response induced by cisplatin was associated with reduced food (−98.7% at day 3) and water consumption (−70.2% at day 3), and progressive weight loss (−12.0% at day 3). Olvanil did not prevent either emesis or the weight loss, or negative effects on food and water consumption, but instead appeared to potentiated the cisplatin-induced inhibition of food consumption by ∼20 %.Citation117 While cisplatin alone was without effect on temperature, hypothermia was recorded in animals concomitantly treated with olvanil; the animals also appeared more active. We concluded that olvanil would be unlikely to be useful clinically for the control of the gastrointestinal side effects induced by cisplatin.Citation117 These result were somewhat disappointing given that in previous studies, RTX 10 (μg, s.c.) given at 36 h could antagonize cisplatin (5 mg/kg, i.p.)-induced delayed emesis in ferrets.Citation62
Undesirable effects of pungent and non-pungent vanilloids on temperature, grooming, and blood pressure
The suggestion that TRPV1 channels are involved in the control of body temperature was initially based on the early findings that capsaicin provoked hypothermia in rats, acting within the hypothalamus.Citation118,119 Hypothermic effects of both capsaicin and RTX were later confirmed in the mouse,Citation120 rat,Citation121 ferret,Citation60 and Suncus murinus (Wan and Rudd, 2004, unpublished observations) (). Likewise, a non-pungent vanilloid, olvanil, may have caused hypothermia by central mechanisms in ferrets.Citation116,117 On the other hand, expected hyperthermic effects of selective TRPV1 antagonists were reported in laboratory animalsCitation122-124 and humans.Citation122 These effects provide evidence that some TRPV1 channels are tonically active and involved in the temperature control at rest.
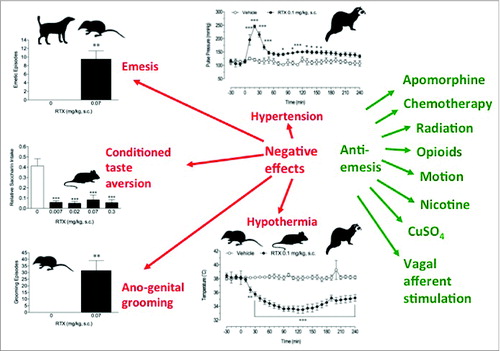
Figure 3. A summary of the biological effects of resiniferatoxin (RTX) given subcutaneously (s.c.) in a range of species indicated by the silhouette. RTX may cause undesirable effects (red) including emesis, genesis of conditioned taste aversion (CTA), hypertension, stimulation of intense ano-genital grooming and hypothermia. However, RTX also has the desirable effect of being a broad-spectrum anti-emetic agent with the range of emetic stimuli affected shown on the right hand side (green). The primary aim of much of the research described in the review is to identify the mechanism of the anti-emetic effect and identify compounds capable of anti-emesis and devoid of the undesirable effects.Symbols:Suncus murinus: Dog: Ferret: Rat: Further details and references to published data are given in the text: CTA is from rat using a 2 bottle choice design with saccharin (Rudd et al. unpublished); Emesis and ano-genital grooming data is from Suncus murinus plotted (Rudd et al., unpublished; blood pressure and core temperature data is from conscious ferrets implanted with a radio-telemetery transmitter derived from data shown in Chu et al., 2010. Neuropharmacology 58, 383–91.
The functional neuroanatomy of thermoregulatory pathways is relatively well understood, at least in rats. Information from central (brain) and peripheral (cutaneous and visceral) thermosensors is integrated in the preoptic anterior hypothalamus (POAH) and then forwarded to the dorsomedial hypothalamus, a major integrative center for autonomic output. From there, descending presympathetic pathways relay in the medullary raphe/parapyramidal area and in the spinal cord, where separate populations of sympathetic neurones control 2 thermoeffectors – brown adipose tissue responsible for non-shivering thermogenesis and cutaneous vascular bed responsible for heat dissipation/conservation.Citation125,126 Neural pathways controlling sweating are less well studied. Recent brain imaging work confirmed the POAH is also involved in mediating sweating responses in humans.Citation127
It is now well established that the 2 principal locations of the thermoregulation-related TRPV1 are in the first order sensory neurons in the dorsal root ganglia and in the POAH thermosensitive neurones. A recent comprehensive review by Romanovsky and coworkers provides excellent coverage of the topic and resolves some earlier controversies regarding central vs. peripheral action of TRPV1 ligands.Citation128 The authors proposed that both agonists and antagonists act on both POAH and DRG neuronal populations, but that the effects of agonists are mediated predominantly in the POAH, whereas the effects of antagonists are mediated predominantly in the DRG neurons. Such relative specificity could be explained if one assumes that POAH TRPV1 channels are inactive (and thus insensitive to the antagonists) in normal conditions whereas DRG channels are active.
From the above data it is clear that there are a limited number of targets where neural signals generated by emetic stimuli could interact with the descending thermoregulatory pathways. The fact that the nausea state is associated with changes in subjective perception of ambient temperature and causes preference for a cooler environment suggests that this interaction may occurs quite high in the neuraxis, and the appropriate candidate could well be the POAH.Citation129 If so, it would be not unreasonable to suggest that the TRPV1 channels in this region represent a critical link mediating hypothermic responses to emetic stimuli.
The precise mechanism of RTX to induce ano-genital grooming in Suncus murinus is not knownCitation68,69 but TRPV1 in the hypothalamus, medial preoptic area, and limbo -striatal system are conceivably involved, as erections were sometimes observed in Suncus murinus (Wan and Rudd, 2004, unpublished observations) and these brain areas are known to be involved in coordinating sexual behavior.Citation29,30,130 In fact, ginger is purportedly used as an aphrodisiac in traditional Arabic medicine,Citation131 but studies have recorded ano-genital grooming with isolated gingerols in Suncus murinus.Citation92 In Suncus murinus, olvanil and PPAHV do not induce ano-genital grooming (Wan and Rudd, 2004, unpublished observations). It is conceivable that the mechanisms involved relate to pungency (although capsaicin is inactive) and/or brain penetration, and possibly a role of substance P, which itself can facilitate sexual behavior from other studies in rats.Citation132,133
Capsaicin is more familiarly known to cause activation of vagal reflexes leading to bradycardia and hypotension.Citation25 This is clearly different from the data obtained in our studies in the ferret using radiotelemetry, and in the original anaesthetised ferret studies where one out of 4 animals tested also exhibited transient hypertension to RTX.Citation60 It is interesting, however, that epicardial administration of capsaicin can increase blood pressure in anaesthetized rat, effects prevented by pretreatment with RTX and iodo-RTX.Citation134 Further, in guinea pigs, RTX induces hypotension, and then hypertension, whereas capsaicin only caused hypotension.Citation134 It seems that the spread of penetration, and possibly anesthesia may mask effects. In a study in anaesthetised rats, an intravenous bolus dose of capsaicin induced an initial fall and then an increase in blood pressure, but a slower infusion only resulted in a pressor response.Citation135 The lack of effect of olvanil on blood pressure in the conscious ferret and retention of anti-emetic effects demonstrates the feasibility of dissociating the unwanted cardiovascular effects form emesis control.Citation116
The emetic and anti-emetic effects of vanilloids: Hypotheses and conclusions
Emetic effects ()
The studies reviewed above covering 4 species with an emetic reflex (dog, ferret, Suncus murinus, Cryptotis parva) revealed the emetic and anti-emetic potential of selected vanilloids. The emetic effect of RTX when given subcutaneously were clear in Suncus murinusCitation68,70 and Cryptotis parvaCitation74 but were not apparent in dogsCitation62 or ferretsCitation60 when given by the same route. As topical application of capsaicin to dorsal brainstem of the decerebrate dogCitation72 induced transient emesis it demonstrates that if a sufficient concentration of a pungent vanilloid accesses the TRPV1 sites thought to be located in the nucleus tractus solitarius, and to a lesser extent the dorsal motor vagal nucleus and area postrema,Citation115 then emesis can ensue. We hypothesize that in Suncus murinus, the rate of absorption of RTX from the skin is rapid and that the blood-brain barrier is relatively permeable so that a high concentration of RTX reaches the critical site(s) in the brainstem. This hypothesis is supported by the observation that the latency of the emetic response decreases with increasing dose given subcutaneously and that the latency of the emetic response via subcutaneous, intra-arterial and intracerebroventricular routes is comparable. Although the data is very limited the latency to i.c.v. capsaicin is similar in both Suncus murinus and dog ().
The mechanism by which RTX (and capsaicin and olvanil) induces emesis is proposed to be via TRPV1 in the brainstem (NTS, AP) inducing the rapid release of substance P (and probably a non-peptide co-transmitter such as L-glutamate.Citation136 Evidence supporting this hypothesis includes:
Rapid (<5 min) release of substance P from Suncus murinus brainstem slices consistent with the timing for RTX induced substance P and CGRP release from bladder, spinal cord and ear in other species;Citation137,138
Absence of response to RTX in adult Suncus murinus treated neonatally with capsaicin;
Reduction or blockade of the emetic response to RTX by the non-selective TRPV1 antagonist ruthenium red; however, the selective antagonist capsazepine was without effect. Interestingly, the genital grooming induced by i.c.v. RTX was antagonised by both ruthenium red and capsazepine and although RTX, Z-capsaicin and E-capsaicin all induced emesis when given i.c.v., only RTX induced genital grooming;Citation69
Blockade of the emetic response to RTX by NK1 (substance P receptor antagonists (). The anti-emetic effect of morphine and 8-OH-DPAT is not unexpected as both substances have “broad spectrum” anti-emetic effects in Suncus murinus (and other species) via an agonist action on endogenous anti-emetic pathways.Citation86 Both morphine and 8-OH-DPAT are presumed to reduce neurotransmitter release in the emetic pathway by a presynaptic inhibitory effect.Citation85 Even at the highest doses of RTX given subcutaneously or RTX and capsaicin given i.c.v., the emetic responses are relatively short-lived with episodes lasting ∼5–10 min. suggesting a self-limiting mechanism. The in vitro brainstem slice study also showed that release of substance P reached a plateau within 5 min of RTX application and sustained for a further 10 min but subsequently began to decline and was close to baseline levels by 30 min despite continued application of RTX. Although the in vitro brainstem slice studies in Suncus murinus used a relatively high concentration of RTX (1 μM) the amount of substance P released was ∼50 % of that release by a pulse of K+ ([50 mM]) over the same time course suggesting that the TRPV1 releasable pool is smaller than the total available. These in vitro studies must be treated with caution, as overall turnover of substance P was not measured so it is not known if the decline in release truly represents net depletion from presynaptic stores. One explanation for the difference between RTX and K+ induced substance P release is that only a proportion of the substance P containing neurones in the brainstem possess TRPV1. Although data are not available for Suncus murinus, the NTS in the ferret has a high level of TRPV1 compared to other dorsal brain stem areas,Citation115 and a high density of NK1 receptors indicative of substance P containing neuronesCitation23 consistent with the presence of substance P immunoreactivity throughout the dorsal vagal complex (NTS, area postrema, dorsal vagal motor nucleus.Citation139) The release of substance P is most likely mediated by an influx of calcium ions into the pre-synaptic-terminal via TRPV1, but actions on postsynaptic receptors cannot be excluded and has been proposed as one of the effects of RTX in the substantia gelatinosa of the spinal cord.Citation136
Studies in the rat spinal cord dorsal horn showed a relationship between substance P release and internalisation of NK1 receptors on dorsal horn neurones.Citation138,140 The authors noted that NK1 receptor internalisation is a saturable process and that substance P release can continue beyond a concentration at which NK1 receptor internalisation saturates. Internalisation is a general property of NK1 receptors and is described in both the peripheral (e.g., myenteric neuronesCitation141) and central nervous system (e.g., dorsal hornCitation138) and hence it is highly likely that such internalisation occurs in the dorsal brain stem and the nucleus tractus solitarius in particular. We propose that while emesis is induced by TRPV1 mediated release of substance P acting on NK1 receptors in the nucleus tractus solitarius, the substance P release eventually runs down the releasable pool, the saturation of the internalisation process is the initial reason for the self-limiting of the emetic response. In vitro studies indicate that recycling of the NK1 receptors to the cells surface takes 30–60minCitation141 and if a similar time course operates in vivo, it is conceivable that by the time NK1 receptors are available for activation, the pool of substance P has been depleted and this would also explain why a second dose of RTX fails to induce an emetic response in Suncus murinus. This hypothesis is testable by administration of selective NK1 receptor agonist which should evoke an emetic response in Suncus murinus at the time when it is unresponsive to other stimuli. Prolonged (24 h) exposure to substance P has been shown to reduce the affinity of NK1 receptors in neonatal rat spinal neuronesCitation142 and in theory if RTX induced sustained release of substance P and such an effect (homologous regulation) could further contribute to the acute anti-emetic effects although at 48 h an increase in binding/receptor density was reported.
Capsaicin, RTX and olvanil differ markedly in many of their pharmacological properties, for example: in their lipophilicity (∼5 log unit range); the kinetics of their effect on Ca2+fluxes in CHO cells expressing recombinant TRPV1 receptorsCitation143,144 kinetics of desensitization (seeCitation144 for capsaicin vs RTX); their potential to induce eye wipingCitation143; the degree of activation of TRPV1 receptors located on the endoplasmic reticulum (capsaicin>olvanil, seeCitation145) in spinal cord slides while both capsaicin (2 μM) and RTX (0.5 μM) increased spontaneous excitatory post synaptic currents in substantia gelatinosa neurones, olvanil (10 μM) was without effect despite being given at a concentration capable of maximally activating TRPV1 located on the cell body of the primary afferent neurone in the dorsal root ganglion.Citation136 Although the data is very limited there does not appear to be a characteristic shared by all 3 compounds except that they all have a potential to induce emesis in Suncus murinus when give subcutaneously and are anti-emetic in ferret and Suncus murinus. To define which pharmacological or physicochemical property of vanilloids/capsaicinoids accounts for the emetic effect will require studies of a wider range of compounds in Suncus murinus including investigating the emetic (and anti-emetic) potential of capsinoids (e.g., capsiate.Citation145)
Anti-emetic effects ()
The anti-emetic effect of RTX was identified in the ferret prior to identification of its emetic potential in other species.Citation60,67-69,146 Based upon the known ability of RTX (and related vanilloids) to deplete substance P from neurones it was hypothesized that its broad spectrum effects were due to this effect with the depletion proposed to occur in the brainstem at a site (probably nucleus tractus solitarius) critical for induction of emesis.
This hypothesis is consistent with the subsequent studies in the decerebrate dog showing that the anti-emetic effect takes some time to develop; for example in the ferret it is about 20–30 min when RTX is given subcutaneouslyCitation60,116 and in the dog when RTX was given i.c.v. ∼40 min was required for blockade of vagal afferent induced retching.Citation72 Using radio-telemetry in the ferret the rise in blood pressure following subcutaneous injection of RTX at the same dose (100 μg/kg) that is effective as an anti-emetic takes 15–30 min to peak and declines by 60 min, although at a level sustained above the pre-injection level.Citation116 These timings are also consistent with studies of the time course of the onset of the analgesic effect of subcutaneously given RTX in the rat.Citation61
Ascribing the acute anti-emetic effect of RTX and other vanilloids to depletion of substance P at a critical site in the brainstem (most likely the NTS) is plausible and is consistent with the “broad spectrum” anti-emetic effects of both RTX and NK1 receptor antagonists (). However, the evidence is circumstantial and other possibilities should not be excluded and could include a selective (recoverable) toxic effect on neurones at a critical point in central or peripheral emetic pathways, impaired synthesis of new substance P following the initial depletion, homologous down regulation of NK1 receptors by substance P142, and elevation of intracellular cGMPCitation147 in the NTS increasing the emetic threshold.
Preliminary unpublished studies in Suncus murinus provide an indication that a single dose of RTX may have an anti-emetic effect against cisplatin and copper sulfate lasting a month. These preliminary observations show the potential for an anti-emetic agent which following a single dose could cover several cycles of chemotherapy but the mechanism is unclear although it may reflect the known neurotoxicity of RTX.Citation61
If the emetic effect of the vanilloids/capsaicinoids is due to rapid access of a high concentration of the agonist reaching the dorsal brainstem to release substance P (or other transmitters) then to minimise the potential for this effect to occur in humans a slow release formulation would be desirable to optimise the anti-emetic effect and reduce the emetic liability. However, the studies in the ferret with olvanil show that it is possible to achieve an anti-emetic effect using a non-pungent vanilloid but further studies need to be undertaken to understand why the efficacy differs from that attained by RTX.
Lessons for anti-emetic research
The identification of the anti-emetic and emetic effect of RTX 20 y ago raises a number of lessons for research in this area:
When we first investigated RTX in ferretCitation60,146 studies of the effects of vanilloids in vivo had been performed almost exclusively in rodents and hence neither emetic nor anti-emetic effects of the compounds would have been readily detected as these species together with lagomorphs (rabbits) lack an emetic reflex.Citation1,56 Specific studies of pica or conditioned taste aversion considered to be indices of activation of pathways in rodents that in species with an emetic reflex would induce emesis might have identified the emetic potentialCitation6;
We had hypothesized that RTX would be effective against emesis induced by intragastric copper sulfate and “low dose” total body X-radiation as in the ferret both are mediated predominantly (possibly solely) by the abdominal vagi.Citation54 If we had confined the initial study to these stimuli we would not have identified the potential for RTX to affect emesis induced by a stimulus (loperamide) acting via the area postrema with no involvement of the vagi.Citation59 This observation was particularly significant because although the discovery of the anti-emetic effect of 5-HT3 receptor antagonists had a dramatic effect on this adverse effect of anti-cancer chemotherapy these agents had a limited spectrum of anti-emetic efficacy so a need for broad spectrum anti-emetic remained;
In an attempt to identify if RTX was also anti-emetic against motion sickness (the vestibular system is the third of the major pathways via which the emesis can be induced together with the area postrema and the abdominal vagal afferents) we investigate the effect in Suncus murinus which at the time was an emergent model for motion sickness; we also investigated if RTX could also inhibit nicotine-induced emesis in this species. The emetic effect of RTX in Suncus murinus was unexpected but provided important support that RTX given s.c. induced release of substance P from the brainstem in a novel species and subsequently confirmed the broad spectrum anti-emetic effect including the potential for long duration (1month) blockade of emesis;
The identification of non-pungent vanilloids (olvanil) enabled us to investigate whether anti-emetic efficacy was related to pungency. The initial studies of anti-emesis were undertaken using direct observation of the animals but the development of radiotelemetry techniques in the ferretCitation148 enabled characterization of the effect of vanilloids on temperature and the cardiovascular system,Citation116 which had previously only been possible in anaesthetised ferrets.Citation60 While identification of the anti-emetic potential of the non-pungent vanilloid olvanil was a key finding of equal importance was the ability to identify the differential effects of RTX, olvanil and AM404 on blood pressure and core temperature as such measurement are critical in selecting compounds or pharmacological targets for progression to minimise the potential for an adverse profile when studied in humans.
Concluding Remarks
It is clear that vanilloids may have anti-emetic effects across several species. The precise mechanisms involved are difficult to interpret given the selectivity of the compounds used and the complexity of distinguishing actions relative to pungency and lipophilicity. The testing of highly selective TRPV1 ligands that are truly ‘non-pungent’ would be required to more confidently ascertain their use as anti-emetics, or as adjuncts to standard anti-emetic regimens. In the interim it may be possible to gain an insight into the involvement of endogenous TRPV1 in humans by examining emetic sensitivity in populations with a cuisine using large amounts of chilli pepper as protracted consumption could lead to chronic down-regulation. This review has focused on TRPV1 in emetic mechanisms but a study of vanilloids and several other compounds at other TRP sites is required to fully understand the complexity of pharmacological action, especially in cases where responses have been noted as being resistant to the classical TRPV1 antagonist, capsazepine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
About the Authors
John A Rudd's research has investigated emetic responses resistant to 5-HT3 receptor antagonists, the discovery of broad spectrum inhibitory anti-emetic drugs, and the mechanism of antiemetic action of dexamethasone. Prof. Rudd subsequently developed the ferret model of cisplatin-induced acute and delayed emesis and revealed the potential of NK1 receptor antagonists to reduce chemotherapy-induced delayed emesis. His Emesis Research Group has strong links with the international pharmaceutical industry. Paul LR Andrews' research has focused on the pre-clinical neuropharmacology of emesis including comparative aspects and together with Norio Matsuki, who pioneered the use as Suncus murinus as a model for emesis worked on the anti-emetic effects of resinferatoxin. Current research is investigating nonanimal methodologies to identify emetic liability of potential new drugs and the mechanisms of nausea. Eugene Nalivaiko is a thermoregulatory physiologist who has recently identified novel thermoregulatory responses to emetic stimuli. Christina Wan performed the initial studies on the central effects of pungent/ non-pungent TRPV1 ligands in Suncus murinus and is currently a family doctor working for the hospital authority, Hong Kong.
References
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One 2013; 8:e60537; PMID:23593236; http://dx.doi.org/10.1371/journal.pone.0060537
- Sanger GJ, Broad J, Andrews PLR. The relationship between gastric motility and nausea: gastric prokinetic agents as treatments. Eur J Pharmacol 2013; 715:10-4; PMID:23831391; http://dx.doi.org/10.1016/j.ejphar.2013.06.031
- Andrews PLR, Sanger GJ. Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol 2014; 722:108-21; PMID:24157981; http://dx.doi.org/10.1016/j.ejphar.2013.09.072
- Stern RM, Koch KL, Andrews PLR. Nausea: mechanisms and management. New York, U.S.A.: Oxford University Press; 2011.
- Borison HL, Wang SC. Physiology and pharmacology of vomiting. Pharmacol Rev 1953; 5(2):193-230; PMID:13064033
- Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PLR. Opportunities for the replacement of animals in the study of nausea and vomiting. Br J Pharmacol 2009; 157:865-80; PMID:19371333; http://dx.doi.org/10.1111/j.1476-5381.2009.00176.x
- Shupak A, Gordon CR. Motion sickness: advances in pathogenesis, prediction, prevention, and treatment. Aviat Space Environ Med 2006; 77:1213-23; PMID:17183916
- Marin J, Ibanez MC, Arribas S. Therapeutic management of nausea and vomiting. Gen Pharmacol 1990; 21:1-10; PMID:2404830; http://dx.doi.org/10.1016/0306-3623(90)90586-B
- Takeda N, Morita M, Hasegawa S, Horii A, Kubo T, Matsunaga T. Neuropharmacology of motion sickness and emesis. A review. Acta Otolaryngol Suppl 1993; 501:10-5; PMID:8447218; http://dx.doi.org/10.3109/00016489309126205
- Gralla RJ. Metoclopramide. A review of antiemetic trials. Drugs 1983; 25 Suppl 1:63-73; PMID:6682376; http://dx.doi.org/10.2165/00003495-198300251-00007
- Sanger GJ, King FD. From metoclopramide to selective gut motility stimulants and 5-HT3 receptor antagonists. Drug Des Deliv 1988; 3(4):273-95; PMID:3076395
- Reiner PB, Kamondi A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience 1994; 59(3):579-88; PMID:8008209; http://dx.doi.org/10.1016/0306-4522(94)90178-3
- Spinks AB, Wasiak J, Villanueva EV, Bernath V. Scopolamine for preventing and treating motion sickness. Cochrane Database Syst Rev 2015; CD002851; PMID:15266468; http://dx.doi.org/10.1002/14651858.CD002851.pub4
- Pinder RM, Brogden RN, Sawyer PR, Speight TM, Avery GS. Metoclopramide: a review of its pharmacological properties and clinical use. Drugs 1976; 12(2):81-131; PMID:786607; http://dx.doi.org/10.2165/00003495-197612020-00001
- Smith RN. Safety of ondansetron. Eur J Cancer Clin Oncol 1989; 25:S47-50; discussion S1–4; PMID:2533899
- Feyer P, Maranzano E, Molassiotis A, Clark-Snow RA, Roila F, Warr D, Olver I. Radiotherapy-induced nausea and vomiting (RINV): antiemetic guidelines. Support Care Cancer 2005; 13:122-8; PMID:15592688; http://dx.doi.org/10.1007/s00520-004-0705-3
- Rudd JA, Andrews PLR. Mechanisms of acute, delayed and anticipatory vomiting in cancer and cancer treatment. In: Hesketh P, ed. Management of nausea and vomiting in cancer and cancer treatment. New York: Jones and Barlett Publishers Inc; 2004: 15-66.
- Grunberg SM, Koeller JM. Palonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesis. Expert Opin Pharmacother 2003; 4:2297-303; PMID:14640928; http://dx.doi.org/10.1517/14656566.4.12.2297
- Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT(3) and tachykinin NK(1) receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2012; 684:1-7; PMID:22425650; http://dx.doi.org/10.1016/j.ejphar.2012.01.046
- Bountra C, Bunce K, Dale T, Gardner C, Jordan C, Twissell D, Ward P. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 1993; 249:R3-4; PMID:7506663; http://dx.doi.org/10.1016/0014-2999(93)90673-6
- Tattersall FD, Rycroft W, Hargreaves RJ, Hill RG. The tachykinin NK1 receptor antagonist CP-99,994 attenuates cisplatin induced emesis in the ferret. Eur J Pharmacol 1993; 250:R5-6; PMID:8119305; http://dx.doi.org/10.1016/0014-2999(93)90649-3
- Tattersall FD, Rycroft W, Francis B, Pearce D, Merchant K, MacLeod AM, Ladduwahetty T, Keown L, Swain C, Baker R, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology 1996; 35:1121-9; PMID:9121615; http://dx.doi.org/10.1016/S0028-3908(96)00020-2
- Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PLR. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol 1995; 115:84-94; PMID:7544198; http://dx.doi.org/10.1111/j.1476-5381.1995.tb16324.x
- Andrews PLR, Rudd JA. The role of tachykinins and the tachykinin receptor in nausea and emesis. In: Holzer P, ed. Handbook of Experimental Pharmacology. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2004: 359-440.
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 1999; 51:159-212; PMID:10353985
- Szallasi A, Blumberg PM. Complex Regulation of TRPV1 by Vanilloids. In: Liedtke WB, Heller S, eds. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL); 2007.
- Rosenbaum T, Simon SA. TRPV1 Receptors and Signal Transduction. In: Liedtke WB, Heller S, eds. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL); 2007.
- Vyklicky L, Knotkova-Urbancova H, Vitaskova Z, Vlachova V, Kress M, Reeh PW. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J Neurophysiol 1998; 79:670-6; PMID:9463430
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 2000; 97:3655-60; PMID:10725386; http://dx.doi.org/10.1073/pnas.97.7.3655
- Szabo T, Biro T, Gonzalez AF, Palkovits M, Blumberg PM. Pharmacological characterization of vanilloid receptor located in the brain. Brain Res Mol Brain Res 2002; 98(1–2):51-7; PMID:11834295; http://dx.doi.org/10.1016/S0169-328X(01)00313-8
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A 2000; 97:6155-60; PMID:10823958; http://dx.doi.org/10.1073/pnas.97.11.6155
- Di Marzo V. Anandamide serves two masters in the brain. Nature neuroscience 2010; 13(12):1446-8; PMID:21102567; http://dx.doi.org/10.1038/nn1210-1446
- Xiong W, Hosoi M, Koo BN, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol 2008; 73:314-22; PMID:17993512; http://dx.doi.org/10.1124/mol.107.039149
- Das UN. Essential Fatty acids - a review. Current pharmaceutical biotechnology 2006; 7:467-82; PMID:17168664; http://dx.doi.org/10.2174/138920106779116856
- Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J 2000; 351:817-24; PMID:11042139; http://dx.doi.org/10.1042/0264-6021:3510817
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. Bmj 2001; 323:16-21; PMID:11440936; http://dx.doi.org/10.1136/bmj.323.7303.16
- Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, Di Marzo V. Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology 2005; 49:502-13; PMID:15921709; http://dx.doi.org/10.1016/j.neuropharm.2005.04.007
- O'Brien LD, Limebeer CL, Rock EM, Bottegoni G, Piomelli D, Parker LA. Anandamide transport inhibition by ARN272 attenuates nausea-induced behaviour in rats, and vomiting in shrews (Suncus murinus). Br J Pharmacol 2013; 170:1130-6; PMID:23991698; http://dx.doi.org/10.1111/bph.12360
- Khairatkar-Joshi N, Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends in molecular medicine 2009; 15:14-22; PMID:19097938; http://dx.doi.org/10.1016/j.molmed.2008.11.004
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews 2006; 58:389-462; PMID:16968947; http://dx.doi.org/10.1124/pr.58.3.2
- Phelps PT, Anthes JC, Correll CC. Cloning and functional characterization of dog transient receptor potential vanilloid receptor-1 (TRPV1). Eur J Pharmacol 2005; 513:57-66; PMID:15878709; http://dx.doi.org/10.1016/j.ejphar.2005.02.045
- Piomelli D. The ligand that came from within. Trends Pharmacol Sci 2001; 22:17-9; PMID:11165666; http://dx.doi.org/10.1016/S0165-6147(00)01602-3
- Vyklicky L, Lyfenko A, Kuffler DP, Vlachova V. Vanilloid receptor TRPV1 is not activated by vanilloids applied intracellularly. Neuroreport 2003; 14:1061-5; PMID:12802203; http://dx.doi.org/10.1097/01.wnr.0000073429.02536.1d
- Lazar J, Braun DC, Toth A, Wang Y, Pearce LV, Pavlyukovets VA, Blumberg PM, Garfield SH, Wincovitch S, Choi HK, et al. Kinetics of penetration influence the apparent potency of vanilloids on TRPV1. Mol Pharmacol 2006; 69:1166-73; PMID:16418338; http://dx.doi.org/10.1124/mol.105.019158
- Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol 1986; 88:497-9; PMID:3742146; http://dx.doi.org/10.1111/j.1476-5381.1986.tb10228.x
- Stables R, Andrews PLR, Bailey HE, Costall B, Gunning SJ, Hawthorn J, Naylor RJ, Tyers MB. Antiemetic properties of the 5HT3-receptor antagonist, GR38032F. Cancer Treat Rev 1987; 14:333-6; PMID:2964267; http://dx.doi.org/10.1016/0305-7372(87)90026-0
- Percie du Sert N, Rudd JA, Apfel CC, Andrews PLR. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer Chemother Pharmacol 2011; 67:667-86; PMID:20509026; http://dx.doi.org/10.1007/s00280-010-1339-4
- Roila F, Herrstedt J, Gralla RJ, Tonato M. Prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: guideline update and results of the Perugia consensus conference. Support Care Cancer 2011; 19:S63-5; PMID:21188425; http://dx.doi.org/10.1007/s00520-010-1044-1
- Andrews PLR. 5-HT3 receptors and anti-emesis. In: King FD, Jones BJ, Sanger GJ, eds. 5-hydroxytryptamine-3 receptor antagonists. Boca Raton (FL); CRC Press; 1994:255-317.
- Wang QP, Guan JL, Pan W, Kastin AJ, Shioda S. A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem Res 2008; 33:2035-43; PMID:18373195; http://dx.doi.org/10.1007/s11064-008-9676-y
- Maolood N, Meister B. Protein components of the blood brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat 2009; 37:182-95; PMID:19146948; http://dx.doi.org/10.1016/j.jchemneu.2008.12.007
- Hu DL, Zhu G, Mori F, Omoe K, Okada M, Wakabayashi K, Kaneko S, Shinagawa K, Nakane A. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol 2007; 9:2267-77; PMID:17517065; http://dx.doi.org/10.1111/j.1462-5822.2007.00957.x
- Niijima A, Jiang ZY, Daunton NG, Fox RA. Effect of copper sulphate on the rate of afferent discharge in the gastric branch of the vagus nerve in the rat. Neurosci Lett 1987; 80:71-4; PMID:3658234; http://dx.doi.org/10.1016/0304-3940(87)90497-6
- Andrews PLR, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 1990; 68:325-45; PMID:2178756; http://dx.doi.org/10.1139/y90-047
- Greenwood B, Fitzakerley J. Desheathing the nodose ganglion is not a reliable method of de-efferentation in the ferret. Life Sci 1988; 43:1393-401; PMID:3242504; http://dx.doi.org/10.1016/0024-3205(88)90306-2
- Sanger GJ, Holbrook JD, Andrews PLR. The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci 2011; 32:402-9; PMID:21531468; http://dx.doi.org/10.1016/j.tips.2011.03.009
- Percie du Sert N, Andrews PLR. The ferret in nausea and vomiting research: Lessons in translation of basic science to the clinic. In: Fox JG, Marinin RP, eds. Biology and Diseases of the Ferret. Third Edition ed.: John Wiley & Sons, Inc; 2014; p. 735-78.
- Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 1990; 255:923-8; PMID:2243359
- Bhandari P, Bingham S, Andrews PLR. The neuropharmacology of loperamide-induced emesis in the ferret: the role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology 1992; 31:735-42; PMID:1326727; http://dx.doi.org/10.1016/0028-3908(92)90034-M
- Andrews PLR, Bhandari P. Resinferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferret. Neuropharmacology 1993; 32:799-806; PMID:8413843; http://dx.doi.org/10.1016/0028-3908(93)90189-A
- Szallasi A, Joo F, Blumberg PM. Duration of desensitization and ultrastructural changes in dorsal root ganglia in rats treated with resiniferatoxin, an ultrapotent capsaicin analog. Brain Res 1989; 503:68-72; PMID:2611660; http://dx.doi.org/10.1016/0006-8993(89)91705-8
- Yamakuni H, Sawai-Nakayama H, Imazumi K, Maeda Y, Matsuo M, Manda T, Mutoh S. Resiniferatoxin antagonizes cisplatin-induced emesis in dogs and ferrets. Eur J Pharmacol 2002; 442:273-8; PMID:12065081; http://dx.doi.org/10.1016/S0014-2999(02)01541-8
- Ueno S, Matsuki N, Saito H. Suncus murinus as a new experimental model for motion sickness. Life Sci 1988; 43:413-20; PMID:2899827; http://dx.doi.org/10.1016/0024-3205(88)90520-6
- Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci 1987; 41:513-8; PMID:3600192; http://dx.doi.org/10.1016/0024-3205(87)90229-3
- Barnes NM, Bunce KT, Naylor RJ, Rudd JA. The actions of fentanyl to inhibit drug-induced emesis. Neuropharmacology 1991; 30:1073-83; PMID:1661861; http://dx.doi.org/10.1016/0028-3908(91)90136-Y
- Lucot JB, Crampton GH. 8-OH-DPAT suppresses vomiting in the cat elicited by motion, cisplatin or xylazine. Pharmacol Biochem Behav 1989; 33:627-31; PMID:2531422; http://dx.doi.org/10.1016/0091-3057(89)90399-7
- Rudd JA, Nylor RJ. The effect of broad spectrum anti-emetic drugs on nicotine-induced emesis in the Suncus murinus. 1995; 114:385P
- Andrews PLR, Okada F, Woods AJ, Hagiwara H, Kakaimoto S, Toyoda M, Matsuki N. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol 2000; 130:1247-54; PMID:10903962; http://dx.doi.org/10.1038/sj.bjp.0703428
- Rudd JA, Wai MK. Genital grooming and emesis induced by vanilloids in Suncus murinus, the house musk shrew. Eur J Pharmacol 2001; 422:185-95; PMID:11430930; http://dx.doi.org/10.1016/S0014-2999(01)01041-X
- Cheng FHM, Andrews PLR, Moreaux B, Ngan MP, Rudd JA, Sam TS, Wai MK, Wan C. Evaluation of the anti-emetic potential of anti-migraine drugs to prevent resiniferatoxin-induced emesis in Suncus murinus (house musk shrew). Eur J Pharmacol 2005; 508:231-8; PMID:15680276; http://dx.doi.org/10.1016/j.ejphar.2004.12.022
- Rudd JA, Chu KM, Wai MK, ANdrews PLR. Comparison of resiniferatoxin and olvanil on blood pressure, temperature and feeding in the ferret. Canadian Journal of Clinical Pharmacology 2008; 15:638
- Shiroshita Y, Koga T, Fukuda H. Capsaicin in the 4th ventricle abolishes retching and transmission of emetic vagal afferents to solitary nucleus neurons. Eur J Pharmacol 1997; 339:183-92; PMID:9473134; http://dx.doi.org/10.1016/S0014-2999(97)01370-8
- Percie du Sert N, Rudd JA, Moss R, Andrews PLR. The delayed phase of cisplatin-induced emesis is mediated by the area postrema and not the abdominal visceral innervation in the ferret. Neurosci Lett 2009; 465:16-20; PMID:19733218; http://dx.doi.org/10.1016/j.neulet.2009.08.075
- Darmani NA, Chebolu S, Zhong W, Trinh C, McClanahan B, Brar RS. Additive antiemetic efficacy of low-doses of the cannabinoid CB(1/2) receptor agonist Delta(9)-THC with ultralow-doses of the vanilloid TRPV1 receptor agonist resiniferatoxin in the least shrew (Cryptotis parva). Eur J Pharmacol 2014; 722:147-55; PMID:24157976; http://dx.doi.org/10.1016/j.ejphar.2013.08.051
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull 1998; 47:395-406; PMID:10052567; http://dx.doi.org/10.1016/S0361-9230(98)00092-6
- Torii Y, Saito H, Matsuki N. Selective blockade of cytotoxic drug-induced emesis by 5-HT3 receptor antagonists in Suncus murinus. Jpn J Pharmacol 1991; 55:107-13; PMID:2041220; http://dx.doi.org/10.1254/jjp.55.107
- Torii Y, Saito H, Matsuki N. 5-Hydroxytryptamine is emetogenic in the house musk shrew, Suncus murinus. Naunyn Schmiedebergs Arch Pharmacol 1991; 344:564-7; PMID:1725807; http://dx.doi.org/10.1007/BF00170653
- Andrews PLR, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur J Pharmacol 1996; 307:305-13; PMID:8836619; http://dx.doi.org/10.1016/0014-2999(96)00275-0
- Smith JE, Paton JF, Andrews PLR. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol 2002; 87:563-74; PMID:12481931; http://dx.doi.org/10.1113/eph8702424
- Mannari T, Morita S, Furube E, Tominaga M, Miyata S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 2013; 61:957-71; PMID:23468425; http://dx.doi.org/10.1002/glia.22488
- Andrews PLR, Dovey E, Hockaday J, Hoyle CH, Woods AJ, Matsuki N. The development of the emetic reflex in the house musk shrew, Suncus murinus. Brain Res Dev Brain Res 2000; 121:29-34; PMID:10837890; http://dx.doi.org/10.1016/S0165-3806(00)00022-5
- Hay M, Hasser EM, Lindsley KA. Area postrema voltage-activated calcium currents. J Neurophysiol 1996; 75:133-41; PMID:8822547
- Szallasi A, Blumberg PM, Nilsson S, Hokfelt T, Lundberg JM. Visualization by ; 3Hresiniferatoxin autoradiography of capsaicin-sensitive neurons in the rat, pig and man. Eur J Pharmacol 1994; 264:217-21; PMID:7851486; http://dx.doi.org/10.1016/0014-2999(94)00526-5
- Darmani N. Mechanisms of broad-spectrum antiemetic efficacy of cannabinoids against chemotherapy-induced acute and delayed vomiting. Pharmaceuticals 2000; 3:2930-55; http://dx.doi.org/10.3390/ph3092930
- Rudd JA, Naylor RJ. Opioid receptor involvement in emesis and anti-emesis. In: DJM Reynolds PLRA, CJ Davis, ed. Serotonin and the scientific basis of anti-emetic therapy. London: Oxford Clincal Communications; 1995:208-19.
- Johnston KD, Lu Z, Rudd JA. Looking beyond 5-HT(3) receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol 2014; 722:13-25; PMID:24189639; http://dx.doi.org/10.1016/j.ejphar.2013.10.014
- Zabara J, Chaffee RB, Jr., Tansy MF. Neuroinhibition in the regulation of emesis. Space Life Sci 1972; 3:282-92; PMID:4338867
- Geraghty DP, Mazzone SB, Carter C, Kunde DA. Effects of systemic capsaicin treatment on TRPV1 and Tachykinin NK(1) receptor distribution and function in the nucleus of the solitary tract of the adult rat. Pharmacology 2011; 87:214-23; PMID:21430411; http://dx.doi.org/10.1159/000324530
- Kissin I, Szallasi A. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr Top Med Chem 2011; 11:2159-70; PMID:21671878; http://dx.doi.org/10.2174/156802611796904924
- Jancso N. Desensitization with capsaicin and related acylamides as a tool for studying the function of pain receptors. In: Lin KH, Armstrong D, Pardo EG, eds. Pharmacology of Pain. Oxford: Pergamon Press; 1968: 33-55.
- Dedov VN, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, Roufogalis BD. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol 2002; 137:793-8; PMID:12411409; http://dx.doi.org/10.1038/sj.bjp.0704925
- Yamahara J, Rong HQ, Naitoh Y, Kitani T, Fujimura H. Inhibition of cytotoxic drug-induced vomiting in Suncus by a ginger constituent. Journal of ethnopharmacology 1989; 27:353-5; PMID:2615441; http://dx.doi.org/10.1016/0378-8741(89)90010-X
- Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol 1997; 57:93-6; PMID:9254112; http://dx.doi.org/10.1016/S0378-8741(97)00054-8
- Kawai T, Kinoshita K, Koyama K, Takahashi K. Anti-emetic principles of Magnolia obovata bark and Zingiber officinale rhizome. Planta Med 1994; 60:17-20; PMID:8134409; http://dx.doi.org/10.1055/s-2006-959399
- Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. European journal of pharmacology 2006; 530:136-43; PMID:16364290; http://dx.doi.org/10.1016/j.ejphar.2005.10.049
- Qian QH, Yue W, Chen WH, Yang ZH, Liu ZT, Wang YX. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin Med J (Engl) 2010; 123(4):478-84; PMID:20193490
- Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res 2009; 32:565-73; PMID:19407975; http://dx.doi.org/10.1007/s12272-009-1413-9
- Gonlachanvit S, Chen YH, Hasler WL, Sun WM, Owyang C. Ginger reduces hyperglycemia-evoked gastric dysrhythmias in healthy humans: possible role of endogenous prostaglandins. J Pharmacol Exp Ther 2003; 307:1098-103; PMID:14534370; http://dx.doi.org/10.1124/jpet.103.053421
- Matthews A, Haas DM, O'Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2014; 3:CD007575; PMID:24659261
- Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005; 12(9):684-701; PMID:16194058; http://dx.doi.org/10.1016/j.phymed.2004.07.009
- Lee J, Oh H. Ginger as an antiemetic modality for chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Oncol Nurs Forum 2013; 40(2):163-70; PMID:23448741; http://dx.doi.org/10.1188/13.ONF.163-170
- Szallasi A. Vanilloid receptor ligands: hopes and realities for the future. Drugs Aging 2001; 18(8):561-73; PMID:11587243; http://dx.doi.org/10.2165/00002512-200118080-00001
- Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol 2002; 118(1):110-21; PMID:12109845; http://dx.doi.org/10.1309/7AYY-VVH1-GQT5-J4R2
- Urban L, Campbell EA, Panesar M, Patel S, Chaudhry N, Kane S, Buchheit K, Sandells B, James IF. In vivo pharmacology of SDZ 249–665, a novel, non-pungent capsaicin analogue. Pain 2000; 89(1):65-74; PMID:11113294; http://dx.doi.org/10.1016/S0304-3959(00)00349-3
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annual review of neuroscience 2001; 24:487-517; PMID:11283319; http://dx.doi.org/10.1146/annurev.neuro.24.1.487
- Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology 2002; 42(7):873-88; PMID:12069898; http://dx.doi.org/10.1016/S0028-3908(02)00047-3
- Wu ZZ, Chen SR, Pan HL. Signaling mechanisms of down-regulation of voltage-activated Ca2+ channels by transient receptor potential vanilloid type 1 stimulation with olvanil in primary sensory neurons. Neuroscience 2006; 141(1):407-19; PMID:16678970; http://dx.doi.org/10.1016/j.neuroscience.2006.03.023
- Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, Gazzieri D, Ligresti A, Campi B, Fontana G, et al. Development of the first ultra-potent “capsaicinoid” agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther 2005; 312(2):561-70; PMID:15356216; http://dx.doi.org/10.1124/jpet.104.074864
- Dray A, Dickenson A. Systemic capsaicin and olvanil reduce the acute algogenic and the late inflammatory phase following formalin injection into rodent paw. Pain 1991; 47(1):79-83; PMID:1771095; http://dx.doi.org/10.1016/0304-3959(91)90014-O
- Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003; 44(7):958-67; PMID:12726827; http://dx.doi.org/10.1016/S0028-3908(03)00100-X
- Geraghty DP, Mazzone SB. Respiratory actions of vanilloid receptor agonists in the nucleus of the solitary tract: comparison of resiniferatoxin with non-pungent agents and anandamide. Br J Pharmacol 2002; 137(6):919-27; PMID:12411424; http://dx.doi.org/10.1038/sj.bjp.0704931
- Beltramo M, Piomelli D. Anandamide transport inhibition by the vanilloid agonist olvanil. Eur J Pharmacol 1999; 364(1):75-8; PMID:9920187; http://dx.doi.org/10.1016/S0014-2999(98)00821-8
- De Petrocellis L, Bisogno T, Ligresti A, Bifulco M, Melck D, Di Marzo V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam Clin Pharmacol 2002; 16(4):297-302; PMID:12570018; http://dx.doi.org/10.1046/j.1472-8206.2002.00094.x
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett 1998; 436:449-54; PMID:9801167; http://dx.doi.org/10.1016/S0014-5793(98)01175-2
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci 2007; 25:2773-82; PMID:17459108; http://dx.doi.org/10.1111/j.1460-9568.2007.05521.x
- Chu KM, Ngan MP, Wai MK, Yeung CK, Andrews PLR, Percie du Sert N, Rudd JA. Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret. Neuropharmacology 2010; 58:383-91; PMID:19825380; http://dx.doi.org/10.1016/j.neuropharm.2009.10.002
- Chu KM, Ngan MP, Wai MK, Yeung CK, Andrews PLR, Percie du Sert N, Lin G, Rudd JA. Olvanil, a non-pungent vanilloid enhances the gastrointestinal toxicity of cisplatin in the ferret. Toxicol Lett 2010; 192:402-7; PMID:19931602; http://dx.doi.org/10.1016/j.toxlet.2009.11.015
- Jancso-Gabor A, Szolcsanyi J, Jancso N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol 1970; 208:449-59; PMID:5500735; http://dx.doi.org/10.1113/jphysiol.1970.sp009130
- Jancso-Gabor A, Szolcsanyi J, Jancso N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol 1970; 206:495-507; PMID:5498502; http://dx.doi.org/10.1113/jphysiol.1970.sp009027
- de Vries DJ, Blumberg PM. Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci 1989; 44:711-5; PMID:2927241; http://dx.doi.org/10.1016/0024-3205(89)90382-2
- Szikszay M, Obal F, Jr., Obal F. Dose-response relationships in the thermoregulatory effects of capsaicin. Naunyn Schmiedebergs Arch Pharmacol 1982; 320:97-100; PMID:7121619; http://dx.doi.org/10.1007/BF00506307
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr., Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 2007; 27:3366-74; PMID:17392452; http://dx.doi.org/10.1523/JNEUROSCI.4833-06.2007
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 2007; 27:7459-68; PMID:17626206; http://dx.doi.org/10.1523/JNEUROSCI.1483-07.2007
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluorom ethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther 2008; 326:218-29; PMID:18420600; http://dx.doi.org/10.1124/jpet.107.132233
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 2000; 31:S157-61; PMID:11113018; http://dx.doi.org/10.1086/317521
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci 2000; 85:18-25; PMID:11189023; http://dx.doi.org/10.1016/S1566-0702(00)00216-2
- Farrell MJ, Trevakis D, McAlle RM. Preoptic activation and connectivity during thermal sweating in humans. Temperature. Temperature 2014; 1:135-41; http://dx.doi.org/10.4161/temp.29667
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 2009; 61:228-61; PMID:19749171; http://dx.doi.org/10.1124/pr.109.001263
- Nobel G, Tribukait A, Mekjavic IB, Eiken O. Effects of motion sickness on thermoregulatory responses in a thermoneutral air environment. European Journal of Applied Physiology 2012; 112:1717-23; PMID:21892631; http://dx.doi.org/10.1007/s00421-011-2142-6
- Giuliano F, Allard J. Dopamine and male sexual function. Eur Urol 2001; 40:601-8; PMID:11805404; http://dx.doi.org/10.1159/000049844
- Qureshi S, Shah AH, Tariq M, Ageel AM. Studies on herbal aphrodisiacs used in Arab system of medicine. Am J Chin Med 1989; 17:57-63; PMID:2589237; http://dx.doi.org/10.1142/S0192415X89000103
- Dornan WA, Malsbury CW. Peptidergic control of male rat sexual behavior: the effects of intracerebral injections of substance P and cholecystokinin. Physiol Behav 1989; 46:547-56; PMID:2482982; http://dx.doi.org/10.1016/0031-9384(89)90034-6
- Dornan WA, Malsbury CW. Neuropeptides and male sexual behavior. Neurosci Biobehav Rev 1989; 13:1-15; PMID:2671829; http://dx.doi.org/10.1016/S0149-7634(89)80046-6
- Porszasz R, Szolcsanyi J. Circulatory and respiratory effects of capsaicin and resiniferatoxin on guinea pigs. Acta biochimica et biophysica Hungarica 1991; 26:131-8; PMID:1844797
- Chahl LA, Lynch AM. The acute effects of capsaicin on the cardiovascular system. Acta Physiol Hung 1987; 69:413-9; PMID:2444070
- Kumamoto E, Fujita T, Jiang CY. TRP Channels Involved in Spontaneous L-Glutamate Release Enhancement in the Adult Rat Spinal Substantia Gelatinosa. Cells 2014; 3:331-62; PMID:24785347; http://dx.doi.org/10.3390/cells3020331
- Maggi CA, Patacchini R, Tramontana M, Amann R, Giuliani S, Santicioli P. Similarities and differences in the action of resiniferatoxin and capsaicin on central and peripheral endings of primary sensory neurons. Neuroscience 1990; 37:531-9; PMID:1723514; http://dx.doi.org/10.1016/0306-4522(90)90421-Y
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience 2003; 118:535-45; PMID:12699788; http://dx.doi.org/10.1016/S0306-4522(02)00977-6
- Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil 1996; 8:255-72; PMID:8878086; http://dx.doi.org/10.1111/j.1365-2982.1996.tb00265.x
- Marvizon JC, Wang X, Lao LJ, Song B. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br J Pharmacol 2003; 140:1389-98; PMID:14623771; http://dx.doi.org/10.1038/sj.bjp.0705578
- Southwell BR, Seybold VS, Woodman HL, Jenkinson KM, Furness JB. Quantitation of neurokinin 1 receptor internalization and recycling in guinea-pig myenteric neurons. Neuroscience 1998; 87:925-31; PMID:9759980; http://dx.doi.org/10.1016/S0306-4522(98)00176-6
- Seybold VS, Abrahams LG. Characterization and regulation of neurokinin1 receptors in primary cultures of rat neonatal spinal neurons. Neuroscience 1995; 69(4):1263-73; PMID:8848112; http://dx.doi.org/10.1016/0306-4522(95)00304-2
- Ursu D, Knopp K, Beattie RE, Liu B, Sher E. Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur J Pharmacol 2010; 641(2–3):114-22; PMID:20576527; http://dx.doi.org/10.1016/j.ejphar.2010.05.029
- Toth A, Wang Y, Kedei N, Tran R, Pearce LV, Kang SU, Jin MK, Choi HK, Lee J, Blumberg PM. Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci 2005; 76(25):2921-32; PMID:15820503; http://dx.doi.org/10.1016/j.lfs.2004.10.056
- Morita A, Iwasaki Y, Kobata K, Iida T, Higashi T, Oda K, Suzuki A, Narukawa M, Sasakuma S, Yokogoshi H, et al. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat TRPV1. Life Sci 2006; 79(24):2303-10; PMID:16950406; http://dx.doi.org/10.1016/j.lfs.2006.07.024
- Bhandari P, Andrews PLR. Resiniferatoxin: a broad spectrum antiemetic in the ferret. In: Bianchi A, L. G, A.D. M, King GL, eds. Mechanisms and Control of Emesis. London: John Libbey Eurotext Ltd; 1992:239-40.
- Winter J, Dray A, Wood JN, Yeats JC, Bevan S. Cellular mechanism of action of resiniferatoxin: a potent sensory neuron excitotoxin. Brain Res 1990; 520:131-40; PMID:2169951; http://dx.doi.org/10.1016/0006-8993(90)91698-G
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PLR. Reduced normogastric electrical activity associated with emesis: a telemetric study in ferrets. World J Gastroenterol 2009; 15:6034-43; PMID:20027675; http://dx.doi.org/10.3748/wjg.15.6034
- Costall B, Domeney AM, Naylor RJ, Owera-Atepo JB, Rudd JA, Tattersall FD. Fluphenazine, ICS 205–930 and dl-fenfluramine differentially antagonise drug-induced emesis in the ferret. Neuropharmacology 1990; 29:453-62; PMID:1972549; http://dx.doi.org/10.1016/0028-3908(90)90167-P
- Miner WD, Sanger GJ, Turner DH. Evidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesis. Br J Cancer 1987; 56:159-62; PMID:3311109; http://dx.doi.org/10.1038/bjc.1987.177
- Rudd JA, Naylor RJ. An interaction of ondansetron and dexamethasone antagonizing cisplatin-induced acute and delayed emesis in the ferret. Br J Pharmacol 1996; 118:209-14; PMID:8735616; http://dx.doi.org/10.1111/j.1476-5381.1996.tb15388.x
- Gonsalves S, Watson J, Ashton C. Broad spectrum antiemetic effects of CP-122,721, a tachykinin NK1 receptor antagonist, in ferrets. Eur J Pharmacol 1996; 305:181-5; PMID:8813551; http://dx.doi.org/10.1016/0014-2999(96)00216-6
- Bermudez J, Boyle EA, Miner WD, Sanger GJ. The anti-emetic potential of the 5-hydroxytryptamine3 receptor antagonist BRL 43694. Br J Cancer 1988; 58:644-50; PMID:2851311; http://dx.doi.org/10.1038/bjc.1988.277
- Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol 1992; 59:221-6; PMID:1434118; http://dx.doi.org/10.1254/jjp.59.221
- Milano S, Grelot L, Chen Z, Bianchi AL. Vagal-induced vomiting in decerebrate cat is not suppressed by specific 5-HT3 receptor antagonists. J Auton Nerv Syst 1990; 31:109-18; PMID:2290001; http://dx.doi.org/10.1016/0165-1838(90)90067-S
- Gardner CJ, Twissell DJ, Dale TJ, Gale JD, Jordan CC, Kilpatrick GJ, Bountra C, Ward P. The broad-spectrum anti-emetic activity of the novel non-peptide tachykinin NK1 receptor antagonist GR203040. Br J Pharmacol 1995; 116:3158-63; PMID:8719790; http://dx.doi.org/10.1111/j.1476-5381.1995.tb15118.x
- Rudd JA, Ngan MP, Wai MK. Inhibition of emesis by tachykinin NK1 receptor antagonists in Suncus murinus (house musk shrew). Eur J Pharmacol 1999; 366:243-52; PMID:10082206; http://dx.doi.org/10.1016/S0014-2999(98)00920-0
- Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK(1) receptor antagonists. Brain Res 2008; 1214:58-72; PMID:18471804; http://dx.doi.org/10.1016/j.brainres.2008.03.077
