Abstract
Psychogenic fever is a stress-related, psychosomatic disease especially seen in young women. Some patients develop extremely high core body temperature (Tc) (up to 41°C) when they are exposed to emotional events, whereas others show persistent low-grade high Tc (37–38°C) during situations of chronic stress. The mechanism for psychogenic fever is not yet fully understood. However, clinical case reports demonstrate that psychogenic fever is not attenuated by antipyretic drugs, but by psychotropic drugs that display anxiolytic and sedative properties, or by resolving patients' difficulties via natural means or psychotherapy. Animal studies have demonstrated that psychological stress increases Tc via mechanisms distinct from infectious fever (which requires proinflammatory mediators) and that the sympathetic nervous system, particularly β3-adrenoceptor-mediated non-shivering thermogenesis in brown adipose tissue, plays an important role in the development of psychological stress-induced hyperthermia. Acute psychological stress induces a transient, monophasic increase in Tc. In contrast, repeated stress induces anticipatory hyperthermia, reduces diurnal changes in Tc, or slightly increases Tc throughout the day. Chronically stressed animals also display an enhanced hyperthermic response to a novel stress, while past fearful experiences induce conditioned hyperthermia to the fear context. The high Tc that psychogenic fever patients develop may be a complex of these diverse kinds of hyperthermic responses.
Abbreviations
| A | = | adrenaline |
| BAT | = | brown adipose tissue |
| CFS | = | chronic fatigue syndrome |
| CRP | = | C-reactive protein |
| DBP | = | diastolic blood pressure |
| DMH | = | dorsomedial hypothalamus |
| FMS | = | fibromyalgia syndrome |
| HMS | = | hypothalamic-medullary-sympathetic |
| HR | = | heart rate |
| 5-HT | = | 5-hydroxytryptamine |
| IL | = | interleukin |
| NA | = | noradrenaline |
| NSAIDs | = | nonsteroidal antiinflammatory drugs |
| PG | = | Prostaglandin |
| PSH | = | psychological stress-induced hyperthermia |
| POA | = | preoptic area of the hypothalamus |
| SBP | = | systolic blood pressure |
| SSRI | = | selective serotonin reuptake inhibitor |
| Tc | = | core body temperature |
| UCP1 | = | uncoupling protein1. |
What is Psychogenic Fever?
Among those who develop episodic or persistent high core body temperature (Tc) without any inflammatory causes, there are patients whose high Tc is associated with psychological stress.Citation1-14 Some patients develop a high fever (up to 41°C) when they are exposed to emotional events (), whereas others show a persistent low-grade fever (37–38°C) lasting months and even years, either during or after situations of chronic stress () (for review, see).Citation15,16 The existence of such patients has been recognized since the early twentieth centuryCitation17 and their high Tc has been called “psychogenic fever”Citation2,18,19 or “neurogenic fever.”Citation20,21 Psychogenic fever is bothersome for both patients and physicians because, although many patients consider the fever to be disabling, there is no abnormal finding to account for their high Tc and antipyretic drugs do not reduce their fever. Moreover, there are still physicians who do not recognize the fact that psychological stress can cause high Tc.
Figure 1. Prominent psychogenic fever observed in a 15-year-old schoolgirl. She was referred from a pediatrician to my outpatient clinic because she repeatedly developed antipyretic drug-resistant fever of unknown causes. I asked the patient to record her axillary temperature (Ta) using an electrothermometer 4 times a day (8 a.m., 12 a.m., 4 p.m., and 8 p.m.) and the events of the day in a “fever diary” to better understand mind (stressor)-body (temperature) relationships. I also asked her mother and school nurse to make sure the temperature she recorded was accurate. The fever diary demonstrated that she developed a high Ta up to 39°C only on the days when she went to school (underlined black bar). (Unpublished observation.)
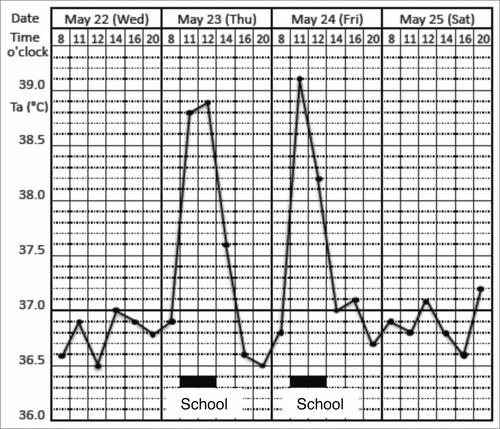
Figure 2. Chronic psychological stress-associated, persistent low-grade high axillary temperature (Ta) observed in a 56-year-old head nurse. She had antipyretic drug-resistant, low-grade (37–38°C) high Ta for more than 3 months. © Japanese Society of Psychosomatic Internal Medicine. Reproduced by permission of Japanese Society of Psychosomatic Internal Medicine. Permission to reuse must be obtained from the rightsholder.
Therefore, to obtain a better understanding of patients with psychogenic fever, this article reviews how psychological stress affects Tc in laboratory animals, healthy human subjects, and clinical populations.
Acute Psychological Stress-Induced Hyperthermia in Laboratory Animals
Animal studies have demonstrated that many, but not all, types of acute psychological stress increase Tc. For example, exposing rats or mice to stressors such as being placed into an unfamiliar space or an open field (novelty stress),Citation22-24 changing home cages (cage-change stress or cage switch stress),Citation25-27 restraint/immobilization,Citation28-31 removing cage-mates (cage-mate removal stress),Citation27,32-34 and exposure to dominant animals (social defeat stress)Citation35-38 or an intruderCitation39 increases Tc. shows that social defeat stress, i.e., exposing rats to a dominant conspecific, increases Tc by up to 2°C within 30 min.37 As represented by this model, a single exposure to psychological stress induces a transient, monophasic increase in Tc, known as psychological stress-induced hyperthermia (PSH). Existence of PSH has been observed not only in rats and mice but also in rabbits,Citation40,41 tree shrews,Citation42,43 sheep,Citation44 squirrels,Citation45,46 chimpanzees,Citation47 impalas,Citation48 Pekin ducks,Citation49 and pigeons.Citation50
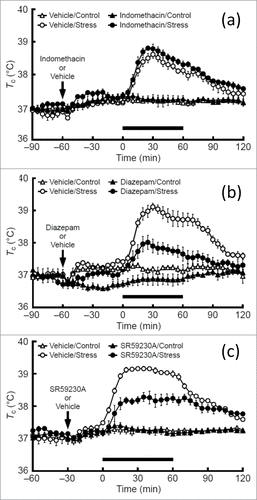
Figure 3. Effects of indomethacin (A), diazepam (B), and SR59230A (C), on social defeat stress-induced hyperthermia in rats. Rats received an intraperitoneal injection of indomethacin, a cyclooxygenase inhibitor (5 mg/kg), diazepam, an anxiolytic drug (4 mg/kg), SR59230A, a β3-adrenoceptor antagonist (5 mg/kg), or their respective vehicles at the time point indicated by arrows and were subsequently exposed to social defeat stress (Stress) or left undisturbed (Control) during the period indicated by the horizontal bars. © John Wiley and Sons. Reproduced by permission of John Wiley and Sons. Permission to reuse must be obtained from the rightsholder.
Psychological or emotional stress increases Tc via mechanisms that are distinct from fever that animals develop when they suffer from infectious and inflammatory diseases (for review, see).Citation51,52 Infection- and inflammation-induced fever is induced when PGE2 acts on neurons in the preoptic area of the hypothalamus (POA).Citation53,54 When animals suffer from infectious diseases, fever is initiated by the release of brain-permeable PGE2 from hepatic and pulmonary macrophages.Citation55,56 Macrophages also release proinflammatory cytokines such as interleukin-1β and interleukin-6 and these cytokines stimulate synthesis and release of acute phase proteins such as C-reactive protein (CRP) from hepatocytes. Furthermore, macrophage-derived proinflammatory cytokines stimulate synthesis of PGE2 from endothelial cells of the brain vesselsCitation57,58 or perivascular cellsCitation59 and cause prolonged fever.Citation60,61 Activation of the dorsomedial hypothalamus (DMH)–medullary raphe region (including the rostral raphe pallidus and adjacent raphe magnus nuclei)–sympathetic (hypothalamic-medullary-sympathetic, HMS) axis increases Tc by activating β3-adrenoceptor-mediated non-shivering thermogenesis in brown adipose tissue (BAT) and α-adrenoceptor-mediated peripheral vasoconstriction to inhibit heat loss.Citation62,63 Stimulation of the DMH and the medullary raphe region also induces shivering thermogenesis in skeletal muscles.Citation64-66 Usually, the POA sends tonic inhibitory input to the HMS axis. PGE2 causes fever by inhibiting the POA neurons, i.e., by disinhibiting the HMS axis.Citation67-69 Consequently, fever is attenuated by nonsteroidal antiinflammatory drugs (NSAIDs), which block PGE2 synthesis ().
Figure 4. Possible mechanisms of psychological stress-induced hyperthermia in comparison with infectious fever. Infectious fever is induced by warmth-seeking behavior and shivering thermogenesis of the skeletal muscles, as well as sympathetic nerve-mediated non-shivering thermogenesis in brown adipose tissue and peripheral vasoconstriction. The HMS axis is known to mediate both sympathetic activation and shivering. In contrast, the brain region responsible for warmth-seeking behavior is currently unknown. Evidence suggests that neither the POA nor the DMH mediate warmth-seeking behavior.Citation129 Infectious/inflammatory fever is accompanied with elevated acute-phase proteins such as CRP and sickness behavior. By contrast, psychological stress increased Tc without accompanying sickness-related symptoms because it increases Tc via cytokines and PGE2-independant manner. So far, it is not known how psychological stress activates the DMH neurons to increase Tc or how the POA and other brain regions are involved in the psychological stress-induced hyperthermia. BAT, brown adipose tissue; CRP, C-reactive protein; DMH, dorsomedial hypothalamic nucleus; HMS, hypothalamic-medullary-sympathetic; IML, intermediolateral cell column; IL, interleukin; Mφ, macrophage; PG, prostaglandin; POA, preoptic area; rRPa, rostral raphe pallidus nucleus; Tc, core body temperature. Reprinted from Advances in Neuroimmune Biology, Vol 3, Oka T, Oka K, Mechanisms of psychogenic fever, Pages 3-17. © IOS Press. Reproduced by permission of IOS Press. Permission to reuse must be obtained from the rightsholder.
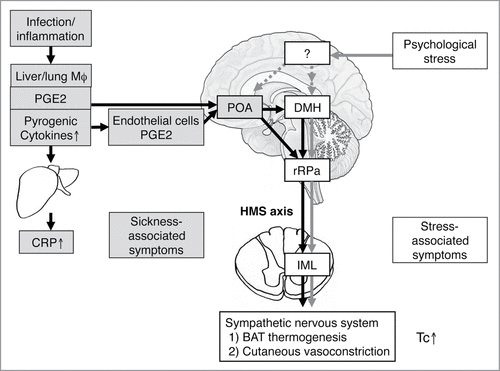
By contrast, recent studies have demonstrated that acute psychological stress also activates the HMS axis and increases Tc,Citation37-39,70-72 albeit via proinflammatory cytokine- and PGE2-independent mechanisms.Citation27,37,38,72,73 Therefore, systemic administration of cyclooxygenase inhibitors, such as indomethacin, do not inhibit PSH,Citation33,52 while anxiolytic drugs such as diazepam or 5-HT1A agonistsCitation74-76 and β3-adrenoceptor antagonists, such as SR59230A, do attenuate PSH ().Citation37 Other brain regions, such as the prefrontal cortex,Citation77 the POA,Citation78,79 the medial amygdala,Citation80 or the lateral habenula,Citation81 in addition to orexin neuronsCitation82 are also suggested to be involved in the development of PSH. However, so far, it is not fully understood how psychological stress activates DMH neurons or how other brain regions affect the HMS axis during psychological stress.
Effects of Repeated and Chronic Stress on Tc
Regardless of the source of stress, acute PSH is represented by a transient, monophasic increase in Tc, and the high Tc returns to baseline levels within several hours if the stressor is terminated. In contrast, repeated or chronic exposure to psychological stress has complex effects on Tc. First, repeated exposure to uncontrollable stressors such as daily confrontation with a dominant rat at fixed time intervals induces anticipatory or learned hyperthermia, i.e., Tc becomes higher during the hour preceding the scheduled time of stress application or during the hour when animals have been exposed to dominant rats even if they are kept in their home cages without stress exposure.Citation36,83,84 Second, repeated application of stressors (for more than several weeks) either reduces diurnal changes in Tc, mostly by increasing Tc in the light (inactive) period,Citation85 or slightly increases Tc (around 0.2–0.3°C) throughout the day.Citation36,86 Third, repeated or chronic stress enhances the magnitude of the hyperthermic effect induced by a novel stressorCitation87 or intravenous administration of noradrenaline (NA).Citation88 Fourth, these rats display depressive-like behavior rather than increased anxiety-like behavior.Citation36,89 Fifth, these changes can be observed even several days after cessation of the final stress exposure.Citation36,85,86
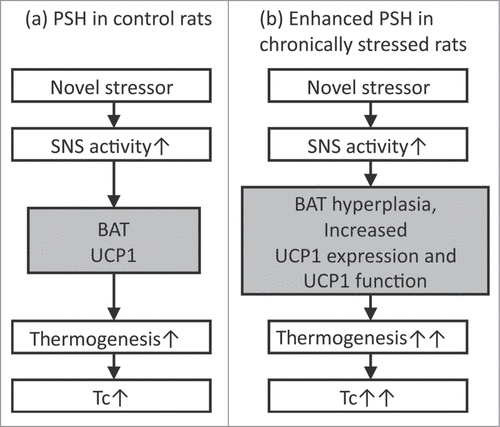
Hyperthermic responses in rats exposed to repeated or chronic stress do not seem to be induced by exactly the same mechanisms as acute PSH. First, the hyperthermic response during conditioned fear does not appear to involve activation of BAT.Citation90 Contextual conditioned fear does not induce the expression of Fos, a marker of neuronal activation, in the DMH, but does increase Fos in spinally projecting neurons in the perifornical area of the hypothalamus.Citation91 Second, after repeated immobilization, the magnitude of the NA-induced increase in Tc, interscapular BAT temperature, and oxygen consumption become greater in stressed rats versus controls.Citation88 As repeated immobilization stress induces interscapular BAT hyperplasiaCitation92 and increases uncoupling protein 1 (UCP1), a protein that generates heat according to its expression and functionCitation30 in BAT, these changes may lead to prominent PSH ().Citation92,93 Thirdly, psychological stress induces microglial activationCitation94-96 and subsequent proinflammatory cytokine productionCitation97 in the central nervous system. As brain-derived cytokines also increase TcCitation98,99 and induce depressive-like behavior,Citation100-102 there is a possibility that hyperthermia and depressive-like behaviors in rats exposed to chronic stress are mediated, at least in part, by activated microglia and subsequent proinflammatory cytokines within the brain. However, additional studies are necessary to make sure if this is the case.
Stress-Induced Hyperthermia in Healthy Subjects
As in laboratory animals, psychological stress increases the Tc in healthy humans. Previous studies have demonstrated that Tc just before emotional events is higher than Tc after these events or at the same hour of the day under non-stressful conditions.Citation103-110 For example, the mean oral temperature before boxing contests (37.55°C) in 12 school boys (12–14 years old) was 0.8°C higher than that taken at home at the same hour of the day (36.75°C).Citation107 The mean oral temperature on movie-watching days in separate groups of females in their teens and twenties (37.55°C and 37.46°C) was 0.53°C and 0.27°C higher than that of the same hour on preceding or following days (37.03°C and 37.19°C), respectively.Citation104 The hyperthermic effect of examination stress is reported to be weaker than the effects of the emotional events described above. For example, the mean oral temperature of 40 subjects immediately before taking a nurses' registration examination (37.17°C) was 0.34°C higher than observed after the examination (36.83°C).Citation103 The mean axillary temperature of 22 residents (26 – 33 years old), 10 to 15 min before a yearly university examination (37.00°C), was 0.6°C higher than the temperature taken 2 to 3 weeks later after having sat and relaxed for at least 30 min (36.40°C).Citation108 The mean oral temperature of 108 medical students (18 – 27 years old) immediately before examination (37.4°C) was 0.18°C higher than what was taken at the same hour of the day 3 days after the exam (37.22°C).Citation109 The mean oral temperature of medical students (17 – 19 years old) 5 – 7 days before examination (36.91°C) was 0.17°C higher than that at the same time 5 – 7 days after the examination (36.74°C).Citation110 In contrast, one study demonstrated that exposing healthy subjects to a standardized laboratory stress task (the Trier Social Stress Test) did not change temporal artery temperature and also decreased intestinal temperature, both of which are assumed to reflect Tc.Citation111
Psychogenic Fever
In 1930, Falcon-LessesCitation18 made precise descriptions of a 20-year-old woman who exhibited a high oral temperature around 37.8°C when she visited the clinic but a normal temperature at home. Her temperature increased following venipuncture, a visit by physicians, or vaginal examination in the hospital as well as during arguments with her sister at home. For example, venipuncture increased her Tc from 36.61°C to 37.39°C (a 0.78°C increase), occurring within 5 min. Falcon-Lesses termed these stress-induced hyperthermic responses of this patient “psychogenic fever.”
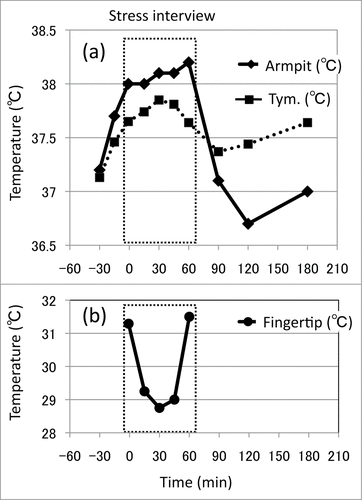
Psychogenic fever is comprised of several subtypes in terms of magnitude and duration. I would like to describe some patients I have treated. indicates an acute onset, short-lasting, prominent psychogenic fever in a 15-year-old schoolgirl. Like this case, some patients develop a high Tc abruptly (up to 41°C) when they are exposed to emotional events. She repeatedly developed an antipyretic drug-resistant high axillary temperature around 39°C only on the days when she went to school (underlined black bar) that returned to around 36.5°C after coming back home and remained normal on days when she stayed at home. There were no inflammatory signs even when she exhibited a high temperature. The fever was not factitious, either. She was a courteous, obedient, and good girl. Via diagnostic interview, she said she wanted to go to school but felt very tense and sad at school because some classmates teased and bullied a friend who had a physical handicap. She hated to see it, but could not do anything. While she wanted to stay at school even when she had a high temperature, she gradually felt hotter and fatigued as her temperature increased. Consequently, her school nurse regularly asked her to go home or to the hospital. Eventually, she changed to another school. Thereafter, her “school fever” disappeared. In addition to the emotional events that provoke negative affect such as anxiety, anger, or fear, other psychological stressors that induce remarkable hyperthermia include separation from nurturing persons (emotional deprivation)Citation1,19 and suppression of negative emotion.Citation3 Stress interviews, i.e., recalling and talking about stressful life events, also increases Tc ().Citation13,14
Figure 6. Effects of stress interview on core and peripheral temperatures in a 26-year-old CFS patient. Changes in axillary (armpit) and tympanic membrane (tym.) temperatures (A) and fingertip temperature (B) during and after a 60-minute stress interview. Stress interview was conducted for one hour (0 min – 60 min). © BioMed Central. Reproduced by permission of BioMed Central. Permission to reuse must be obtained from the rightsholder.
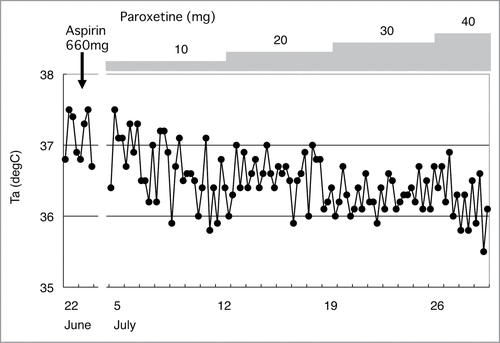
In contrast, other patients show a persistent low-grade fever (37–38°C) lasting months and even years, either during or after situations of chronic stress. shows the chronic psychological stress-associated, persistent low-grade high Tc observed in a 56-year-old head rheumatology nurse. She suffered from NSAIDs- and adrenocorticosteroid-resistant, low-grade (37–38°C) high Tc for more than 3 months. Her doctor, a rheumatologist, conducted thorough medical tests but could not discern any findings to account for her fever. For diagnostic purposes, the doctor asked her to take NSAIDs and corticosteroids, but they were ineffective in reducing her fever. Subsequently, she was referred to my outpatient clinic. Through a diagnostic interview, I realized that she was in a physically and psychologically demanding situation because of cumulative stressful life events at the time she noticed the low-grade high Tc in April. She had been working as a nurse for more than 30 years while at home taking care of her father with dementia in recent years. In January, she was shocked to hear that her younger sister was diagnosed with breast cancer. In March, one hospital nurse suddenly quit and the patient had to substitute for her and had to work an overnight duty as well. Her Tc showed diurnal changes but was 37.4–37.8°C in the afternoon. There were no inflammatory signs accounting for her high Tc. Although it was just a slightly elevated Tc, she felt strong discomfort and increased fatigue when the Tc increased above 37.0°C. Therefore, she was suspended from her job. However, even after taking sufficient time off for recuperation for more than 3 months, her high Tc did not decrease until she began to take paroxetine, a selective serotonin reuptake inhibitor (SSRI).Citation112
Certain forms of psychogenic fever have been given additional labels, e.g., prolonged low-grade high Tc in nervous patients has been termed “habitual hyperthermia”Citation113 and abrupt increases in Tc in hysterical patients was previously called “hysterical fever.”Citation114,115
Differences Between PSH in Healthy Subjects and Psychogenic Fever
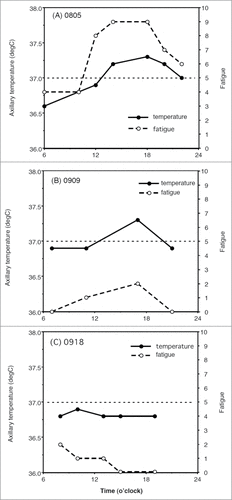
The clinical significance of high Tc in patients with psychogenic fever is different from PSH in healthy subjects in several ways.Citation116 Remarkable differences include the magnitude of increase in Tc and the associated symptoms. First, in healthy subjects, although emotional events increase Tc, its magnitude is <1°C and the maximal Tc they show is <37.5°C in most cases.Citation103-110 By contrast, in some patients with psychogenic fever, emotional events increase Tc to 39–41°C ().Citation4,6,8 Such differences may arise according to the severity of stressors. However, as was shown in animal studies, it is also possible that chronic stressors that the patient has experienced cause the induction of a prominent hyperthermic response when the patient is exposed to emotional events. Second, healthy subjects do not complain of symptoms even when they exhibit high Tc. By contrast, although some patients have no complaints except for the high Tc, others complain of numerous symptoms in addition to high Tc. These symptoms include insomnia, fatigue, headache, nausea, and/or abdominal pain. As increases in Tc are frequently associated with these symptoms (), patients consider the high Tc disabling. Some patients are neurotic and have high anxiety.Citation20 Psychogenic fever is also observed in patients who have traumatic experiences in their early livesCitation115 and with psychiatric disorders such as anxiety (panic and post-traumatic stress) disorders,Citation8 mood (depressive and bipolar) disorders,Citation7,11 somatoform (conversion) disorders,Citation115 catatonia,Citation5,117 and borderline personality disorders.Citation6 For these reasons, they worry about their high Tc and may consult their physicians asking for treatment.
Figure 7. Inhibitory effects of tandospirone, a 5-HT1A receptor agonist, on the axillary temperature (Ta) and severity of fatigue in a 30-year-old woman with psychogenic fever. Vertical lines show axillary temperature (black line) and fatigue level (dotted line, with numerical rating scale in which 10 represents the most severe fatigue imaginable and 0 represents none). (A) Before the treatment (August 5th), (B) After tandospirone treatment Sep. 9th, and (C) After tandospirone treatment Sep. 18th. The patient started to take tandospirone, a 5-HT1A agonist, 30 mg from Sep. 2nd and 60 mg from Sep. 9th. © Japanese Society of Psychosomatic Internal Medicine. Permission to reuse must be obtained from the rightsholder. Before the treatment, as her Ta induced 0.5°C increased from 36.8°C to 37.3°C, her fatigue level increased remarkably from 4 to 9. She asked for the treatment of her low-grade fever hypochondriacally (A). However, after the treatment with tandospirone, she became less concerned about her low-grade fever, when although her Ta increased from 36.8°C to 37.3°C, her fatigue level increased from just 1 to 2 (B). Her Ta did not exceed 37°C (C).
Low-Grade Fever in Patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome
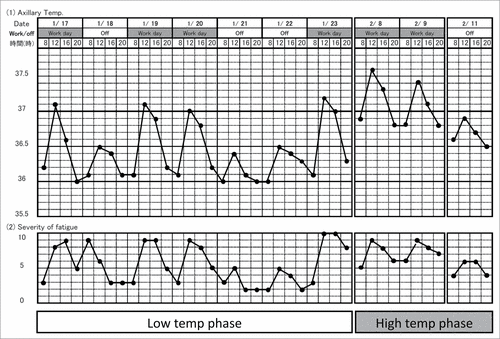
Patients with chronic fatigue syndrome (CFS) and fibromyalgia syndrome (FMS) also exhibit low-grade fever of unknown causes.Citation14,118 It is well known that psychological stress exacerbates their symptoms, and this may be the case with their low-grade fever. For example, some patients show “workday hyperthermia,” i.e., higher Tc on working days compared with holidays.Citation14 shows the record of Tc and severity of fatigue of a 24-year-old woman having both CFS and FMS. It demonstrates that her Tc and fatigue scores were higher during working days compared to days off. As she was a telephone operator, she remained sitting almost all day but kept concentrating on numerous phone conversations. Therefore, the higher Tc may not be due to increased activity during the working day, but due to psychological strain.
Figure 8. Fever and fatigue chart recorded by a 24-year-old patient with chronic fatigue syndrome and fibromyalgia syndrome. She worked as a telephone operator, a sedentary job. This chart tells that her axillary temperature is higher on the workday than on a day-off, showing that “workday hyperthermia” and the increase in axillary temperature is associated with increased fatigue. Reprinted from Advances in Neuroimmune Biology, Vol 4, Oka T, Influence of psychological stress on chronic fatigue syndrome, Pages 301-9. © IOS Press. Reproduced by permission of IOS Press. Permission to reuse must be obtained from the rightsholder.
Another example is the remarkable psychological stress-induced hyperthermic response in these patients. A 26-year-old female nurse with CFS noticed that her Tc became higher (up to 38.5°C) when she felt stressed at work. To investigate the mechanisms for her PSH, we conducted a 60-minute stress interview, in which we asked her to recall and talk about her difficult life.Citation14 Her Tc at baseline was 37.2°C, and increased to 38.2°C (a 1.0°C increase) by the end of the interview. In contrast, her fingertip temperature decreased during the interview (). During the stress interview, blood levels of pyretic cytokines, such as IL-1β and IL-6, or antipyretic cytokines, such as TNF-α and IL-10, did not change but heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and plasma levels of NA and adrenaline (A) increased. These results suggest that stress interview-induced hyperthermia is not mediated by pyretic cytokine production but by emotional expression-associated sympathetic activation. Considering these findings on the effects of chronic stress on acute PSH in animals, it is possible that the patient's difficult daily life acts as a chronic stressor, leading the patient to exhibit robust increases in Tc when she/he is exposed to emotional events.
How Does Psychological Stress Increase Tc in Humans?
Animal studies have demonstrated that psychological stress increases Tc via PGE2- and proinflammatory cytokine-independent mechanisms. This seems to be the case in patients with psychogenic fever. First, NSAIDs do not attenuate the high Tc in these patients. Second, stress interview-induced hyperthermia is not associated with changes in blood levels of PGE2 or proinflammatory cytokines.Citation13,14
In rodents, BAT thermogenesis plays a crucial role in the development of PSH. In humans, BAT is distributed exclusively in an interscapular region in infantsCitation119 and in thyroid/tracheal, mediastinal, paracervical/supraclavicular, parathoracical, supra- and peri-renal regions in adults.Citation120-123 As in rodents, BAT induces non-shivering thermogenesis when humans are exposed to a cold environment. Therefore, it is possible that psychological stress increases Tc via sympathetic nerve-mediated non-shivering thermogenesis in BAT in humans as well. Recently, the author found that patients with psychogenic fever exhibit greater HR response to orthostatic stress and increased prevalence of postural orthostatic tachycardia syndrome, one form of orthostatic intolerance, compared with healthy subjects,Citation124,125 suggesting a heightened sympathetic response to stress in patients with psychogenic fever. This might account for prominent hyperthermic responses to stressful events in patients with psychogenic fever. However, a role of BAT in PSH in healthy subjects or high Tc in patients with psychogenic fever has not yet been elucidated.
Previous clinical case reports have demonstrated that patients who exhibit persistent low-grade high Tc were treated successfully with drugs that produce sedative effects such as opiumCitation113 or phenobarbital,Citation6,7 serotonergic tricyclic antidepressants such as amitriptyline and clomipramine,Citation8,126 SSRIs such as paroxetine (),Citation112 or serotonin 1A receptor agonists such as tandospirone ().Citation127 Furthermore, relaxation training,Citation8,12 solving their difficulties, and psychotherapy to ventilate suppressed negative emotion and conflicts verballyCitation6,115 or non-verballyCitation10 are also helpful strategies.
About the Name “Psychogenic Fever.” Why not “Functional Hyperthermia?”
I propose to call psychological stress-associated high Tc “functional hyperthermia” instead of psychogenic fever. This is in part because psychological stress-associated high Tc is induced by mechanisms distinct from infection/inflammation-associated fever, where proinflammatory mediators play a pivotal role. Furthermore, “psychogenic” sounds stigmatic for some patients and their families. I do not want to call their high Tc emotional hyperthermia, either, because it sounds like a physiological response, which does not require treatment. I prefer to call it functional because in the clinical setting the naming of diseases including the term “functional,” such as functional dyspepsia, functional gastrointestinal disorders, or functional somatic syndrome, connotes both stress-related pathology and impaired functioning of the autonomic nervous system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
About the Author
Takakazu Oka is a physician specializing in psychosomatic medicine. He exclusively treats patients with psychosomatic diseases, i.e., physical diseases and conditions affected by psychosocial factors. One such disease is psychogenic fever. When he was a resident in psychosomatic medicine and internal medicine, he met some patients with fever of unknown causes that developed during highly stressful situations. In spite of repeated and thorough medical tests, abnormal findings were not detected and antipyretic drugs failed to attenuate their high body temperature. However, their high temperature was normalized after psychotherapy sessions. Since then, he has been conducting basic research on the mechanisms of psychogenic fever as well as seeing patients as a clinician.

Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (number 23390189 to TO).
References
- Bakwin H. Emotional deprivation in infants. J Pediatr 1949; 35:512-21; PMID:18143946; http://dx.doi.org/10.1016/S0022-3476(49)80071-0
- White KL, Long WN, Jr. The incidence of psychogenic fever in a university hospital. J Chronic Dis 1958; 8:567-86; PMID:13587612; http://dx.doi.org/10.1016/0021-9681(58)90050-X
- Meyer R, Beck D. Psychodynamics of psychogenic fever. Zeitschrift fur Psychosomatische Medizin und Psychoanalyse 1976; 22:169-70; PMID:941539
- Malleson N. Panic and phobia; a possible method of treatment. Lancet 1959; 1:225-7; PMID:13631975; http://dx.doi.org/10.1016/S0140-6736(59)90052-2
- O'Toole JK, Dyck G. Report of psychogenic fever in catatonia responding to electroconvulsive therapy. Dis Nerv Syst 1977; 38:852-3; PMID:908250
- McNeil GN, Leighton LH, Elkins AM. Possible psychogenic fever of 103.5 degrees F in a patient with borderline personality disorder. Am J Psychiatry 1984; 141:896-7; PMID:6731643; http://dx.doi.org/10.1176/ajp.141.7.896
- Weinstein L. Clinically benign fever of unknown origin: a personal retrospective. Rev Infect Dis 1985; 7:692-9; PMID:4059757; http://dx.doi.org/10.1093/clinids/7.5.692
- Timmerman RJ, Thompson J, Noordzij HM, van der Meer JW. Psychogenic periodic fever. Neth J Med 1992; 41:158-60; PMID:1470287
- Miric D, Venet R, Aproh E, Nguyen-Duc H. Psychogenic fever or psychogenic hyperthermia? J Am Geriatr Soc 1997; 45:1287-8; PMID:9329503; http://dx.doi.org/10.1111/j.1532-5415.1997.tb03796.x
- Araki T, Oka T, Oyama N, Akamine M, Kubo C. A case of psychogenic fever treated successfully with art therapy. Jpn J Psychosom Med 2004; 44:289-94
- Nozu T, Uehara A. The diagnoses and outcomes of patients complaining of fever without any abnormal findings on diagnostic tests. Intern Med 2005; 44:901-2; PMID:16157998; http://dx.doi.org/10.2169/internalmedicine.44.901
- Kura N, Oka T, Ando T, Ishikawa T, Kubo C, Ago Y. A case of psychogenic fever treated successfully with tandospirone in combination with psychotherapy and autogenic training. Jpn J Psychosom Med 2004; 44:297-303
- Hiramoto T, Oka T, Yoshihara K, Kubo C. Pyrogenic cytokines did not mediate a stress interview-induced hyperthermic response in a patient with psychogenic fever: a case report. Psychosom Med 2009; 71:932-6; PMID:19875636; http://dx.doi.org/10.1097/PSY.0b013e3181bfb02b
- Oka T, Kanemitsu Y, Sudo N, Hayashi H, Oka K. Psychological stress contributed to the development of low-grade fever in a patient with chronic fatigue syndrome: a case report. Biopsychosoc Med 2013; 7:7; PMID:23497734; http://dx.doi.org/10.1186/1751-0759-7-7
- Oka T, Oka K. Age and gender differences of psychogenic fever: a review of the Japanese literature. Biopsychosoc Med 2007; 1:11; PMID:17511878; http://dx.doi.org/10.1186/1751-0759-1-11
- Kaneda Y, Tsuji S, Oka T. Age distribution and gender differences in psychogenic fever patients. Biopsychosoc Med 2009; 3:6; PMID:19379524; http://dx.doi.org/10.1186/1751-0759-3-6
- Friedmann M, Kohnstmann O. Zur Pathogenese und Psychotherapie bei Basodowsher Krankheit. Ztschr f d ges Neurol u Psychiat 1914; 23:357; http://dx.doi.org/10.1007/BF02867687
- Falcon-Lesses M, Proger SH. Psychogenic fever. N Engl J Med 1930; 203:1034-6; http://dx.doi.org/10.1056/NEJM193011202032113
- Bakwin H. Psychogenic fever in infants. Am J Dis Child 1944; 67:176-81
- Kintner AR, Rowntree LG. Long continued, low grade, idiopathic fever. JAMA 1934;102:889-92; http://dx.doi.org/10.1001/jama.1934.02750120001001
- Wolf S, Wolf HD. Intermittent fever of unknown origin. Arch Internal Med 1942;70:293-302; http://dx.doi.org/10.1001/archinte.1942.00200200113007
- Singer R, Harker CT, Vander AJ, Kluger MJ. Hyperthermia induced by open-field stress is blocked by salicylate. Physiol Behav 1986; 36:1179-82; PMID:3725924; http://dx.doi.org/10.1016/0031-9384(86)90497-X
- Soszynski D, Kozak W, Kluger MJ. Endotoxin tolerance does not alter open field-induced fever in rats. Physiol Behav 1998; 63:689-92; PMID:9523916; http://dx.doi.org/10.1016/S0031-9384(97)00515-5
- Butterweck V, Prinz S, Schwaninger M. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav Brain Res 2003; 144:49-56; PMID:12946594; http://dx.doi.org/10.1016/S0166-4328(03)00059-7
- Long NC, Vander AJ, Kunkel SL, Kluger MJ. Antiserum against tumor necrosis factor increases stress hyperthermia in rats. Am J Physiol 1990; 258:R591-5; PMID:2316707
- Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J Physiol 1993; 460:221-9; PMID:8487193; http://dx.doi.org/10.1113/jphysiol.1993.sp019468
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 2003; 551:945-54; PMID:12837930; http://dx.doi.org/10.1113/jphysiol.2003.048140
- Shibata H, Nagasaka T. Contribution of nonshivering thermogenesis to stress-induced hyperthermia in rats. Jpn J Physiol 1982; 32:991-5; PMID:7169705; http://dx.doi.org/10.2170/jjphysiol.32.991
- Shibata H, Nagasaka T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn J Physiol 1984; 34:103-11; PMID:6727066; http://dx.doi.org/10.2170/jjphysiol.34.103
- Gao B, Kikuchi-Utsumi K, Ohinata H, Hashimoto M, Kuroshima A. Repeated immobilization stress increases uncoupling protein 1 expression and activity in Wistar rats. Jpn J Physiol 2003; 53:205-13; PMID:14529581; http://dx.doi.org/10.2170/jjphysiol.53.205
- Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 2008; 11:125-33; PMID:18311601; http://dx.doi.org/10.1080/10253890701638303
- Borsini F, Lecci A, Volterra G, Meli A. A model to measure anticipatory anxiety in mice? Psychopharmacology (Berl) 1989; 98:207-11; PMID:2502791; http://dx.doi.org/10.1007/BF00444693
- Zethof TJ, Van der Heyden JA, Tolboom JT, Olivier B. Stress-induced hyperthermia as a putative anxiety model. Eur J Pharmacol 1995; 294:125-35; PMID:8788424; http://dx.doi.org/10.1016/0014-2999(95)00520-X
- Vinkers CH, van Bogaert MJ, Klanker M, Korte SM, Oosting R, Hanania T, Hopkins SC, Olivier B, Groenink L. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur J Pharmacol 2008; 585:407-25; PMID:18420191; http://dx.doi.org/10.1016/j.ejphar.2008.02.097
- Beig MI, Baumert M, Walker FR, Day TA, Nalivaiko E. Blockade of 5-HT2A receptors suppresses hyperthermic but not cardiovascular responses to psychosocial stress in rats. Neuroscience 2009;159: 1185-91; PMID:19356699; http://dx.doi.org/10.1016/j.neuroscience.2009.01.038
- Hayashida S, Oka T, Mera T, Tsuji S. Repeated social defeat stress induces chronic hyperthermia in rats. Physiol Behav 2010; 101:124-31; PMID:20438740; http://dx.doi.org/10.1016/j.physbeh.2010.04.027
- Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 2011; 34:1442-52; PMID:21978215; http://dx.doi.org/10.1111/j.1460-9568.2011.07863.x
- Lkhagvasuren B, Oka T, Nakamura Y, Hayashi H, Sudo N, Nakamura K. Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience 2014; 272:34-57; PMID:24797330; http://dx.doi.org/10.1016/j.neuroscience.2014.04.047
- Mohammed M, Ootsuka Y, Blessing W. Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am J Physiol Regul Integr Comp Physiol 2014; 306:R394-400; PMID:24452545; http://dx.doi.org/10.1152/ajpregu.00475.2013
- Yokoi Y. Effect of ambient temperature upon emotional hyperthermia and hypothermia in rabbits. J Appl Physiol 1966; 21:1795-8; PMID:5929304
- Snow AE, Horita A. Interaction of apomorphine and stressors in the production of hyperthermia in the rabbit. J Pharmacol Exp Ther 1982; 220:335-9; PMID:7199085
- Kohlhause S, Hoffmann K, Schlumbohm C, Fuchs E, Flugge G. Nocturnal hyperthermia induced by social stress in male tree shrews: relation to low testosterone and effects of age. Physiol Behav 2011; 104:786-95; PMID:21827778; http://dx.doi.org/10.1016/j.physbeh.2011.07.023
- Schmelting B, Corbach-Sohle S, Kohlhause S, Schlumbohm C, Flugge G, Fuchs E. Agomelatine in the tree shrew model of depression: effects on stress-induced nocturnal hyperthermia and hormonal status. Eur Neuropsychopharmacol 2014; 24:437-47; PMID:23978391; http://dx.doi.org/10.1016/j.euroneuro.2013.07.010
- Pedernera-Romano C, Ruiz de la Torre JL, Badiella L, Manteca X. Effect of perphenazine enanthate on open-field test behaviour and stress-induced hyperthermia in domestic sheep. Pharmacol Biochem Behav 2010; 94:329-32; PMID:19799930; http://dx.doi.org/10.1016/j.pbb.2009.09.013
- Muchlinski AE, Baldwin BC, Padick DA, Lee BY, Salguero HS, Gramajo R. California ground squirrel body temperature regulation patterns measured in the laboratory and in the natural environment. Comp Biochem Physiol A Mol Integr Physiol 1998; 120:365-72; PMID:9773514; http://dx.doi.org/10.1016/S1095-6433(98)10037-5
- Lee BY, Padick DA, Muchlinski AE. Stress fever magnitude in laboratory-maintained California ground squirrels varies with season. Comp Biochem Physiol A Mol Integr Physiol 2000; 125:325-30; PMID:10794961; http://dx.doi.org/10.1016/S1095-6433(00)00157-4
- Parr LA, Hopkins WD. Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiol Behav 2000; 71:363-71; PMID:11150569; http://dx.doi.org/10.1016/S0031-9384(00)00349-8
- Meyer LC, Fick L, Matthee A, Mitchell D, Fuller A. Hyperthermia in captured impala (Aepyceros melampus): a fright not flight response. J Wildl Dis 2008; 44:404-16; PMID:18436672; http://dx.doi.org/10.7589/0090-3558-44.2.404
- Gray DA, Maloney SK, Kamerman PR. Restraint increases afebrile body temperature but attenuates fever in Pekin ducks (Anas platyrhynchos). Am J Physiol Regul Integr Comp Physiol 2008; 294:R1666-71; PMID:18337310; http://dx.doi.org/10.1152/ajpregu.00865.2007
- Bittencourt Mde A, Melleu FF, Marino-Neto J. Stress-induced core temperature changes in pigeons (Columba livia). Physiol Behav 2015; 139:449-58; PMID:25479572; http://dx.doi.org/10.1016/j.physbeh.2014.11.067
- Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med 2001; 63:476-86; PMID:11382276; http://dx.doi.org/10.1097/00006842-200105000-00018
- Vinkers CH, Groenink L, van Bogaert MJ, Westphal KG, Kalkman CJ, van Oorschot R, Oosting RS, Olivier B, Korte SM. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav 2009; 98:37-43; PMID:19375439; http://dx.doi.org/10.1016/j.physbeh.2009.04.004
- Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E(2)-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol 2002; 283:R1104-17; PMID:12376404; http://dx.doi.org/10.1152/ajpregu.00347.2002
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 2004; 9:1977-93; PMID:14977603; http://dx.doi.org/10.2741/1383
- Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, et al. Cellular and molecular bases of the initiation of fever. PLoS Biol 2006; 4:e284; PMID:16933973; http://dx.doi.org/10.1371/journal.pbio.0040284
- Romanovsky AA, Steiner AA, Matsumura K. Cells that trigger fever. Cell Cycle 2006; 5:2195-7; PMID:16969135; http://dx.doi.org/10.4161/cc.5.19.3321
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci 2001; 21:2669-77; PMID:11306620
- Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 2003; 6:1137-8; PMID:14566340; http://dx.doi.org/10.1038/nn1137
- Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 2003; 8:s1321-9; PMID:12957837; http://dx.doi.org/10.2741/1211
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 1991; 71:93-127; PMID:1986393
- Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol 2002; 92:2648-55; PMID:12015385; http://dx.doi.org/10.1152/japplphysiol.01005.2001
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 2004; 24:5370-80; PMID:15190110; http://dx.doi.org/10.1523/JNEUROSCI.1219-04.2004
- Rathner JA, Madden CJ, Morrison SF. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol 2008; 295:R343-54; PMID:18463193; http://dx.doi.org/10.1152/ajpregu.00115.2008
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci 2003; 23:4657-66; PMID:12805305
- Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol 2006; 572:569-83; PMID:16484305; http://dx.doi.org/10.1113/jphysiol.2005.102400
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 2011; 589:3641-58; PMID:21610139; http://dx.doi.org/10.1113/jphysiol.2011.210047
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 2002; 22:4600-10; PMID:12040067
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 2007; 10:1131-3; PMID:17676060; http://dx.doi.org/10.1038/nn1949
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011; 16:74-104; PMID:21196160; http://dx.doi.org/10.2741/3677
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci 2006; 126–127:106-19; PMID:16580890; http://dx.doi.org/10.1016/j.autneu.2006.02.010
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007; 292:R47-63; PMID:16959861; http://dx.doi.org/10.1152/ajpregu.00498.2006
- Kataoka N, Hioki H, Kaneko T, Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 2014; 20:346-58; PMID:24981837
- Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol 2005; 288:R1100-7; PMID:15677520; http://dx.doi.org/10.1152/ajpregu.00872.2004
- Lecci A, Borsini F, Volterra G, Meli A. Pharmacological validation of a novel animal model of anticipatory anxiety in mice. Psychopharmacology (Berl) 1990; 101:255-61; PMID:1971957; http://dx.doi.org/10.1007/BF02244136
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Olivier B. Stress-induced hyperthermia in mice: effects of flesinoxan on heart rate and body temperature. Eur J Pharmacol 2000; 400:59-66; PMID:10913585; http://dx.doi.org/10.1016/S0014-2999(00)00387-3
- Olivier B, Bouwknecht JA, Pattij T, Leahy C, van Oorschot R, Zethof TJ. GABAA-benzodiazepine receptor complex ligands and stress-induced hyperthermia in singly housed mice. Pharmacol Biochem Behav 2002; 72:179-88; PMID:11900786; http://dx.doi.org/10.1016/S0091-3057(01)00759-6
- Pecoraro N, de Jong H, Ginsberg AB, Dallman MF. Lesions of the medial prefrontal cortex enhance the early phase of psychogenic fever to unexpected sucrose concentration reductions, promote recovery from negative contrast and enhance spontaneous recovery of sucrose-entrained anticipatory activity. Neuroscience 2008; 153:901-17; PMID:18455879; http://dx.doi.org/10.1016/j.neuroscience.2008.03.043
- Pae YS, Lai H, Horita A. Hyperthermia in the rat from handling stress blocked by naltrexone injected into the preoptic-anterior hypothalamus. Pharmacol Biochem Behav 1985; 22:337-9; PMID:4039067; http://dx.doi.org/10.1016/0091-3057(85)90400-9
- Egawa M, Yoshimatsu H, Bray GA. Preoptic area injection of corticotropin-releasing hormone stimulates sympathetic activity. Am J Physiol 1990; 259:R799-806; PMID:2221147
- Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, Olivier B. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiol Behav 2010; 99:395-401; PMID:20006965; http://dx.doi.org/10.1016/j.physbeh.2009.12.006
- Ootsuka Y, Mohammed M. Activation of the habenula complex evokes autonomic physiological responses similar to those associated with emotional stress. Physiol Rep 2015; 3:e12297; PMID:25677551; http://dx.doi.org/10.14814/phy2.12297
- Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol 2010; 588:4117-29; PMID:20807795; http://dx.doi.org/10.1113/jphysiol.2010.195099
- Eikelboom R. Learned anticipatory rise in body temperature due to handling. Physiol Behav 1986; 37:649-53; PMID:3749329; http://dx.doi.org/10.1016/0031-9384(86)90299-4
- Pardon MC, Kendall DA, Perez-Diaz F, Duxon MS, Marsden CA. Repeated sensory contact with aggressive mice rapidly leads to an anticipatory increase in core body temperature and physical activity that precedes the onset of aversive responding. Eur J Neurosci 2004; 20:1033-50; PMID:15305872; http://dx.doi.org/10.1111/j.1460-9568.2004.03549.x
- Kant GJ, Bauman RA, Pastel RH, Myatt CA, Closser-Gomez E, D'Angelo CP. Effects of controllable vs. uncontrollable stress on circadian temperature rhythms. Physiol Behav 1991; 49:625-30; PMID:2062941; http://dx.doi.org/10.1016/0031-9384(91)90289-Z
- Endo Y, Shiraki K. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiol Behav 2000; 71:263-8; PMID:11150557; http://dx.doi.org/10.1016/S0031-9384(00)00339-5
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 2006; 18:13-24; PMID:16451216; http://dx.doi.org/10.1111/j.1365-2826.2005.01375.x
- Nozu T, Okano S, Kikuchi K, Yahata T, Kuroshima A. Effect of immobilization stress on in vitro and in vivo thermogenesis of brown adipose tissue. Jpn J Physiol 1992; 42:299-308; PMID:1434095; http://dx.doi.org/10.2170/jjphysiol.42.299
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flugge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol 2008; 19:183-96; PMID:18469536; http://dx.doi.org/10.1097/FBP.0b013e3282fe8871
- Marks A, Vianna DM, Carrive P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am J Physiol Regul Integr Comp Physiol 2009; 296:R1239-47; PMID:19211724; http://dx.doi.org/10.1152/ajpregu.90723.2008
- Carrive P, Gorissen M. Premotor sympathetic neurons of conditioned fear in the rat. Eur J Neurosci 2008; 28:428-46; PMID:18702716; http://dx.doi.org/10.1111/j.1460-9568.2008.06351.x
- Kuroshima A, Habara Y, Uehara A, Murazumi K, Yahata T, Ohno T. Cross adaption between stress and cold in rats. Pflugers Arch 1984; 402:402-8; PMID:6522247; http://dx.doi.org/10.1007/BF00583941
- Kuroshima A, Yahata T. Changes in the colonic temperature and metabolism during immobilization stress in repetitively immobilized or cold-acclimated rats. Jpn J Physiol 1985; 35:591-7; PMID:4068366; http://dx.doi.org/10.2170/jjphysiol.35.591
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience 2007; 146:1388-99; PMID:17433555; http://dx.doi.org/10.1016/j.neuroscience.2007.02.043
- Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 2012; 22:1442-54; PMID:21878486; http://dx.doi.org/10.1093/cercor/bhr229
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 2013; 23:1784-97; PMID:22710611; http://dx.doi.org/10.1093/cercor/bhs151
- Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol 2013; 254:161-4; PMID:22944319; http://dx.doi.org/10.1016/j.jneuroim.2012.08.007
- Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 β induces hyperalgesia in rats. Brain Res 1993; 624:61-8; PMID:8252417; http://dx.doi.org/10.1016/0006-8993(93)90060-Z
- Oka T, Oka K, Hosoi M, Hori T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res 1995; 692:123-8; PMID:8548295; http://dx.doi.org/10.1016/0006-8993(95)00691-I
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27:24-31; PMID:16316783; http://dx.doi.org/10.1016/j.it.2005.11.006
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:760-8; PMID:20600462; http://dx.doi.org/10.1016/j.pnpbp.2010.06.020
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65:732-41; PMID:19150053; http://dx.doi.org/10.1016/j.biopsych.2008.11.029
- Wynn FR. The psychic factor as an element in temperature disturbance. JAMA 1919; 73:31-4; http://dx.doi.org/10.1001/jama.1919.02610270035010
- Kleitman N. The effect of motion pictures on body temperature. Science 1945; 102:430-1; PMID:17730629; http://dx.doi.org/10.1126/science.102.2652.430
- Gotsev T, Ivanov A. Psychogenic elevation of body temperature in healthy persons. Acta Physiol Hung 1950; 1:53-62; PMID:14782995
- Aschoff J, Fatranska M, Gerecke U, Giedke H. Twenty-four-hour rhythms of rectal temperature in humans: effects of sleep-interruptions and of test-sessions. Pflugers Arch 1974; 346:215-22; PMID:4856415; http://dx.doi.org/10.1007/BF00595708
- Renbourn ET. Body temperature and pulse rate in boys and young men prior to sporting contests. A study of emotional hyperthermia: with a review of the literature. J Psychosom Res 1960; 4:149-75; PMID:14437326; http://dx.doi.org/10.1016/0022-3999(60)90008-8
- Marazziti D, Di Muro A, Castrogiovanni P. Psychological stress and body temperature changes in humans. Physiol Behav 1992; 52:393-5; PMID:1326118; http://dx.doi.org/10.1016/0031-9384(92)90290-I
- Briese E. Emotional hyperthermia and performance in humans. Physiol Behav 1995; 58:615-8; PMID:8587973; http://dx.doi.org/10.1016/0031-9384(95)00091-V
- Shah SJ, PAtel HM. Effect of examination stress on parameters of autonomic functions in medical students. Int J Sci Res 2014; 3:273-6
- Vinkers CH, Penning R, Hellhammer J, Verster JC, Klaessens JH, Olivier B, Kalkman CJ. The effect of stress on core and peripheral body temperature in humans. Stress 2013; 16:520-30; PMID:23790072; http://dx.doi.org/10.3109/10253890.2013.807243
- Oka T, Kaneda Y, Takenaga M, Hayashida S, Tamagawa Y, Kodama N, Tsuji S. Efficacy of paroxetine for treating chronic stress-induced low-grade fever. Jpn J Psychosom Intern Med 2006; 10:5-8
- Reimann HA. The problem of long, continued, low grade fever. JAMA 1936;107:1089-94; http://dx.doi.org/10.1001/jama.1936.02770400001001
- Ziegler LH, Cash PT. A study of the influence of emotions and affects on the surface temperature of the human body. Am J Psychiatr 1938;95:677-96; http://dx.doi.org/10.1176/ajp.95.3.677
- Duras FP. Hyperpyrexia due to hysteria. Lancet 1942; 11:39; http://dx.doi.org/10.1016/S0140-6736(00)62135-9
- Oka T, Oka K. Mechanisms of psychogenic fever. Adv Neuroimmune Biol 2012; 3:3-17
- Bohorfoush JG, Craig JB, Patterson HS. Catatonia as a cause of fever of undetermined origin. J Med Assoc Ga 1965; 54:324-5; PMID:5887902
- Buchwald D, Goldenberg DL, Sullivan JL, Komaroff AL. The “chronic, active Epstein-Barr virus infection” syndrome and primary fibromyalgia. Arthritis Rheum 1987; 30:1132-6; PMID:2823835; http://dx.doi.org/10.1002/art.1780301007
- Enerback S. Human brown adipose tissue. Cell Metab 2010; 11:248-52; PMID:20374955; http://dx.doi.org/10.1016/j.cmet.2010.03.008
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360:1500-8; PMID:19357405; http://dx.doi.org/10.1056/NEJMoa0808718
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509-17; PMID:19357406; http://dx.doi.org/10.1056/NEJMoa0810780
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360:1518-25; PMID:19357407; http://dx.doi.org/10.1056/NEJMoa0808949
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58:1526-31; PMID:19401428; http://dx.doi.org/10.2337/db09-0530
- Lkhagvasuren B, Masuno T, Kanemitsu Y, Sudo N, Kubo C, Oka T. Increased prevalence of postural orthostatic tachycardia syndrome in psychogenic fever patients. Psychother Psychosom 2013; 82:269-70; PMID:23735890; http://dx.doi.org/10.1159/000345171
- Lkhagvasuren B, Tanaka H, Sudo N, Kubo C, Oka T. Characteristics of the orthostatic cardiovascular response in adolescent patients with psychogenic fever. Psychother Psychosom 2014; 83:318-9; PMID:25116930; http://dx.doi.org/10.1159/000360999
- Oka T. Mechanism and treatment of psychogenic fever. Jpn J Psychosom Intern Med 2005; 9:117-21.
- Oka T, Hayashida S, Kaneda Y, Kodama N, Hashimoto T, Tsuji S. Why do chronic stress-induced hyperthermia patients worry about slightly elevated body temperature? Jpn J Psychosom Intern Med 2006; 10:243-6
- Oka T. Influence of psychological stress on chronic fatigue syndrome. Adv Neuroimmune Biol 2013; 4:301-9
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA: Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One 2006; 1:e1; PMID:17183631; http://dx.doi.org/10.1371/journal.pone.0000001