Abstract
Heat acclimation results in systemic and cellular adaptions that reduce the negative effect of heat and, consequently, the risk of heat illness. Although the classical changes observed with heat acclimation lead to increased tolerance to exercise in the heat by reducing heat storage (reflected in reduced core and skin temperatures) and increasing whole-body capacity for heat dissipation (greater plasma volume, sweat output, and skin blood flow), it appears that heat acclimation also induces changes at the cellular level that might increase tolerance of the whole organism to a higher core temperature for the development of fatigue. Thermotolerance is a process that involves increased resilience to an otherwise lethal heat stress that follows a sublethal exposure to heat. Thermotolerance is believed to be the result of increased content of heat shock proteins (Hsp), specially a member of the 70 kDa family, Hsp72 kDa. In humans, we and others have reported that heat acclimation increases intracellular Hsp72 levels. This increase in intracellular Hsp72 could improve whole-body organism thermotolerance by maintaining intestinal epithelial tight junction barriers, by increasing resistance to gut-associated endotoxin translocation, or by reducing the inflammatory response. In this review, we will initially provide an overview of the physiological adaptations induced by heat acclimation and emphasize the main cellular changes that occur with heat acclimation associated with intracellular accumulation of Hsp72. Finally, we will present an argument for a role of whole-body heat acclimation in augmenting cellular thermotolerance, which may protect vital organs from deleterious effects of heat stress in humans.
Abbreviations
| Apaf-1 | = | Apoptotic protease activating factor 1 |
| FiO2 | = | fraction of inspired oxygen |
| HSE | = | Heat Shock Element |
| HSF-1 | = | heat shock transcription factor |
| HSP | = | heat shock proteins |
| Hsp70 | = | heat shock protein 70-kDa |
| IkB | = | Inhibitor kappa B |
| IL-1 | = | interleukin 1 |
| IL-12 | = | 12 interleukin |
| IL-18 | = | 18 interleukin |
| NF-κB | = | nuclear factor kappa B |
| PBMC | = | peripheral blood mononuclear cells |
| TNF-α | = | tumor necrosis factor alpha |
| VO2max | = | maximum oxygen consumption |
Introduction
Heat acclimation or acclimatization results from chronic exposure to heat stress, generally resulting in physiological and functional changes that reduce the negative effect of heat and, consequently, the risk of heat illness (for a review see ref. 1 and 2). The classical changes observed with heat acclimation includes a series of physiological adaptions such as higher and earlier sweating and skin blood flow (for a given core temperature), lowered body temperatures (resting and exercise), improved body fluid and electrolyte balance.Citation3 Although heat acclimation is known to reduce heat storage by increasing body heat dissipation capacity and tolerance to exercise in the heat with less strain, it is not well investigated the impact of heat acclimation on cellular adaptation and thermotolerance in humans.Citation4 In isolated cellsCitation5 and animal studies,Citation6 thermotolerance (also called heat preconditioning) is characterized as a process that involves increased resilience to an otherwise lethal heat stress that follows a sublethal exposure to heat. Thermotolerance is believed to be the result of increased content of heat shock proteins (Hsp), specially a member of the 70 kDa family, Hsp72 kDa.Citation5 In humans, weCitation7-9 and othersCitation10-16 have reported that heat acclimation increases intracellular Hsp72 levels. This increase in intracellular Hsp72 could improve whole-body organism tolerance to exercise in hot environment (mirroring thermotolerance) by maintaining intestinal epithelial tight junction barriers, by increasing resistance to gut-associated endotoxin translocation, or by reducing the inflammatory response. This review will initially provide a brief overview of the physiological adaptations induced by heat acclimation, and subsequently emphasize the cellular changes that occur with heat acclimation associated with intracellular accumulation of Hsp72. Finally, this review will present an argument for a role of whole-body heat acclimation in augmenting cellular thermotolerance, which may protect vital organs from deleterious effects of heat stress in humans.
Methods
A literature search was done employing the PubMed and MEDLINE database using a combination of the following keywords: “heat acclimation," “cellular adaptations," “heat shock protein," “hsp72," and “thermotolerance." Papers from the authors personal collections were included if they suited the search parameters. Inclusion and exclusion criteria for manuscripts were defined before the search was conducted. This review will address only human heat acclimation studies.
Heat acclimation
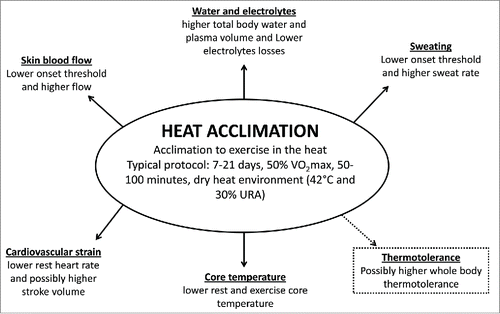
Heat acclimation or acclimatization is a process of adaptation resulting from frequent, continuous or intermittent heat exposures, in natural or artificial environments (e.g., controlled laboratory setting), respectively, which cause transient increases in core and skin temperatures and promote moderate to profuse sweating.Citation17 Heat acclimation can be induced by passive exposure to hot environments, or by exercise in hot environments.Citation18 The magnitude of heat acclimation adaptations depends on environmental characteristics (dry versus wet heat), intensity, duration, frequency, and number of heat exposures.Citation3 Protocols involving repeated exposure to moderate intensity exercise in the heat are often usedCitation1 and thus in this review we will refer to this process as acclimation to exercise in the heat.
Heat acclimated humans have a higher tolerance to exercise in the heat usually expressed by a series of physiological changes: 1) lower cardiovascular strain, indicated by reduced heart rate for a given workloadCitation17,19,Citation20; 2) cutaneous vasodilatation begins at a lower core temperature threshold, and skin blood flow is higher for a given core temperature, which may enhance core-to-skin heat transferCitation21; 3) sweating begins at a lower core temperature threshold and there is an increase in sweat rate per degree rise in core temperature, increasing the potential for evaporative heat loss and reducing core and skin temperaturesCitation17,21; 4) less accumulation of body heat during exercise performed at the same relative intensity and environment before acclimationCitation1,17,Citation20; 5) a possible increase in stroke volumeCitation17,23; 6) an increase in plasma volumeCitation24; 7) a decrease in sweat sodium concentration lossCitation17,20 (). Together all these adjustments increase exercise tolerance in the heat. For a detailed review on the effects of heat acclimation on exercise performance in the heat as well as on the physiological mechanisms involved (for a review see refs. 1, 2, and 25).
However, less investigated is the change induced by whole-body heat acclimation to tolerate higher core temperature during exercise, postponing heat-induced fatigue. The only studyCitation23 that investigated the effects of heat acclimation on final core temperature observed that heat acclimation did not change the final core temperature (∼39.8°C) when endurance trained athletes reached voluntary fatigue.Citation The authors justified that high core temperature was the critical factor for fatigue in heat stress, both before and after heat acclimation. However, it is worth observing that there was no reduction in muscle or skin blood flow, lactate concentration or ability to recruit motor units measured at the point of fatigue during a maximal voluntary contraction test. Additionally, the final heart rate was lower after (153 ± 6 bpm) than before (164 ± 6 bpm) heat acclimation. It might be speculated that the lack of motivation associated with heat stress could have induced a prematurely exercise termination and not core temperature.
Although it is not clear, heat acclimated subjects may tolerate higher core temperature, delaying heat-induced fatigue development and thermal injury.Citation3 Whereas untrained subjects seem to tolerate lower levels of exercise-induced hyperthermia and develop fatigue symptoms with an average core temperature around 38–39°C,Citation31 highly trained individuals (supposedly also heat acclimated) may tolerate core temperature around 40°C, with individual values over 41°C being reported in the literature.Citation28,32 Our speculation is based on previous reports, in which individuals who frequently perform exercise in the heat (assumed as heat acclimated), for example marathon runners and cyclists athletes, can achieve and tolerate higher core temperatures (>41°C) with no symptoms of heat illness.Citation26-30 This capacity is not only related to high heat dissipation and lower heat storage, but it appears to result from additional adaptations that allow cells and tissues to function with high internal temperatures, resembling the experimental thermotolerance observed in cell studies.
Intracellular heat shock proteins
On a cellular level, temporary modifications in gene expression to cope with stress (heat, oxidative stress and hypoxia) have been attributed to HSP. The HSP are a highly conserved group of proteins expressed in both prokaryote and eukaryote organisms. These proteins are classified by their molecular weight (ranging from 27 to 110 kDa) and grouped into families (for a review see ref. 33). They are found in different cellular compartments and play key roles in physiological conditions and cellular stress, involving both individual cellsCitation4 and the whole-body organism.Citation34 In response to cellular stressors, such as direct effect of heat, HSP gene transcription is activated and intracellular levels of HSP accumulate. Although the precise function of each HSP family member is still being uncovered, some studies have shown that HSPs are involved in many regulatory pathways (protein trafficking across cell membrane, cell apoptosis prevention, and immune function) and behave as molecular chaperones for other cellular proteins.Citation33
The 70-kDa-family (HSP70) consists of proteins with a molecular mass of 72, 73, 75, and 78-kDa. The 72-kDa family member (Hsp72) is the most heat sensitive and highly inducible HSP. In unstressed conditions, heat shock transcription factor (HSF-1) is maintained in a monomeric state, attached to Hsp72 in the cytoplasm. In stressful conditions, such as heat shock, thermally denatured and malfolded proteins can accumulate in the cytoplasm, inducing the dissociation of the HSF-Hsp72 complex. The free Hsp72 then binds to the denatured proteins and facilitates refolding to restore cellular homeostasis by maintaining the cytoskeletal structure and ensuring cell function and survivability. The unbound HSF-1 monomer will trimerize, be phosphorylated, and migrate to the nucleus where it will attach to the Heat Shock Element (HSE) located in the promoter region of heat shock protein genes, leading to increase in transcription and expression of mRNA of heat shock genes. Protein translation will increase intracellular levels of Hsp72.Citation35 This increase in intracellular concentration of Hsp72 can result in a state of thermotolerance, which has been described as the ability to induce transient resistance to an otherwise lethal heat stress. The thermotolerance process consists of first exposing a cell or whole organism to a sublethal heat shock, which makes it tolerant to a second otherwise lethal heat exposure. This thermotolerant state correlates with the level of intracellular Hsp72.Citation5 Interestingly, the induction of Hsp72 is associated with an increased resistance to other stressors as well, including hypoxia, acidosis, ischemia-reperfusion, and reactive oxygen species.Citation33
During extreme heat stress it is proposed that heat exposure inhibits gene expression, altering transcriptional and translational kinetics in the cell.Citation36 Also proteins are partially denatured, exposing hydrophobic sites, which interact to form insoluble aggregates.Citation37 It is suggested that thermotolerant cells present an accelerated translational activity, increased Hsp72 synthesis, and a more rapid recovery to the normal 37°C translational pattern, as compared to non-heat tolerant cells.Citation35 Liu et al.Citation38 demonstrated that the expression of human Hsp72 in Rat-1 fibroblast cells facilitates the ability of these cells to recover from heat-induced inhibition in protein and RNA synthesis. After heating the cells to 45°C for 25 min, the time required for the recovery of normal RNA and protein synthesis was considerably shorter in cells with higher levels of Hsp72 compared to control cells.
Although Hsp72 is known to function as a protein chaperone, studies have shown that Hsp72 also can inhibit cellular apoptosis.Citation39,40 In this regard, Hsp72 inhibits caspase-dependent and caspase-independent apoptotic stimuli. Hsp72 has been reported to block stress kinases, including JNK, the formation and activation of the Apaf-1 complex and, the subsequent activation of caspase-9.Citation40 Li and colleaguesCitation41 investigated the effect of Hsp72 on apoptotic processes using heat shocked cells (42°C for 30 min) and transfected cells over-expressing Hsp72 during in vitro experiments. After a lethal heat shock treatment (45°C for 60-80 min), the authors observed that Caspase-3 cleavage and DNA fragmentation were detected in cytosolic fractions from control cells, but not from thermotolerant cells or gene-transfected cells. Moreover, the addition of purified recombinant Hsp72 to normal cytosolic fractions prevented caspase-3 cleavage and DNA fragmentation, suggesting that Hsp72 prevents apoptosis upstream of caspase-3 processing. The authors concluded that Hsp72 acts as a strong suppressor of apoptosis acting downstream of cytochrome c release and upstream of caspase-3 activation.
Induction of Hsp72 may also alter proteins and genes recognized to be involved in inflammatory responses. The nuclear factor kappa B (NF-κB) transcription factor is involved in immune and inflammatory responses, altering the expression of cytokines, chemokines, cell adhesion molecules, growth factors, and immunoreceptors.Citation42 Inactive NF-κB is normally found in the cytoplasm bound to its inhibitory protein, IκB (Inhibitor kappa B). NF-κB is activated by a number of incoming signals from the cell surface, including ischemia oxidative stress, and endotoxin exposure.Citation40 These signals lead to activation of IκB kinase, which phosphorylates IκB, allowing NF-κB to translocate into the nucleus and bind to its target genes. The targeted genes include those that activate the inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), chemokines, and inducible nitric oxide synthase. It has been speculated that Hsp72 could interact with NF-κB inhibitor protein, IκB, and prevent NF-κB dissociation.Citation40 Jo and colleagues,Citation43 using a model of acute renal failure in rats, demonstrated that ischemia/reperfusion–induced NFκB activation was suppressed by Hsp72 accumulation (42°C for 15 min), with a subsequent decrease in inflammatory mediators. Heat preconditioning also suppressed the accumulation of phosphorylated inhibitory IκB, indicating that Hsp72 blocked the activation of the IκB kinase complex. Induction of Hsp72 by heat shock or Hsp72 over-expression can be an important factor reducing mortality in experimental models of septic shock and heat stroke, by down-regulating expression of inflammatory genes, such as TNF-α, IL-1, interleukin 12 (IL-12), and interleukin 18 (IL-18).Citation44
Cellular adaptation and heat acclimation
As noted above, intracellular Hsp72 plays a critical role in the ability of cells, tissues and whole organisms in becoming thermotolerant. The process of heat acclimation is known for reducing the risk of complications caused by heat exposure.Citation1 Therefore, it has been speculated that Hsp72 might be involved in the adaptations caused by heat acclimation.Citation4 Thus, it is surprising that although heat acclimation reduces thermal stress and avoids heat complications, and is recommended to athletes and workers from occupational activities in the heat such as miners, rural and construction workers, soldiers and firefighters, relatively few studies have investigated intracellular alterations regarding Hsp72 in response to heat acclimation in humans.Citation7-16 An even less studied and perhaps more relevant issue is what are the functional roles that these cellular adaptations play in response to heat acclimation.Citation9,13,Citation14
The results of the studies involving the effects of heat acclimation on intracellular Hsp72 in humans are not consistent (). Some studies did not observe alterations in intracellular Hsp72 after a short period of heat acclimation. Marshall et al.,Citation10 for example, showed no increase in intracellular Hsp72 in peripheral blood mononuclear cells (PBMC) after 2 days of heat acclimation, and Watkins et al.Citation12 did not observe any difference in intracellular Hsp72 in the vastus lateralis muscle after 7 days of 30 minutes/day of heat acclimation. However, the short length protocols, as well as the session duration, might not have been enough to induce adaptations at the cellular level. On the other hand, results from our groupCitation7-9 and from others Citation11-16 have repeatedly shown that longer and more stressful heat acclimations protocols increase intracellular Hsp72 content in humans. For example, McClung et al.Citation11 and Yamada et al.Citation7 used a 10-day, fixed-intensity exercise heat acclimation protocol, and Magalhaes et al.Citation8 used an 11-day, controlled-hyperthermia technique heat acclimation and showed increases in intracellular Hsp72 content in PBMC and total leukocytes. Recently, Gibson et al.Citation45 demonstrated that 3 different heat acclimation protocols (fixed-intensity – 50% maximum oxygen consumption (VO2max), continuous isothermic – targeted 38.5°C, and progressive isothermic targeted ~39.0°C) during short (5 days) and long term (10 days) heat acclimation showed similar changes in leukocyte Hsp72 mRNA expression. Therefore, it appears that to induce cellular changes related to the increased content of Hsp72 in response to heat acclimation, longer (>7 days) heat acclimation protocols should be used.
Table 1. Summary of heat acclimation studies that measured heat shock protein 72 in humans.
To address the question as to whether the increase in intracellular Hsp72 content induced by heat acclimation replicate the thermotolerant state induced in vitro in cells, McClung et al. Citation11 and Amorim et al.Citation9 exposed PBMC before and after heat acclimation to an in vitro heat shock treatment (>42°C) or control temperature (37°C). Both studies showed similar results and described that Hsp72 content in the non-acclimated cells increased more after the heat shock treatment than in the acclimated ones. Similarly, Magalhaes et al.Citation7 described that after 11 days of heat acclimation the basal levels of Hsp72 in total leukocytes was increased and also that the increase in Hsp72 induced by a 90 minutes heat stress test was blunted after the heat acclimation protocol. Altogether, these results suggest that heat acclimation induces a state of thermotolerance involving an increase in baseline Hsp72 content, similar to that observed in cells or experimental animals.
In a more recent study, Gibson et al.Citation16 observed that heat acclimation increased basal Hsp72 mRNA and Hsp90 mRNA expressions and blunted the Hsp72 mRNA increase to a 30 minute hypoxic tolerance test (FiO2 = 0.12; 10 min rest, 10 min of exercise at 40% VO2max, 10 min of exercise at 65% VO2max). Interestingly, Hsp90 mRNA increase was not different comparing pre- and post-heat acclimation to the hypoxic tolerance test. Reductions in Hsp72 mRNA in post-heat acclimation hypoxic test are compatible with the majority of studies showing that heat acclimation leads to accumulation of Hsp72 proteinCitation7,9,Citation11,13-15 and reduced requirements for further gene transcription and mRNA translation. Although it has been demonstrated that heat acclimation also leads to the accumulation of Hsp90 in PBMC,Citation11 and, as stated above, in vitro heat shock led to no further increase in the protein content of Hsp9010, the results from Gibson et al.Citation16 seem to contradict these previous results. It has been demonstrated that Hsp90 basal content was lower than Hsp72,Citation11 and therefore, it can be suggested that basal Hsp72 might have been sufficient to cope with the hypoxic stress post heat acclimation, while further increase in Hsp90 may have been necessary. However, differences in the methods (mRNA expression vs. protein content) also make comparisons between these 2 studies difficult. mRNA content might not necessarily reflect increased translation and augmented protein content,Citation46 and therefore, one cannot exclude the possibility of post-transcriptional regulation of Hsp90 mRNA affecting its translation and protein content. Another explanation for these contradictory results might stem from the difference in the stimuli used for the induction of the heat shock response. While McClung et al.Citation11 applied an in vitro heat shock to PBMC from heat acclimated subjects, Gibson et al.Citation16 used an in vivo hypoxic tolerance test. Therefore, the different nature of these stimuli (whole body hypoxia exposure versus in vitro heat stress) might induce different cellular responses regarding the expression of Hsp72 and Hsp90. Although it has been shown that heat shock and hypoxia/ischemia induce similar changes in expression of various genes,Citation47 including increases in Hsp72 and erythopoietin,Citation48,49 leading to the proposal of the cross-tolerance hypothesis.Citation50 Taylor et al.,Citation51 for example, showed that 10 consecutive days of hypoxic exposures (75 min at 2,980 m) increase monocyte Hsp72 expression. For a detailed review on hypoxia-heat cross-tolerance, please see Ely et al.Citation52
A response unknown until recently was whether the increase in Hsp72 content could be somehow related to the whole-body systemic adaptations observed in response to heat acclimation. Kuennen et al.Citation13 tackled this question using a drug that inhibited the increase in Hsp72 during the heat acclimation protocol (quercetin). In that study, while in the placebo group all classical systemic adaptations were observed after the heat acclimation protocol, in the subjects treated with quercetin internal temperature during exercise was not reduced, suggesting that the inhibition of Hsp72 increase is related to this classical systemic heat acclimation adaptation. Furthermore, the authors observed that the gastrointestinal barrier permeability remained elevated and that circulating cytokines IL-6 and IL-10 levels were not altered in the quercetin group after heat acclimation. These results and the ones described previously show that heat acclimation and the thermotolerant state share a common mechanism: the increase in Hsp72 content.
Conclusions and future directions
The studies above indicate that having a greater supply of intracellular Hsp72 available presumably allows the cell or whole-body to cope with greater stress. However, it remains to be seen whether cellular changes with heat acclimation are able to change the core temperature threshold for cellular injury observed during heat stroke in humans.
Further research is necessary to address issues related to heat acclimation, and cellular changes including Hsp72 regulation. Some key questions include: a) Does heat acclimation increase cellular resistance to stresses commonly described during heat stroke, including heat, hypoxia, cytokines, and combined effects? b) Does heat acclimation increase tolerance to higher core temperature during exercise? c) Is there a dose-response relationship for increased Hsp72 to augment benefits at the cellular, systemic or whole body level? d) Are sex differences in temporal patterning to heat acclimation also evident at the cellular level? e) Do other heat shock proteins participate in these processes? f) Are there other cellular system related to thermotolerance affected by heat acclimation? Answers to these questions may lead to a better understanding of how internal and/or external environmental stressors might provoke cellular adaptations to improve whole-body tolerance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Fabiano Amorim gratefully acknowledges the financial support from CNPq (process # 404201/2013-0).
References
- Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol 2011; 1:1883-928; PMID:23733692
- Chalmers S, Esterman A, Eston R, Bowering KJ, Norton K. Short-term heat acclimation training improves physical performance: a systematic review, and exploration of physiological adaptations and application for team sports. Sports Med 2014; 44:971-88; PMID:24817609; http://dx.doi.org/10.1007/s40279-014-0178-6
- Sawka MN, Wenger CB, Pandolf KB. Thermoregulatory responses to acute exercise – heat stress and heat acclimation. In: Blatteis CM, Fregley MJ, editors. Handbook of Physiology. Environmental Physiology. Bethesda, MD: American Physiological Society, 1996; sect. 4, vol. I.
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol 1997; 83:1413; PMID:9375300
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res 1982; 42:2457-61; PMID:7074623
- Li GC, Meyer JL, Mak JY, Hahn GM. Heat-induced protection of mice against thermal death. Cancer Res 1983; 43:5758-60; PMID:6640528
- Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol 2007; 103:1196-204; PMID:17615280; http://dx.doi.org/10.1152/japplphysiol.00242.2007
- Magalhães FC, Amorim FT, Passos RL, Fonseca MA, Oliveira KP, Lima MR, Guimarães JB, Ferreira-Júnior JB, Martini AR, Lima NR, et al. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones 2010; 15:885-95; PMID:20414820; http://dx.doi.org/10.1007/s12192-010-0197-7
- Amorim F, Yamada P, Robergs R, Schneider S, Moseley P. Effects of whole-body heat acclimation on cell injury and cytokine responses in peripheral blood mononuclear cells. Eur J Appl Physiol 2011; 111:1609-18; PMID:21191798; http://dx.doi.org/10.1007/s00421-010-1780-4
- Marshall HC, Campbell SA, Roberts CW, Nimmo MA. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol 2007; 32:341-8; http://dx.doi.org/10.1016/j.jtherbio.2007.04.003
- McClung J, Hasday J, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 2008; 294:R185; PMID:17977914; http://dx.doi.org/10.1152/ajpregu.00532.2007
- Watkins AM, Cheek DJ, Harvey AE, Blair KE, Mitchell JB. Heat acclimation and HSP-72 expression in exercising humans. Int J Sports Med 2008; 29:269; PMID:17879884; http://dx.doi.org/10.1055/s-2007-965331
- Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol 2011; 301:R524-33; PMID:21613575; http://dx.doi.org/10.1152/ajpregu.00039.2011
- Hom LL, Lee EC, Apicella JM, Wallace SD, Emmanuel H, Klau JF, Poh PY, Marzano S, Armstrong LE, Casa DJ, et al. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones 2012; 17:29-39; PMID:21796498; http://dx.doi.org/10.1007/s12192-011-0283-5
- Lee BJ, Mackenzie RW, Cox V, James RS, Thake CD. Human monocyte heat shock protein 72 responses to acute hypoxic exercise after 3 days of exercise heat acclimation. Biomed Res Int 2015; 849809; PMID:25874231
- Gibson OR, Turner G, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Heat Acclimation attenuates physiological strain and the Hsp72, but not Hsp90α mRNA response to acute normobaric hypoxia. J Appl Physiol 2015; 119:889-99; PMID:26205540; http://dx.doi.org/10.1152/japplphysiol.00332.2015
- Wenger CB. Human heat acclimatization. In: Pandolf KB, Sawka MN, Gonzalez RR, eds. Human performance physiology and environmental medicine at terrestrial extremes. Indianapolis, IN: Benchmark Press, 1988, 153-97.
- Regan JM, Macfarlane DJ, Taylor NAS. The role of body core and skin temperatures during heat adaptation. Acta Physiol Scand 1996; 158:365-75; PMID:8971258; http://dx.doi.org/10.1046/j.1365-201X.1996.561311000.x
- Garrett A, Rehrer N, Patterson M. Induction and decay of short term heat acclimation in moderately and highly trained athletes. Sports Med 2011; 41:757-71; PMID:21846164; http://dx.doi.org/10.2165/11587320-000000000-00000
- Garrett AT, Goosens NG, Rehrer NJ, Patterson MJ, Cotter JD. Induction and decay of short-term heat acclimation. Euro J Appl Physiol 2009; 107:659-70; PMID:19727796; http://dx.doi.org/10.1007/s00421-009-1182-7
- Lorenzo S, Minson CT. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J Appl Physiol 2010; 109:1736-43; PMID:20864556; http://dx.doi.org/10.1152/japplphysiol.00725.2010
- Magalhães FC, Machado-Moreira CA, Vimieiro-Gomes AC, Silami-Garcia E, Lima NR, Rodrigues LO. Possible biphasic sweating response during short-term heat acclimation protocol for tropical natives. J Physiol Anthropol 2006; 25:215-9; PMID:16763363; http://dx.doi.org/10.2114/jpa2.25.215
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 1993; 460:467-85; PMID:8487204; http://dx.doi.org/10.1113/jphysiol.1993.sp019482
- Senay LC, Mitchell D, Wyndham CH. Acclimatization in a hot, humid environment: body fluid adjustments. J Appl Physiol 1976; 40:786-96; PMID:931907
- Taylor NAS. Human heat adaptation. Compr Physiol 2014; 4:325-65; PMID:24692142; http://dx.doi.org/10.1002/cphy.c130022
- Adams WC, Fox RH, Fry AJ, MacDonald IC. Thermoregulation during marathon running in cool, moderate, and hot environments. J Appl Physiol 1975; 38:1030-7; PMID:1141115
- Maron MB, Wagner JA, Horvath SM. Thermoregulatory responses during competitive marathon running. J Appl Physiol 1977; 42:909-14; PMID:881391
- Pugh LG, Corbett JL, Johnson RH. Rectal temperatures, weight losses, and sweat rates in marathon running. J Appl Physiol 1967; 23:347-52; PMID:6047956
- Byrne C, Lee JK, Chew SA, Lim CL, Tan EY. Continuous thermoregulatory responses to mass-participation distance running in heat. Med Sci Sports Exerc 2006; 38:803-10; PMID:16672830; http://dx.doi.org/10.1249/01.mss.0000218134.74238.6a
- Ronneberg K, Roberts WO, McBean AD, Center BA. Temporal artery temperature measurements do not detect hyperthermic marathon runners. Med Sci Sports Exerc 2008; 40:1373-5; PMID:18614958; http://dx.doi.org/10.1249/MSS.0b013e31816d65bb
- Cheung SS, McLellan TM. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol 1998; 84:1731-9; PMID:9572824
- González-Alonso J, Teller C, Andersen S, Jensen F, Hyldig T, Nielsen B. Influence of body temperature on the development of fatique during prolonged exercise in the heat. J Appl Physiol 1999; 86:1032-9
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002; 92:2177-86; PMID:11960972; http://dx.doi.org/10.1152/japplphysiol.01267.2001
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 1992; 72:1063-81; PMID:1438579
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia 1995; 11:459-88; PMID:7594802; http://dx.doi.org/10.3109/02656739509022483
- Li GC, Petersen NS, Mitchell HK. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys 1982; 8:63-7; PMID:7199521; http://dx.doi.org/10.1016/0360-3016(82)90386-8
- Pelham HR. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 1986; 26: 46:959-61; PMID:2944601; http://dx.doi.org/10.1016/0092-8674(86)90693-8
- Liu RY, Li X, Li L, Li GC. Expression of human hsp70 in rat fibroblasts enhances cell survival and facilitates recovery from translational and transcriptional inhibition following heat shock. Cancer Res 1992; 52:3667-73; PMID:1377596
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res 1996; 223:163-70; PMID:8635489; http://dx.doi.org/10.1006/excr.1996.0070
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci 2005; 1053:74-83; PMID:16179510; http://dx.doi.org/10.1196/annals.1344.007
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem 2000; 18; 275:25665-71; PMID:10806214; http://dx.doi.org/10.1074/jbc.M906383199
- Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol 1998; 38:981-93; PMID:9824778; http://dx.doi.org/10.1177/009127009803801101
- Jo SK, Ko GJ, Boo CS, Cho WY, Kim HK. Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-{kappa}B–mediated inflammation and on tubular cell injury. J Am Soc Nephrol 2006; 17:3082-92; PMID:17021270; http://dx.doi.org/10.1681/ASN.2005101077
- Chen D, Pan J, Du B, Sun D. Induction of the heat shock response in vivo inhibits NF-kappaB activity and protects murine liver from endotoxemia-induced injury. J Clin Immunol 2005; 25:452-61; PMID:16160914; http://dx.doi.org/10.1007/s10875-005-5636-3
- Gibson OR, Mee JA, Taylor L, Tuttle JA, Watt PW, Maxwell NS. Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 Mrna. Scand J Med Sci Sports 2015; 25:259-68; PMID:25943677; http://dx.doi.org/10.1111/sms.12430
- Tuttle JA, Castle PC, Metcalfe AJ, Midgley AW, Taylor L, Lewis MP. Downhill running and exercise in hot environments increase leukocyte Hsp72 (HSPA1A) and Hsp90α (HSPC1) gene transcripts. J Appl Physiol 2015; 118; 996-1005; PMID:25722377; http://dx.doi.org/10.1152/japplphysiol.00387.2014
- Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. Stress-related genomic responses during the course of heat acclimation and its association with ischemic-reperfusion cross-tolerance. J Appl Physiol 2004; 97:1496-507; PMID:15155711; http://dx.doi.org/10.1152/japplphysiol.00306.2004
- Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. Heat acclimation increases hypoxia-inducible factor 1 alpha and erythropoietin receptor expression: Implication for neuroprotection after closed head injury in mice. J Cerebr Blood Flow Metab 2005; 25:1456-65; PMID:15902197; http://dx.doi.org/10.1038/sj.jcbfm.9600142
- Shein NA, Horowitz M, Shohami E. Heat acclimation: a unique model of physiologically mediated global preconditioning against traumatic brain injury. Prog Brain Res 2007; 161:353-63.
- Horowitz M, Assadi H. Heat acclimation-mediated cross-tolerance in cardioprotection: do HSP70 and HIF-1α play a role? Ann N Y Acad Sci 2010; 1188:199-206; PMID:20201904; http://dx.doi.org/10.1111/j.1749-6632.2009.05101.x
- Taylor L, Midgley AW, Chrismas B, Hilman AR, Madden LA, Vince RV, McNaughton LR. Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids 2011; 40:393-401; PMID:20552383; http://dx.doi.org/10.1007/s00726-010-0644-x
- Ely BR, Loveringa AT, Horowitz M, Minson CT. Heat acclimation and cross tolerance to hypoxia Bridging the gap between cellular and systemic responses. Temperature 2014; 1:107-14; http://dx.doi.org/10.4161/temp.29800