Abstract
Nausea and vomiting are among the most basic of human experiences and unfortunately accompany a wide variety of clinical treatments as side effects. Despite decades of research, the neural mechanisms of nausea and vomiting (emesis) remain elusive. TRPV1 represents a possibly overlooked and understudied pharmacological target with anti-emetic potential.
Abbreviations
| TRPV1 | = | transient receptor potential vanilloid receptors |
| CINV | = | chemotherapy-induced nausea and vomiting |
| 5-HT3 | = | serotonin type-3 |
| NK-1 | = | neurokinin-1 |
| RTX | = | resiniferatoxin |
| SP | = | substance P |
| CTA | = | conditioned taste aversion. |
This Editorial Comment focuses on the recent article reviewing the role of TRPV1 in emesis and anti-emesis by John Rudd and Christina Wan (Hong Kong), Eugene Nalivaiko (Australia), Paul Andrews (UK), and Norio Matsuki (Japan).Citation1 In this comprehensive review, the authors summarize several animal models of emesis targeting TRPV1 and in doing so highlight a greater issue in the development of new anti-emetic drugs, namely that translatability and species congruency, even within closely-related species, are not always straightforward.
In a generalized pharmaceutical approach to drug development, it is not uncommon for thousands of initially screened compounds to move through a systematic process of elimination whereby the models for drug therapeutic targets become increasingly more complex, financially burdensome, and dependent on large numbers of animals. The same type of systematic analysis for emetic research does not exist. To this end, the study of nausea and vomiting, from the perspective of pharmacological safety, requires more robust and systematic attention, as these side effects can reduce quality of life and adherence to curative therapies; and nausea and vomiting are also off-target effects that limit drug development.Citation2,3 Despite such obvious importance, nausea and vomiting mechanisms remain largely understudied in research globally.
There are 2 key questions in the translational relevance and determination of anti-emetic efficacy following drug treatment: 1) which species model(s) is most appropriately concordant with clinical outcomes?, and 2) how can we best systematically assess the emetic and/or anti-emetic properties of a drug?
Historically, the ferret has been the focus of preclinical work in anti-emetic drug development because it is a less expensive and more available species compared to the dog and cat. Research on the ferret produced significant advances in the treatment of CINV via the introduction of 5-HT3 antagonists in the early 1990s.Citation4 The 5-HT3 antagonists provided strong efficacy to reduce vomiting within 24 hours (acute phase) of chemotherapy and radiotherapy, while in parallel illustrating that the delayed phase of emesis was much less well controlled, which led to the development of NK-1 receptor antagonists for delayed emesis and role of SP in these responses, often using the ferret model.
The current review from Rudd et al. in this issue explores numerous hypotheses and experiments summarizing the role of vanilloids, and more specifically the central TRPV1 receptor and a naturally occurring agonist RTX, in emesis and anti-emesis in several vomiting models. The authors highlight the need for a systematic study of emesis and accompanying behaviors in several species in order to draw sound conclusions on a specific neurochemical target. In particular, RTX is discussed in the context of species differences in emesis induction which point to the need for greater elucidation of RTX mechanism(s) of action.
When injected subcutaneously, varying doses of RTX can induce emesis in the musk shrew (Suncus murinus), but not the dog or the ferret; critically, it was the work of Prof. Matsuki and his colleagues in Japan who were instrumental in establishing the use of Suncus in emesis research.Citation5 It is presumed that these behavioral differences are due to a relatively higher permeability of the blood-brain barrier in the shrew to RTX, and thus a more robust activation of hindbrain TPRV1 receptors. Intriguingly, the emetic effect of RTX likely involves SP/NK-1 signaling as RTX has been in shown in shrew brainstem slices to induce release of SP, and in addition, RTX-induced emesis can be blocked by NK-1 antagonists.
From an anti-emesis perspective, RTX may also show therapeutic potential in the reduction or blockade of emesis where SP/Nk-1 receptor signaling is implicated. While seemingly paradoxical at first, the authors' suggest that given TRPV-1 activation in the brainstem induces robust SP release, a congruent speculative notion is that SP “depletion” in the brainstem following TRPV1 activation would create a neurochemical environment where subsequent emetic stimuli would show reduced or absent induction of emesis; indeed, as the authors present in Table 1, this may partially clarify the “broad spectrum” anti-emetic effect of RTX. Long-term down-regulation of SP signaling would help to explain the anti-emetic property of RTX pretreatment.
In a larger context, the discussion by Rudd et al. helps to inform the process of research as much as delineates the pathways of emesis. Essentially, the program of research discussed tells us that single species approaches to emesis research (even vomiting species) offer limited translational potential. In order to effectively increase translational potential we need to examine the findings of several pre-clinical animal models of emesis, multiple species and behavioral approaches. Given the resources required to do so, the field has long faced a dilemma of a lack of systematic approach to advancement. Indeed, the data summarized in the current review span decades, attempting to construct a detailed picture with fragments spread across the literature.
Emesis and related behaviors, such as CTA and pica (ingestion of a non-nutritive substance; e.g., clay) should be addressed not only by researchers of emesis, but by disciplines researching energy balance and ingestive behavior. For example, rats and mice (rodents) do not vomit, but they are the overwhelming choice for genetic models of obesity, energy balance, cancer, heart disease, etc., and also display behaviors validated as reflecting emesis-associated behavior.Citation6,7 We also lack resolution on many of our models. Use of telemetry and predictive models of emesis are not well-established and could provide the field with greater understanding of behavioral patterns and sequences of emesis, and also offer much needed temporal (and special) resolution in experimental design. The choice of species and specific set of behavioral outcome variables, in addition to the tools used to measure them, is not standard across the field.
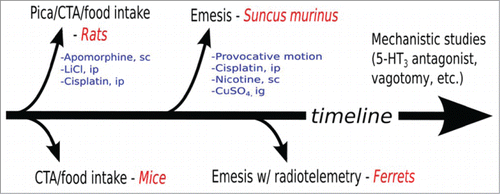
The findings summarized by Rudd et al. here illustrate the importance of these mentioned issues. The study of emesis as a main outcome variable involves a small number of laboratories worldwide, despite enormous clinical and drug discovery impact.Citation3 Establishing a more objective and systematic preclinical development platform for the experimental research of emesis is crucial to developing novel or potentially overlooked substrates of anti-emetic control, such as TRPV1 targets, as discussed by Rudd et al. The authors have listed 4 lessons from their research that may lead to a generalized approach to emesis research, as shown in , including: (1) an emphasis on early testing using rats and mice (non-vomiting species), in CTA or pica studies; (2) the use of multiple emetic stimuli to test activation of different pathways for anti-emetic actions; (3) using Suncus murinus, a small animal model, in the testing pipeline; and (4) implementation of radiotelemetry methods.
Figure 1. A proposed generalized approach timeline to emesis research. Initial research may indicate an emetic liability using rodents (rats and mice) in conditioned taste aversion (CTA) or pica (clay ingestion) experiments. This could be followed by emesis testing in Suncus using stimuli known to activate peripheral and central pathways. Ferrets would be used in later experiments with radio-telemetry to measure abdominal contractions and emesis over potentially a longer time scale. Additional research may proceed with using well established anti-emetic agents and ablation of nerves to test for specific mechanisms. Common test agents and routes of administration are listed (e.g., lithium chloride, LiCl, intraperitoneal, ip; cooper sulfate; nicotine, subcutaneous, sc; CuSO4, intragastric, ig).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rudd JA, et al. Temperature, 2015; 2:258–76; http://dx.doi.org/10.1080/23328940.2015.1043042
- Cavero I. Expert Opin Drug Saf 2015; 14:999-1008; PMID:25872620; http://dx.doi.org/10.1517/14740338.2015.1034104
- Holmes AM, et al. Br J Pharmacol 2009; 157(6):865-80; PMID:19371333; http://dx.doi.org/10.1111/j.1476-5381.2009.00176.x
- Reynolds DJM, et al. Serotonin and the scientific basis of anti-emetic therapy. Oxford, UK: Oxford Clinical Communications, 1995.
- Ueno S, et al. Life Sci, 1987; 41:513-8; http://dx.doi.org/10.1016/0024-3205(87)90229-3
- Andrews PL, et al. Auton Neurosci 2006; 125:100-15; PMID:16556512; http://dx.doi.org/10.1016/j.autneu.2006.01.008
- Kanoski SE, et al. Neuropharmacology 2012; 62:1916-27; PMID:22227019; http://dx.doi.org/10.1016/j.neuropharm.2011.12.022
