ABSTRACT
Physiological systems respond acutely to stress to minimize homeostatic disturbance, and typically adapt to chronic stress to enhance tolerance to that or a related stressor. It is legitimate to ask whether dehydration is a valuable stressor in stimulating adaptation per se. While hypoxia has had long-standing interest by athletes and researchers as an ergogenic aid, heat and nutritional stressors have had little interest until the past decade. Heat and dehydration are highly interlinked in their causation and the physiological strain they induce, so their individual roles in adaptation are difficult to delineate. The effectiveness of heat acclimation as an ergogenic aid remains unclear for team sport and endurance athletes despite several recent studies on this topic. Very few studies have examined the potential ergogenic (or ergolytic) adaptations to ecologically-valid dehydration as a stressor in its own right, despite longstanding evidence of relevant fluid-regulatory adaptations from short-term hypohydration. Transient and self-limiting dehydration (e.g., as constrained by thirst), as with most forms of stress, might have a time and a place in physiological or behavioral adaptations independently or by exacerbating other stressors (esp. heat); it cannot be dismissed without the appropriate evidence. The present review did not identify such evidence. Future research should identify how the magnitude and timing of dehydration might augment or interfere with the adaptive processes in behaviorally constrained versus unconstrained humans.
Introduction
The purpose of training is to elicit adaptations that improve relevant aspects of fitness or health. Adaptations that improve endurance fitness occur in most physiological systems to aid in mobilising, transporting, using or removing respiratory gases, substrates and heat. These physiological systems can be stressed in a variety of ways. Therefore, it is likely that several stimuli contribute to improving endurance fitness and are induced by exercise itself. Supplemental or adjunct stressors used as ergogenic stimuli for adaptation have received little interest by athletes and researchers, other than hypoxia over several decades and more recently whole-body heat stress and localized (muscular) carbohydrate availability. Therefore much more remains unknown than known as to whether any of these targeted stressors enhance either fitness or health per se. Environmental and metabolic heat stress both result in dehydration, which has widespread physiological and psychophysical effects alone and supplemental to those of heat. This review considers whether heat and associated dehydration in exercise training and/or exogenous heat stress might provide any useful stimulus for the adaptations that mediate improved endurance performance. We are unaware of any evidence or rationale by which repeated dehydration might enhance the adaptations underpinning improved strength or power-related fitness; indeed, some impairment is conceivable due to neuro-endocrine or local cell-mediated effects on protein balance. As indicated above, we address dehydration in the context of heat acclimation and/or exercise training, rather than the targeted use of dehydration to reduce body mass for weight-classified sports such as boxing or rowing, or decreasing body mass to improve power to weight ratios for jumping/vaulting sports. In doing so, we highlight how the often-potentiating, but sometimes-confounding effects of individual stressors are rarely differentiated in the contribution to an overall adapted phenotype. The terms ‘adaptation’ and ‘acclimation’ are used here as defined by the Thermal Physiology Commission of the International Union of Physiological sciencesCitation1 and in a recent reviewCitation2; adaptation refers to phenotypic and functional changes that reduce the physiological strain produced by stressful components of the total environment, whereas acclimation refers to phenotypic changes to specific environmentally-induced climatic factors. An important distinction is that adaptation promotes behavioral changes (an often under-appreciated fact;Citation3) whereas acclimation emphasizes physiological changes that may even reflect a consequence of behavioral constraints.
Dehydration is the process of losing fluid, which would typically produce a state of hypohydration (lower-than-normal body water volume). Hypohydration is commonly approximated using change in body mass (% BM), which can be misleading and thus problematic as an index of functional hypohydration in strenuous exerciseCitation4,5 but is also adopted here for simplicity. In a training context, dehydration occurs largely as a consequence of heat stress, or heat load, most of which is endogenous from the thermal energy (∼’heat’) yield of metabolism. Because an athlete's rate of heat production is their metabolic rate minus their work rate (Ḣ = Ṁ – Ẇ;Citation1), the most aerobically-powerful athletes are subjected to the most heat stress endogenously. Heat strain can also be incurred or exacerbated by clothing or from characteristics of the environment that impair the gradient for heat loss via convection, radiation and evaporation in particular (e.g., lack of airflow, sunshine and high humidity, respectively). Heat stress itself is almost certainly a principal stressor for both adaptation and acclimation, and of more importance than dehydration. Therefore, the relation between heat and dehydration is discussed before focusing on the role of each.
Heat and dehydration are strongly inter-connected
Heat stress elevates body tissue temperatures, which stimulate sweating and cutaneous vasodilatation to increase heat dissipation if the environment permits. Warmer environments add to heat stress by reducing (i) internal gradients for convective, conductive and mass flow heat transfer from the core to the skin, and (ii) external gradients for heat transfer from the skin via convection and radiation (conduction is usually negligible). Humid environments add to heat stress by reducing the vapor pressure gradient from the skin to the environment, which reduces the rate at which sweat can evaporate. Evaporation is already the dominant means of heat loss at usual training- or competitive-exercise intensities, so hot or humid environments exacerbate the dehydration that normally accompanies exercise. Dehydration by sweating leaves a smaller volume of more concentrated body fluid, including of the blood plasma, i.e., a hyperosmotic hypovolemic hypohydration; more so in trained and acclimated athletes because of their higher rates of work and concomitant heat production, sweating requirement and capacity for sodium reabsorption.Citation6,7
Not only does heat stress cause hypohydration, but heat and hypohydration each incur wide-ranging physiological and psychophysical strain independently, several examples of which are illustrated in . In many respects their acute effects are synergistic – a notable exception being that hyperosmotic hypovolemia can attenuate vasodilation and sweating,Citation8,9 thereby further increasing heat strain. Thus, each can exacerbate the other. The acute effects of heat strain and hypohydration need to be considered because they could stimulate or impair adaptations. Many effects of heat stress and hypohydration are mediated at least partly via increased tissue temperatures and cardiovascular strain.Citation10-12 Thus, the physiological effects in the left side of tend to drive those on the right. Cardiac output and muscle perfusion can be compromised by the combination of prolonged or intense endurance exercise in warm conditions, in an upright posture, and hypohydrated, at least in laboratory conditions.Citation13-15 Heat strain and hypohydration - even at mild levels in a lab environment (i.e., low air velocity) - can each reduce central venous pressure and stroke volume, and increase glycogenolysisCitation16,17 oxidative stressCitation18,19 and several neuro-endocrine responses.Citation20-22 Given that some of these stimuli can elicit adaptation, as explained below, it seems plausible – but speculative - that hypohydration could therefore potentiate some heat-induced adaptations irrespective of whether it exerts independent effects. It must be acknowledged however that individual stressors may provide interference (e.g., potentially with heat and hypoxia on plasma volume) or an excessive net stressCitation23 and thus act to attenuate adaptive responses. Heat and hypohydration could conceivably interfere hypothalamically (discussed below) but otherwise would seem to have mainly synergistic effects at least systemically.
Figure 1. Heat stress and sweating-induced hypohydration can each cause widespread acute effects, many of which are synergistic. Hypohydration is usually caused by heat stress, but can then oppose heat-induced increases in skin blood flow and sweating to further exacerbate heat strain. Abbreviations: ADH = Anti-diuretic hormone; Aldo = Aldosterone; ANP = Atrial Natriuretic Peptide; BBB = Blood brain barrier; Cats = Catecholamines; LPS = Lipopolysaccharide; ROS = Reactive Oxygen Species.
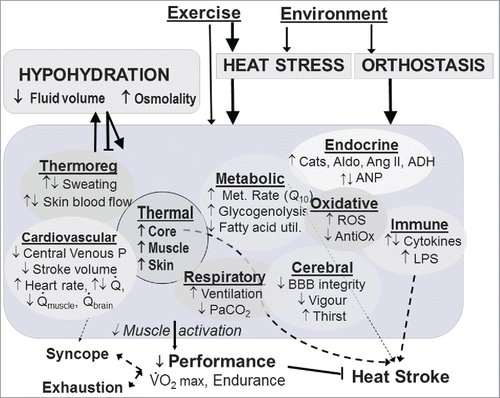
Some differential effects of heat stress and hypohydration are evident (). For example, oxidative stress may be more attributable to hypohydration than heat during exercise.Citation18 Whether this stress would help, hinder or have no effect on adaptation is, to our knowledge, unknown, and might depend on the athlete's age, sex, use of exogenous antioxidantsCitation24,25 and balance between exogenous heat stress and exercise per se.Citation19 Hypohydration is also more important than heat in driving the fluid regulatory hormones, although both hot-dry and warm-wet heat stress potentiate the hypohydration-induced increases in aldosterone during exercise performed at low intensity.Citation22 In contrast, high core temperature per se reduces cerebral perfusion, due in part to effects of hyperventilation-induced hypocapnia and higher cerebrovascular reactivity to CO2 in exercise.Citation26,27 Heat-induced hypocapnia does not seem to be exacerbated by hypohydration, whether at mild levels in exercise (2.5% BM)Citation28 or moderate levels at rest (5%).Citation29 In turn, hypohydration at 2% BM does not measurably exacerbate heat-induced reductions in cerebral perfusion during passive heat stress,Citation30 but 3% BM exacerbates orthostatically-induced reductions in perfusion when normothermic upon standing, independently of blood pressure.Citation31,32 Given that prolonged endurance exercise itself causes marked reduction in orthostatic tolerance,Citation33-35 hypohydration might be considered to have a minor role relative to those of exercise and heat in impaired cerebrovascular perfusion during or immediately following exercise, including the risk of syncope.
Table 1. Acute and adaptive effects of the 3 stressors that typically comprise heat acclimation or heat acclimatization.
Strenuous exercise or exercise in the heat can increase the permeability of tight junctions of the gutCitation36-39 and blood brain barrier.Citation40,41 At least in the case of the blood brain barrier, hypohydration may play a larger role than heat,Citation40 which might account for the lack of an observable effect of exercise in the heat in some studies.Citation42,43 Physiologically-relevant levels of heating increase the permeability of epithelial cell tight junctions of the gut in rats,Citation44 and in gut and kidney cell cultures.Citation45,46 Heat stress also stimulates the important signaling molecule, mTOR,Citation47,48 as well as glucose sensitivity at least in aged muscle, via HSP72 induction.Citation49 Contrary to this, hyperosmotic hypohydration may oppose both of these effectsCitation50 via volume regulatory signaling mechanisms and/or oxidative stress. It is therefore apparent that heat and hypohydration each elicit a wide range of physiological effects as a normal part of exercise or additional stress; many of these effects are synergistic although some are not.
The adaptive responses to exercise or heat acclimation/acclimatization programs are likely facilitated by a combination of muscular activity, heat, orthostasis, and dehydration. The highly interconnected nature of these stressors makes it problematic to attribute adaptations primarily to an individual stressor or a defined combination. Rarely are the effects of each stressor delineated and their individual contributions accounted for. The left side of highlights the acute contributions of heat, dehydration, and orthostasis to overall physiological and psychological strain under exertional stress, and the subsequent functional outcomes. The right side summarizes the state of knowledge regarding the importance of these stressors to adaptation, namely reducing the strain to each of the systems, and improving functional outcomes. Because of the interconnected acute effects of these stressors and a lack of research delineating their contributions in acclimation, their separate and potentially combined roles in the acclimated or adapted phenotype must be scrutinized; hence the purpose, scope and progression of this review.
Why might heat stress be important for adaptation?
Given the many acute effects of heat stress (e.g., ), it is unsurprising that repeated exposure to exogenous heat stress drives adaptations at intracellular, tissue, organ and systemic levels that collectively lessen physiological and perceptual strainCitation2 and improve exercise capacity, at least in the heat. Several such adaptations are qualitatively similar to those arising from aerobic training, which is also unsurprising given the large endogenous heat stress of trainingCitation51 and the cross tolerance provided by some adaptations (described below). For example, acclimation-induced lowering of sympathetic activationCitation52,53 and body tissue temperatures could both contribute to glycogen sparing in exercise.Citation54,55 The exogenous heat stress provides large additional physiological and heat tolerance benefits for untrained and moderately-trained individuals and modest but important gains for well-trained individuals.Citation56,57 The warmer periphery provides several benefits over endogenous heat stress alone; it increases tissue temperatures throughout the body, and signaling therein,Citation58,59 increases central cardiovascular and fluid regulatory strain, functionally adapts heat loss effectors in the skin,Citation60,61 and improves familiarity.
In a wider context, short-term (8 weeks) hot water immersion water has been shown to improve cardiovascular and cerebrovascular health in young healthy individuals,Citation62,63 and may provide similar benefits for diseased populations.Citation64-67 Regular bouts of passive heat stress (sauna bathing) have also been associated with increased longevity among the general population in Finland,Citation68 and of improved clinical outcomes in cardiovascular-diseased cohorts in JapanCitation69 - albeit with orthostatic and perhaps fluid regulatory stressors in both instances. The changes in physiological function with heat acclimation are addressed below.
Thermoregulatory
Heat acclimation widens the core temperature band available for exercise by reducing the resting core temperature at the time of day in which the acclimation bouts are performed.Citation70,71 This benefit is more important in uncompensable heat stress,Citation52 and can be negated mostly by prior activity (e.g., warm up) but also by hypohydration.Citation72 The upper core temperature at which exhaustion occurs in uncompensable heat stress seems not to be raised by short-term acclimation but is higher in aerobically-trained individuals.Citation73 Skin blood flow and sweating adapt over a similarly rapid time course, to concomitantly increase heat loss power due to both local and central adaptations.Citation51,60,74 The effectors activate at a lower core temperature (Tc), have higher sensitivity (relative to Tc), and attain higher maxima (sweating onlyCitation60), even in highly-trained athletes.
Cardiovascular
Adaptations occur in all components of the cardiovascular system. Peripheral heating increases endothelial shear stress by virtue of increased blood flow, and can thereby improve flow-mediated dilationCitation75 and vessel calibre.Citation76 A similarly biphasic adaptation seems to occur in the myocardium, initially showing increased contractility and stress tolerance, then increased metabolic efficiency (in ratsCitation58,77). Microvascular function (using the cutaneous circulation as a model) is improved following prolonged passive heat acclimation at least partly by way of greater nitric oxide (NO) bioavailability.Citation78 Orthostatic tolerance also develops rapidly, in conjunction with a rapid increase in blood volume via expansion of the plasma volume (i.e., hypervolemia). Several factors point to the hypervolemic response to heat acclimation as being a key mediator of improved cardiovascular and thermoregulatory function; e.g., its extent correlates with the acclimation-induced reduction in heart rate and the increases in skin blood flow and sweat rate during exercise in the heat.Citation79 The lower thermal and cardiovascular strain is likely to underlie the other benefits at least in part, such as glycogen sparing and lower perceived exertion. Improved exercise tolerance arises from some unknown combination of these adaptations.
A sustained reduction in central venous pressure (CVP) during and/or after exercise has emerged as a key mediator of adaptive hypervolemia, hence the separated inclusion of orthostatic stress in . is an extension of a model developed by ConvertinoCitation79 to illustrate how exercise may lead to hypervolemia via reduced CVP, especially when supplemented with heat stress. Convertino's model is extended to incorporate subsequent findings and theories. First, experiments from the John B Pierce laboratory confirmed the findings of ConvertinoCitation80 on the importance of reduced CVP, and the roles of albumin synthesis and sodium retention.Citation81-83 Upright compared with supine posture during and following exercise substantially increases the aldosterone response and modestly suppresses the atrial natriuretic peptide response, leading to an expansion of plasma volume within one day.Citation83 Second, a role for erythropoietin release in response to reductions in central venous pressure (independent of hypoxia) has been proposed.Citation84 Third, additional benefits of expanded fluid volumes are incorporated.Citation85,86 The possible roles of hyperosmolality on ADH, leading to hypervolemia, were also proposed by Convertino et al.Citation80 as non-thermal factors.
Figure 2. Factors that acutely and chronically determine blood volume with repeated training bouts, and the consequential effects on the physiology of exercise. This schematic is based mostly on that developed by Convertino,Citation79 extended to incorporate subsequent research on the role of central blood volume on renal-, albumin- and EPO- mediated volume expansion.Citation81-84 Abbreviations: ADH = Anti-diuretic hormone; Aldo = Aldosterone; AngII = Angiotensin II; ANP = Atrial natrietic peptide; BV = Blood volume; CNa = sodium clearance; ECFV = Extra cellular fluid volume; EPO = Erythropoietin; GFR = Glomerular filtration rate; PV = plasma volume; RCM = Red cell mass; SNSA = Sympathetic nervous system activity.
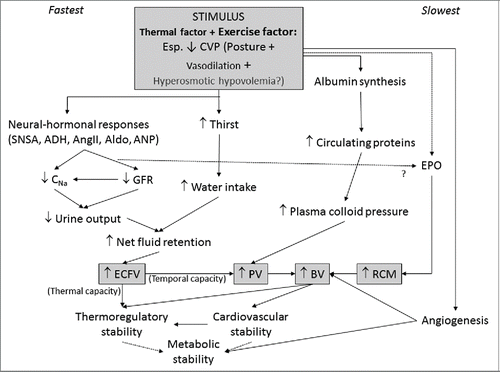
Endurance athletes already have markedly expanded red cell and plasma volumes,Citation87 which limits the stroke volume-related benefit of further expansion for exercise in temperate conditions.Citation88,89 Nonetheless, athletes show further volume expansion from heat acclimation, along with reduced heart rates in submaximal exercise and increased maximal aerobic power, anaerobic threshold, and time trial performance in hot laboratory conditions ().Citation90,91 Individual differences in the hypervolemic response to heat acclimation are pronounced.Citation92 The extent of the increase in exercising, but perhaps not resting, volume correlates with ergogenic variability between individuals.Citation93 On average at least, heat acclimation does not appear to improve the defense of plasma volume during exercise in the heat.Citation94 As yet it is unclear whether the key putative adaptation is an expansion of PV as measured in the resting stateCitation79,95 or the exercising state only.Citation93
Table 2. Heat acclimation studies on highly-trained athletes or on its ergogenic transfer to temperate conditions.
Muscle and epithelia
Heating skeletal muscle either in the absence of exercise or after exercise can stimulate HSP72 and mTOR,Citation47 thereby protecting against disuse atrophy and enhancing muscle regrowth in ratsCitation96 and producing hypertrophy in humans.Citation97 Heating of muscle might therefore seem attractive for strength athletes, but it should be noted that (i) heat per se is much less effective than exercise itself in conditioning muscle against mechanical overload,Citation98 (ii) when applied before exercise, heat actually preconditions muscle to become less perturbedCitation98 and thus less responsive to a short-term overload,Citation99 and (iii) HSP72 is stimulated by intense exercise as well. Incorporating heat into a resistance-training program is therefore not straight forward, even from a conditioning perspective. However, heat acclimation is effective for aerobically-demanding exercise in the heat, and is broadly consistent with the phenotypic responses obtained from aerobic training.
Heat shock proteins have many intracellular roles, one of which is to protect against multiple stressors,Citation45,100 including otherwise-lethal heat stress. The induction of HSP72 in various tissues shows a heat-dose dependency, with thermal intensity being more important than its duration.Citation59,101 In regard to acclimation for performance, it is also noteworthy that cellular stress during sporting competition is characterized by the intensity of multiple stressors rather than the more prolonged, lower-grade stress that characterizes many occupational circumstances. Heat acclimation includes increased HIF-1α in rat hearts - which also appears to upregulate renal mRNA erythropoietin, among other targets - and along with HSP72, strongly protects the myocardium against ischemia/reperfusion injury.Citation102,103 Thus, generalized cellular stress protection, including thermotolerance and ischemia/reperfusion tolerance,Citation102,104 may be as important for athletes as simply being heat acclimated/acclimatized. In contrast, heat acclimation of a systemic nature may suffice for the less intense demands on workers, coaches and other athletes (e.g., bowls or archery), and these can be achieved as much by the volume as the intensity of heat stress.Citation105,106
The muscle energetics of heat acclimation have been eloquently described by Horowitz and colleagues based on extensive research on soleus and cardiac muscle in animal models (reviewed in ref. Citation107). Long-term heat acclimation is characterized by metabolic efficiency, particularly in the heart, achieved without sacrificing autonomic function, and appears to be modulated by sustained low plasma thyroxine concentrations (relative to pre-acclimation and the short-term acclimated phenotype). The relevance of this research for untrained and endurance-trained humans has yet to be determined. For instance, the 30 day heat acclimation used in the rodents has yet to be replicated in humans, much less in endurance-trained humans. As such we are unaware of any human characterization of many features of the long-term heat acclimated rat; such as sustained low plasma thyroxine contributing to altered calcium handling mechanics, and increased myocardial work efficiency of the heart.
Horowitz and colleaguesCitation77,108,109 have also identified the response of the heat acclimated phenotype to novel superimposed hypohydration. Sustained and severe hypohydration (10% BM) abolished the beneficial thermoregulatory characteristics of the heat acclimated rodent,Citation108,109 and disrupted the gene profile developed by long-term heat acclimation.Citation77 While such findings provide valuable mechanistic insight, the extent to which such effects apply to self-autonomous, exercise-conditioned humans is yet to be determined, especially given the self- vs. externally-imposed control of hydration status, its severity, and its novelty.
But does supplemental heat improve adaptation for performance per se?
Several studies have examined whether heat acclimation enhances cardiovascular adaptations and performance in temperate conditions (12–22°C), as summarised in and in recent reviews.Citation110-113 The potential mechanisms are summarised above and in of Corbett et al..Citation110 Heat acclimation was shown 40 y ago to increase V̇O2max in temperate conditions, although early studies used relatively untrained participantsCitation114,115 or uncontrolled designs.Citation114,116 To our knowledge, Morrison et al.Citation92 were the first to address the ergogenic effects in highly-trained athletes, i.e., in an already strongly-stress-adapted phenotype, in a controlled-design study. The matched variable during the heat and control training regimes was the athlete's perception of exertion, which has high ecological validity. Those researchers observed a large variability in both resting hypervolemia and cycling time trial performance outcomes, with little relation between them. Scoon et al.Citation95 and then Creasy et al.Citation117 also used crossover experimental designs, in which endurance athletes undertook sauna bathing to volitional tolerance following most training bouts for 3 weeks. The intention was to maximize cardiovascular and thermal strain while already warmed and vasodilated by the training bout. Scoon et al.Citation95 observed expanded plasma volume (Evans Blue dye), which correlated closely with a modest but meaningful improvement in running performance, whereas Creasy et al.Citation117 found no such benefits on hypervolemia (CO dilution) or performance in highly-trained rowers when tested 2, 5 and 9 d following heat acclimation. The reason for the different outcomes from these studies is unknown, but it is noted that running for ∼15 min and rowing for <7 min place different demands on the cardiovascular system in regard to orthostatic stress and oxygen delivery to exercising muscle. Both cohorts were clearly orthostatically stressed in the heat bouts, but drinking was discouraged in Scoon et al.,Citation95 whereas participants in Creasy et al.Citation117 drank more fluid than they lost in the sauna. Heat stress after training was also employed by Zurawlew et al.Citation118 but using hot-water immersion and a between-subjects design. They observed an increase in 5-km time trial performance in hot (33˚C), but not temperate (18˚C) conditions. While hot water immersion may provide a simple and practical means for athletes to acclimatize, hydrostatic pressure effects of water could conceivably impact hematological adaptations, and the most efficacious method is undetermined (see below).
Lorenzo et al.Citation60 have addressed the ergogenic issue comprehensively in cohorts of well-trained cyclists, with extensive performance and physiological assessments in both warm and temperate conditions. The aerobic measures were improved 5–10% on average in both environments, in conjunction with a typical magnitude of expansion in plasma volume (∼4.5%, at rest) and a substantial increase in maximal cardiac output in both environments. Similar to other studies, the standard deviation between individuals approximated the mean treatment effect, for both physiological and performance variables. Those researchers used matched work rate between heat acclimation and control training conditions, in contrast to the approach used by Morrison et al.,Citation92 also in cyclists. On the other hand, Neal et al.Citation119 administered isothermic short-term heat acclimation to trained cyclists and triathletes and found that temperate performance (peak power output; PPO) tended to increase by ∼2% (P = 0.06). A two-week heat acclimatization of 9 trained cyclists improved hot-weather time-trial performance back to temperate-performance levels, which correlated with the heat-induced hypervolemia,Citation120 but performance in the temperate environment was not improved beyond that of the control group.Citation121 Interestingly, plasma expanded by >10% in both groups. Thus, it remains unclear whether heat acclimation confers cross-tolerance to improve performance in less stressful conditions, and what features of the adapted phenotype convey this (if any) benefit.
The ergogenic effect of heat acclimation via water immersion is similarly unresolved because the data are sparse and conflicting. Bradford et al.Citation122 had swimmers undertake 6 hours of mixed-intensity training (subjectively matched, to volitional tolerance) in hot (33˚C) vs. temperate (28˚C) water in a crossover fashion, and demonstrated clearly trivial effects for swimming performance in both water temperatures, and unclear effects for terrestrial performance. The posture, hydrostatic squeeze and lack of heat strain from swimming in such water were all suggested to account for the lack of any apparent physiological adaptations. Conversely, Hue et al.Citation123 had 6 competitive swimmers undertake 14 swims (∼60 km) in the heat (air 30˚C and water 35˚C), or at altitude (air 4˚C and water 27˚C). The 400-m performance times in temperate water were unchanged 10 d afterward but were improved by “10%” in the tropical group at 30 d afterward.
The ergogenic potential of heat for sports involving variable exercise intensities is also unresolved. Buchheit et al.Citation124,125 and have found that field team sport athletes (Football/soccer and Australian Football League) also seem to gain functionally-important physiological and performance adaptations from heat acclimation. Those studies have strong ecological validity in their conditioning, testing in lab and field settings, and the calibre of athlete tested. Unfortunately the lack of a control group precludes the subtraction of any potential camp effects or early-season training effects in regard to the role of heat as a conditioning stimulus. Chen et al.Citation126 had elite racket sport athletes undergo short-term heat acclimation using matched incremental exercise to that of a control group, and found a trivial increase in time-to-exhaustion in the heat but not in temperate conditions. Finally, while the combination of hypoxia and heat may act synergistically to maximise hematologic adaptations and improve performance in both endurance and field sport competition, the scant findings are inconclusive.Citation115,125
Methods of acclimation: Disparate heat acclimation regimes have been used with athletes and have produced meaningful physiological and performance outcomes (). In fact, so many variables differ between the relatively few studies on athletes that it is impossible to identify an optimal acclimation regime. Presumably the essential components are: achieving prolonged (60-100 min) warming of the skin during or following exercise, perhaps on consecutive days,Citation127 in which core temperature is raised (1–2°C), and remaining upright (i.e., a combination of all component stressors). It is however important to realize that individual differences to acclimation are larger than the differences between protocols (). Thus, any one optimal or most efficacious heat acclimation regime seems unlikely to exist for use across athletes, with little evidence to indicate one method of sustaining thermal strain throughout the regime is more effective than another.Citation128,129
Adaptation to heat stress attenuates performance decrements in similarly stressful environments, secondary to reduced physiological and psychological strain. Important unresolved issues include the magnitude of these effects, particularly in well-trained athletes,Citation112,113 and its usefulness in cross-tolerance to different stressors and environments, such as cold and altitude.Citation130-134 Only one-third of studies (6/19) on this topic have formally compared control against HA effects (), and measurement error is seldom taken into consideration in the design, execution and interpretation of studies (,Citation135); both of which make it even more difficult to establish true effects. Some behavioral responses should be reported throughout the conditioning regime (e.g., dietary behavior before, during and after individual sessions, particularly sodium, carbohydrate and protein intake) to appropriately assess the role of other stressors (e.g., dehydration), or their contribution to individual differences. The right side of illustrates the current knowledge (or lack of) regarding how individual stressors contribute to adaptation. While heat appears to be the primary stimulus in many cases (e.g., in reducing thermal, cardiovascular, and psychological strain), it is likely influenced by synergistic or antagonistic effects of concurrent stressors.
Why consider dehydration?
It would be valuable to know whether the hypohydration incurred during bouts of training or acclimation is harmful, helpful or of no consequence for adaptation,Citation136 at the mild-to-moderate levels (typically <3%) that develop volitionally and become self-limiting during such training and acclimatization. Astoundingly, the roles of mild dehydration in adaptation appear to be unknown despite it being a typical stressor for athletes during fitness training, especially in summer.Citation57,137 Consideration is not given to the resting situation, because healthy individuals with access to water become thirsty and drink before body fluid deficits are incurred.Citation138,139 Similarly, severe hypohydration (>5%) is not considered here for reasons described below and because it is (i) self-limiting, (ii) unpleasant and thus distracting, (iii) therefore unnatural or at least unusual in a non-competitive setting, (iv) possibly unhealthy, and (v) may not stimulate further net compensatory responses especially relative to catabolic effects.
Adolph and colleagues undertook extensive research on dehydration and acclimation more than 75 y ago,Citation140 but they focused more on whether humans could acclimate to dehydration, i.e., in reducing the volume of fluid intake required to offset BM losses during work in the heat. Water requirements were not lessened to any functional extent by repeated dehydration, which is logical and important for physical activity in a hot environment because heat balance is governed by the requirement for evaporation. Other studies have looked at various physiological and genomic impacts of hypohydration before and after heat acclimation.Citation22,72,73,77,108,109,141,142 Notwithstanding the important contributions of such studies, again, they were not designed to address the issue of a possible role of ecologically-valid dehydration in adaptation. The extent of hypohydration imposed in such studies (5–6% in non-athlete humans and 10% in rats) is also larger than would reasonably occur in training, acclimation or acclimatization, and the participants were not permitted any behavioral regulation other than avoidance of further dehydration by volitional cessation of exercise.
Before considering the potential relevance of volitional hypohydration in adaptation, its acute effects on physiology and aerobic performance must be critiqued briefly for three reasons. First, adaptation is the cumulative outcome from additive bouts of stress, so knowledge of the acute effects in the self-regulated setting of training or acclimation is important (e.g., see , and left side of ). Second, if quality conditioning is that which maximizes its work volume (absolute intensity and/or duration), then volitional dehydration might be ill-advised because even these magnitudes are advocated as impairing work tolerance and promoting fatigue.Citation143-146 Third, if ecologically-valid extents of hypohydration have little or no effect on thermal or cardiovascular strain when training outdoors in temperate or warm conditions (see below), then hypohydration would presumably also fail to provide a stimulus for adaptation in training or in heat acclimatizing exercise where airflow is high (e.g., cycling, running, rowing). Hypohydration would still be relevant for heat acclimation though.
Does dehydration affect physiological strain and tolerance? Of relevance to the heat acclimation context, hypohydration has been advised against in a general sense because it can increase Tc at rest and during exercise, and reduce the maximal Tc toleratedCitation57,147 thus potentially truncating the volume of exposure. But, the reduced tolerance to hyperthermia was demonstrated in the setting of a hot lab environment with an imposed prior hypohydration of 8% BM. In free-living field settings, fit individuals will voluntarily exercise intensely at high Tc even at these rarely-encountered magnitudes of hypohydration.Citation148,149 In fact, even during the artificial setting of externally-imposed hypohydration at a more typical magnitude (2–2.5% BM) while encapsulated in protective clothing in a research laboratory, thermal tolerance remains high in fit individuals.Citation73,86 While some aerobic training takes place indoors (e.g., in northern hemisphere winters), the thermal strain caused by being indoors with limited airflow would be at least as important as any impact of volitional hypohydration.Citation150,151 On the other hand, if being indoors is for the purpose of heat acclimating, then the purpose is not to maximize work volume, but to promote cardiovascular and thermal strain. So, effects such as impaired mood, increased glycogenolysis, reduced CVP, increased heart rate, and increased tissue temperatures become relevant - some beneficially and others detrimentally. Further, if hypohydration confers greater strain than when euhydrated, then its' presence may provide a time efficient alternative for conditioning (i.e., same strain in less time). Several recent studies using ecologically-valid designs, along with quantitative reviews (meta-analysis) have shown that hypohydration of 2–3% has little or no measurable effects on physiological strain, and no effect on psychophysical strain or performance.Citation152-156 In contrast, if participants are deprived of fluid before or during exercise against their behavioral drive, are not given opportunity to familiarize to that stressorCitation157 or are tested in lower airflow environments, then physiological, psychophysical strain and tolerance of exercise are all affected substantially (reviewed in ref. Citation158). In summary, volitional dehydration might be relevant in heat acclimation, but less so in outdoor training or acclimatization because its effects are smaller than is typically conveyed in the literature.
Why might dehydration enhance adaptation? One reason is by exacerbating strain () and enhancing compensatory adaptations (e.g., ECFV; ). Another possible candidate for adaptation to dehydration transients would be in fluid regulation, since most physiological systems adapt and at multiple levels in response to repeated stress. Fluid regulatory adaptations could involve afferent, central or efferent structural or functional components.Citation159,160 Renal concentrating ability is markedly (40–50%) increased during short-term (3-d) sustained hypohydration, and is inhibited during short-term over-drinking in humans.Citation161,162 While these renal adaptations would theoretically improve fluid balance during the often-obligatory dehydrative stress of athletic competition, they seem unrealistic because they were demonstrated using a sustained, high magnitude of hypohydration, which might interfere with muscle metabolic control, protein synthesis and hypothalamic adaptations to heat acclimation,Citation50,77,163 not to mention cognitive and psychosocial effects. Secondly, any reduction in renal excretion of water during sporting competition would have only small effects on fluid balance in view of the dominance of sweating in dehydration during competitive-intensity exercise.
Horowitz and colleaguesCitation164,165 highlighted the ability of animals (desert spiny mouse) to acclimate to prolonged dehydration, namely to better maintain plasma volume in the face of acute dehydration. Such defense was attributed to decreased permeability of the vascular capillary bed, and thus less ultrafiltration. Whether such effects would occur in humans is unknown, and may be even less likely for the self-limited magnitudes and durations of hypohydration involved with training and heat acclimatization.
Plasma volume expansion is reported as being observed most commonly after 5 d of exercising upright in the heat, while “properly hydrated.”Citation166 Such appraisals acknowledge the value of exercise, heat and orthostasisCitation166 while precluding that of dehydration. As mentioned above, if dehydration independently, and in combination with heat and exercise, reduces CVP, even in a temperate environment,Citation167 and increases fluid regulatory, thermal, and cardiovascular strain, then it seems reasonable to suggest that its addition could augment the stimulus for adaptation.
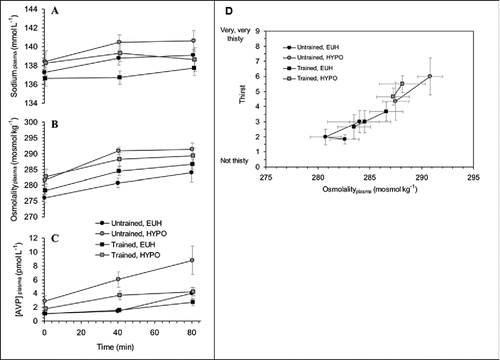
Relative to untrained individuals, athletes have altered neuroendocrine control (ADH vs. plasma osmolality) and are less sensitive to thermal and cardiovascular effects of hypohydration when exercising with high airflow in both temperateCitation160,168 and warm environments,Citation155 but not when exercising with compromised airflow in the heat.Citation72,169 Athletes perceive thirst as sensitively as untrained individuals, and voluntarily rehydrate to a similar extent – at least during cycling in lab trialsCitation168 - so they do not appear to be more predisposed to an insidious progression of dehydration during training or acclimation (). Athletes dehydrate more quickly in outdoor training,Citation168,170 due to higher endogenous heat production, but less markedly so during indoor training and heat acclimation bouts because of the imposed (exogenous) heat exposure (and thus similar sweat rates attained). Even indoors, their higher Tc tolerance, work rates and sweat rates will incur more hypohydration. However, drinking behavior increases as heat acclimation progresses, apparently in relation to the renin-angiotensin-aldosterone (RAAS) pathway more than ADH.Citation170 Hyperosmolality in exercise may contribute to a rise in anti-diuretic hormone (ADH) and to fluid expansion with repeated exposures but the increase in osmolality is at least partly attributable to exercise per se.Citation80 The modest exercise intensities used in prolonged heat acclimation bouts involve little increase in osmolality or ADH.Citation170 In conclusion, some fluid regulatory differences exist for athletes relative to untrained individuals, but not in their behavioral drive to limit dehydration. Although renal concentrating ability can be improved by hypohydration, this adaptation is not warranted. Adaptations to dehydration in training or acclimation should be studied using athletes during heat acclimation, for reasons discussed above.
Figure 3. Plasma sodium (A), osmolality (B), and AVP (C) concentration in trained and untrained groups at rest and during exercise (∼70% V̇O2 peak); and thirst as a function of osmolality (D) during the same exercise when receiving 100% rehydration (EUH) or 20% rehydration. Reproduced with permission from ref. Citation168.
Is there evidence for enhanced or attenuated adaptations from volitional dehydration?
The available studies do not reveal whether voluntary dehydration enhances or impairs adaptation during heat acclimation because the requisite information is not always available (), and the studies are too disparate in other respects (). If an effect exists it is presumably not dramatic because heat acclimation regimes using almost no replacement or full replacement have achieved similar hypervolemic and performance outcomes. Fleming and JamesCitation157 have recently demonstrated that familiarisation can occur within 4 exposures to hypohydration and exercising heat stress, such that its ergolytic effect became non-significant, as mentioned above. It is unclear how much of this recovery in performance was physiological, but participants still showed higher heart rates, RPE and Tc in exercise with hypohydration in that low airflow environment, so the habituation was presumably psychological rather than physiological.
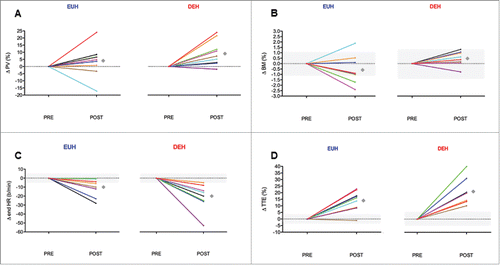
We studied the separated role of dehydration in heat acclimation, using 9 fit male volunteers (V̇O2 peak 60 ±7 mL/kg/min).Citation171 Participants were acclimated on two occasions, once with no dehydration and once with minimal rehydration (0.1 L; achieving ∼2% hypohydration), in crossover fashion, for 7.5 h (90-min/d for 5 d) using controlled hyperthermia (Tc = 38.5°C). Importantly, core (rectal) temperature was clamped to prevent the additional thermal strain that would otherwise ensue in the calm lab conditions, so any difference in outcome was delineated from any such thermal effect. Participants were given no verbal or written expectation as to which direction the effects might be, if any. The acclimation-induced expansion in plasma volume tended to be larger across acclimation with dehydration than euhydration (by 4.5%; 95%CI: −1 to 10%; P = 0.06; ), and be correlated with a rise in the aldosterone response across acclimation. Similarly, the change in body mass from pre to post acclimation was significantly (albeit trivially) larger with dehydration than euhydration (by 0.8 kg; 95%CI: 0.1 to 1.5; P = 0.03; unpublished results; see ), potentially reflecting the higher total body water content subsequent to greater fluid retention. The acclimation-induced reduction in end-exercise heart rate during a standardized heat stress test was also larger across the dehydration acclimation regime (by 11 b/min: −1 to 22; P = 0.05; ). But the differences between hydration regimes were unclear for most endocrine, cardiovascular, psychophysical and ergogenic outcomes (e.g., ). Thus, dehydration to ∼2% BM did not impair heat adaptation in fit males, and may have enhanced some aspects of short-term heat acclimation.Citation119,171 However, in view of the distinct lack of research on the role of hydration when conditioning the cardiovascular, thermoregulatory and fluid regulatory systems, and the inconsistent findings from the two studies, it is clearly not possible to suggest whether dehydration during stress is beneficial, counterproductive, or neither.
Figure 4. Individual responses of resting plasma volume (A), resting body mass (B), end-exercise heart rate (C), and subsequent time-to-exhaustion ((TTE) D) to short-term heat acclimation undertaken with euhydration (EUH) or dehydration (DEH). Mean values are illustrated as a black diamond, offset slightly for visual clarity. The smallest worthwhile difference is shown as a gray band, where able to be calculated. Data for A, C and D are individual responses of data published in ref. Citation171.
Caveats with dehydration: Research from the lab of Dr Hiroshi Nose has demonstrated the importance of replenishing protein and carbohydrate soon after a bout of exercise, especially in older individuals.Citation172-174 Partial rehydration with carbohydrate and amino acid-containing fluids (1.8 g amino acids/kg BM, in 3.2 mL water/kg) increased plasma albumin content and plasma volume restoration following a single bout of interval exercise, in old and young men.Citation173 Post-exercise supplementation, when applied across 8 wk of aerobic training in older men, enabled expansion of plasma albumin content and plasma volume.Citation174 This hypervolemia was somewhat defended during exercise, in conjunction with less cardiovascular strain and enhanced thermoeffector responses, compared to responses obtained from an equivalent fluid volume of placebo replenishment following each training bout. Similar results were obtained with young men training for 5 d in warm conditions.Citation172 Given those findings and existing knowledge that older individuals are slow to perceive hypohydration and subsequently rehydrate,Citation175 early nourishment following exercise seems warranted, especially in older individuals and regardless of the magnitude of hypohydration caused by exercise. Since mild hypohydration can also impair moodCitation176,177 and cognitionCitation178 at rest, full rehydration early following exercise may be merited. This actually remains unresolved because of the multiple problems of validity with such studies (see ref. Citation158), and because of opposing data on effects of early rehydration (see below).
The adaptive stimulus of a given exercise bout, or heat exposure, may be also be determined by the time course and extent of rehydration. Plasma volume expansion is consistently evident in response to acute and repeated exercise bouts, particularly utilizing 8 × 4 min at ∼85% peak V̇O2 with 5-min active recovery between repetitions (John B. Pierce Laboratory). In these and similar studies characterizing post-exercise plasma volume expansion, fluid is not made available during the exercise, and rehydration begins after one hourCitation179,180 or at least 2 hoursCitation82,83,181,182 following the exercise. Yet fluid retention is consistently stimulated, producing a rebound hypervolemic response. It appears that fluid regulatory hormonal responses (particularly plasma aldosterone) is apparent with prolonged maintenance of hypohydration, up to at least 6 hours post exercise,Citation183 and may be attenuated on provision of food and fluid.Citation183 Similarly, Costill et al.Citation184 found an impaired accrual of plasma volume if drinking carbohydrate and electrolyte beverage relative to water only, early after each of 5 daily bouts of dehydrating to 3%. Further, the reduction in CVP following prolonged dehydrating (∼3% BM) exercise is evident even after eating and drinking to satiety 1 hour after exercise cessation,Citation167 and thus the timing of rehydration (and nutrient replenishment) may provide a window for manipulating an adaptive stimulus. As some functional hypohydration is likely following exercise and particularly exogenous heat stress (see in ref. Citation158), individual differences in adaptive responses (particularly hematological) may therefore be influenced by rehydration regimes following the conditioning stimulus. Further research is warranted to determine the role of hydration before, during, and following conditioning sessions on adaptive responses.
Individual differences
The effects of dehydration do not impact all individuals equally. The degree to which dehydration will stimulate behavioral (thirst), and physiological responses (e.g., ADH secretion) differs due to genetic variation in the threshold and sensitivity of the osmoregulatory systems.Citation185 This variability makes some individuals reluctant to drink during prolonged exercise,Citation186 and can be further influenced by their fitness (neuroendocrine response;Citation168) or acclimatization to heat (quantity of fluid consumed;Citation170). It therefore seems likely that if permissive dehydration were beneficial during heat acclimation, or ergogenic in its own right, the degree of dehydration that is beneficial would likely differ between individuals. As indices of hydration used for prescribing drinking are confounded by the same variability,Citation187 it would appear that self-regulated rehydration is as appropriate as a prescribed drinking regime for most individuals.
Sex effects on chronic adaptations to heat stress are conspicuously under researched,Citation188 whereas those on chronic adaptations to hydration stress are still relatively unknown. It appears that females may require more heat acclimation to achieve the same attenuation in cardiovascular and thermoregulatory strain as males,Citation189 and any differences in adaptive responses may be partly explained by body composition.Citation190 The female heart also appears to be less sensitive to heat acclimation-induced HSP induction, possibly due to an inhibitory role of estrogen on HSP transcription and expression (in rats;Citation191). Sex differences therefore seem likely to contribute to variability in adaptive responses to heat, but this requires further research.
Several other issues remain unresolved or unexamined, including the separate and interactive roles of typically-encountered magnitudes of heat and dehydration in regard to (i) intracellular responses in vivo, especially on oxidative stress, energy metabolism and cellular toleranceCitation18,19,50; (ii) adaptation, especially in young versus older adults, and in regard to anti-inflammatory interventionsCitation192,193; (iii) red cell volume, (iv) the large individual differences in cardiovascular and functional impacts of heat acclimation, and (iv) short vs. long term adaptations, especially for endurance athletes. Functional effects should be assessed under psychologically and physically valid conditions; if representing training, these should ideally include blinding and strong airflow, respectively, whereas if representing heat acclimation, indoor environments or encapsulation, then airflow is unwarranted.
Conclusions and perspectives
Voluntary dehydration is an inherent part of exercise, with athletes typically drinking only half of their fluid loss (as approximated from mass loss, with its limitations). It remains unclear whether ad libitum drinking optimizes performance in competition, partly because laboratory-based research has limited validity in addressing that issue. Nonetheless, a far greater number of exercise bouts are performed in training, the major purpose of which is to adapt multiple systems to improve fitness. It is therefore remarkable that almost no research has been undertaken to determine whether dehydration enhances, impairs or does not substantively affect these adaptations. Dehydration is increased by exogenous heat stress, such as heat acclimation or acclimatization. Dehydration exacerbates the magnitude of strain in several physiological systems, and can increase thermal strain by attenuating the heat loss effectors. While heat is almost certainly the stress of major benefit in driving adaptations, more research is needed to delineate the roles of heat and dehydration. The effectiveness of heat acclimation for enhancing adaptations and performance remains unclear for team sport and for endurance athletes despite a surge in studies on this topic. Whether team sport or endurance athletes should drink ad libitum, or more avidly during aerobic training and heat acclimation is also not known, but rehydrating in conjunction with amino acids, carbohydrates, and sodium after training seems valuable, especially in older athletes. It is clear that prolonged orthostasis (during or following exercise or heat exposure) facilitates a more beneficial hormonal profile (for enhanced fluid regulation), however the time course of rehydration (in recovery), and its possible potentiating roleCitation194 in prolonging cardiovascular and fluid regulatory strain remain unclear. As is stands, the effectiveness of heat acclimation as a strategy to enhance adaptation for performance in a cool environment is unclear, as is the role of volitional dehydration within such heat acclimation or within normal training for endurance performance in hot, warm or cool environments.
Abbreviations
| ADH | = | Antidiuretic hormone, or vasopressin |
| BM | = | Body mass |
| CVP | = | Central venous pressure |
| HIF-1α | = | Hypoxia-inducible-factor 1 α |
| HSP72 | = | Heat shock protein 72 |
| mTOR | = | Mammalian target of rapamycin |
| mRNA | = | mRNA (ribonucleic acid) |
| NO | = | Nitric oxide |
| RAAS | = | Renin-angiotensin-aldosterone system |
| Tc | = | Core temperature |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Notes on contributors

Ashley Paul Akerman

Michael Tipton

Christopher T. Minson

James David Cotter
References
- IUPS Thermal Commission. Glossary of terms for thermal physiology. Third ed. Revised by The commission for thermal physiology of the International union of physiological sciences. Jap J Physiol 2001; 51:245-80.
- Taylor NA. Human heat adaptation. Comprehensive Physiol 2014; 4:325-65; PMID:24692142; http://dx.doi.org/10.1002/cphy.c130022
- Edholm OG. The physiology of adaptation. Eugen Rev 1966; 58:136-42; PMID:5913726
- Maughan RJ, Shirreffs SM, Leiper JB. Errors in the estimation of hydration status from changes in body mass. J Sports Sci 2007; 25:797-804; PMID:17454547; http://dx.doi.org/10.1080/02640410600875143
- Pastene J, Germain M, Allevard A, Gharib C, Lacour J. Water balance during and after marathon running. Eur J Appl Physiol Occupational Physiol 1996; 73:49-55; PMID:8861668; http://dx.doi.org/10.1007/BF00262802
- Buono MJ, Ball KD, Kolkhorst FW. Sodium ion concentration vs. sweat rate relationship in humans. J Appl Physiol (1985) 2007; 103:990-4; PMID:17600161; http://dx.doi.org/10.1152/japplphysiol.00015.2007
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol 1963; 166:530-47; PMID:13959046; http://dx.doi.org/10.1113/jphysiol.1963.sp007121
- Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol 1990; 68:1902-8; PMID:2361893
- Bittel J, Henane R. Comparison of thermal exchanges in men and women under neutral and hot conditions. J Physiol 1975; 250:475-89; PMID:1177148; http://dx.doi.org/10.1113/jphysiol.1975.sp011066
- Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc 1992; 24:657-70; PMID:1602938
- Casa DJ. Exercise in the heat. I. Fundamentals of thermal physiology, performance implications, and dehydration. J Athl Training 1999; 34:246.
- Montain SJ. Fluid ingestion during exercise increases skin blood flow independent of increases in blood volume. J Applied Physiol Respir Environ Exerc Physiol 1992; 73:903-10.
- Gonzalez-Alonso J, Mora-Rodriguez R, Coyle EF. Supine exercise restores arterial blood pressure and skin blood flow despite dehydration and hyperthermia. Am J Physiol 1999; 277:H576-H83; PMID:10444482
- Gonzalez-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol (Lond) 1998; 513:895-905; PMID:9824726
- Gonzalez-Alonso J, Calbet JA, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol (Lond) 1999; 520:577-89; PMID:10523424
- Logan-Sprenger HM, Heigenhauser GJ, Killian KJ, Spriet LL. Effects of dehydration during cycling on skeletal muscle metabolism in females. Med Sci Sports Exerc 2012; 44:1949-57; PMID:22543739; http://dx.doi.org/10.1249/MSS.0b013e31825abc7c
- Starkie RL, Hargreaves M, Lambert DL, Proietto J, Febbraio MA. Effect of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol 1999; 84:775-84; PMID:10481233; http://dx.doi.org/10.1111/j.1469-445X.1999.01815.x
- Hillman AR, Vince RV, Taylor L, McNaughton L, Mitchell N, Siegler J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutr Metab 2011; 36:698-706; PMID:21980993; http://dx.doi.org/10.1139/h11-080
- Laitano O, Kalsi KK, Pook M, Oliveira AR, Gonzalez-Alonso J. Separate and combined effects of heat stress and exercise on circulatory markers of oxidative stress in euhydrated humans. Eur J Appl Physiol 2010; 110:953-60; PMID:20658249; http://dx.doi.org/10.1007/s00421-010-1577-5
- Convertino VA, Keil LC, Greenleaf JE. Plasma volume, renin, and vasopressin responses to graded exercise after training. J Appl Physiol Respir Environ Exerc Physiol 1983; 54:508-14; PMID:6339451
- Wright H, Selkirk G, McLellan T. HPA and SAS responses to increasing core temperature during uncompensable exertional heat stress in trained and untrained males. Eur J Appl Physiol 2010; 108:987-97; PMID:19967394; http://dx.doi.org/10.1007/s00421-009-1294-0
- Francesconi RP, Sawka MN, Pandolf KB. Hypohydration and heat acclimation - plasma-renin and aldosterone during exercise. J Applied Physiol 1983; 55:1790-4; PMID:6363364
- Lloyd A, Raccuglia M, Hodder S, Havenith G. Interaction between environmental temperature and hypoxia on central and peripheral fatigue during high-intensity dynamic knee extension. J Appl Physiol 2016; 120:567-79; PMID:26769955; http://dx.doi.org/10.1152/japplphysiol.00876.2015
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kienhntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci 2009; 106:8665-70; http://dx.doi.org/10.1073/pnas.090348-5106
- Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 2013; 98:4507-15.
- Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol 2006; 96:299-304; PMID:16284788; http://dx.doi.org/10.1007/s00421-005-0079-3
- Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol 2002; 93:58-64; PMID:12070186; http://dx.doi.org/10.1152/japplphysiol.00049.2002
- Fujii N, Honda Y, Hayashi K, Kondo N, Nishiyasu T. Effect of hypohydration on hyperthermic hyperpnea and cutaneous vasodilation during exercise in men. J Appl Physiol 2008; 105:1509-18; PMID:18787094; http://dx.doi.org/10.1152/japplphysiol.01206.2007
- Senay LC, Christensen ML. Respiration of dehydrating men undergoing heat stress. J Appl Phsyiol 1967; 22:282-6.
- Fan JL, Cotter JD, Lucas RA, Thomas K, Wilson L, Ainslie PN. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol (1985) 2008; 105:433-45; PMID:18483173; http://dx.doi.org/10.1152/japplphysiol.00010.2008
- Carter R, 3rd, Cheuvront SN, Vernieuw CR, Sawka MN. Hypohydration and prior heat stress exacerbates decreases in cerebral blood flow velocity during standing. J Appl Physiol (1985) 2006; 101:1744-50; PMID:16916922; http://dx.doi.org/10.1152/japplphysiol.00200.2006
- Trangmar SJ, Chiesa ST, Llodio I, Garcia B, Kalsi KK, Secher NH, González-Alonso J. Dehydration accelerates reductions in cerebral blood flow during prolonged exercise in the heat without compromising brain metabolism. Am J Physiol Heart Circulatory Physiol 2015; 309:H1598-607; PMID:26371170; http://dx.doi.org/10.1152/ajpheart.00525.2015
- Murrell CJ, Cotter JD, George K, Shave R, Wilson L, Thomas K, Williams MJ, Ainslie PN. Cardiorespiratory and cerebrovascular responses to head-up tilt II: Influence of age, training status and acute exercise. Exp Gerontol 2011; 46:1-8; PMID:20600780; http://dx.doi.org/10.1016/j.exger.2010.06.004
- Murrell CJ, Cotter JD, George K, Shave R, Wilson L, Thomas K, Williams MJ, Ainslie PN. Syncope is unrelated to supine and postural hypotension following prolonged exercise. Eur J Appl Physiol 2011; 111:469-76; PMID:20886226; http://dx.doi.org/10.1007/s00421-010-1671-8
- Gratze G, Rudnicki R, Urban W, Mayer H, Schlogl A, Skrabal F. Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. J Appl Physiol 2005; 99:1728-35; PMID:16002770; http://dx.doi.org/10.1152/jappl-physiol.00487.2005
- Bosenberg AT, Brock-Utne JG, Gaffin SL, Wells MT, Blake GT. Strenuous exercise causes systemic endotoxemia. J Appl Physiol 1988; 65:106-8; PMID:3403455
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Mild endotoxemia, NF-{kappa}B translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol 2008; 295:R611-23; PMID:18565834; http://dx.doi.org/10.1152/ajpregu.00917.2007
- Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol 1997; 82:571-6; PMID:9049739
- Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol (1985) 2015; 120:692-701; jap 00536 2015; PMID:26359485; http://dx.doi.org/10.1152/japplphysiol.00536.2015
- Watson P, Black KE, Clark SC, Maughan RJ. Exercise in the heat: effect of fluid ingestion on blood-brain barrier permeability. Med Sci Sports Exerc 2006; 38:2118-24; PMID:17146318; http://dx.doi.org/10.1249/01.mss.000-0235356.31932.0a
- Watson P, Shirreffs SM, Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol 2005; 288:R1689-94; PMID:15650123; http://dx.doi.org/10.1152/ajpregu.00676.2004
- Morrison SA, Cheung SS, Hurst RD, Cotter JD. Cognitive function and blood-brain barrier permeability during exercise in the heat: Effect of fitness and bovine colostrum supplementation. J Thermal Biol 2013; 38:374-83; http://dx.doi.org/10.1016/j.jtherbio.2013.05.002
- Cheuvront SN, Chinevere TD, Ely BR, Kenefick RW, Goodman DA, McClung JP, Sawka MN. Serum S-100beta response to exercise-heat strain before and after acclimation. Med Sci Sports Exerc 2008; 40:1477-82; PMID:18614943; http://dx.doi.org/10.1249/MSS.0b013e3181-6d65a5
- Prosser C, Stelwagen K, Cummins R, Guerin P, Gill N, Milne C. Reduction in heat-induced gastrointestinal hyperpermeability in rats by bovine colostrum and goat milk powders. J Appl Physiol 2004; 96:650-4; PMID:14527963; http://dx.doi.org/10.1152/japplp-hysiol.00-295.2003
- Moseley PL, Gapen C, Wallen ES, Walter ME, Peterson MW. Thermal stress induces epithelial permeability. Am J Physiol 1994; 267:C425-C34; PMID:8074177
- Marchbank T, Davison G, Oakes JR, Ghatei MA, Patterson M, Moyer MP, Playford RJ. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol 2011; 300:G477-84; PMID:21148400; http://dx.doi.org/10.1152/ajpgi.00281.2010
- Kakigi R. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J Physiol Sci 2011; 61:131-40; PMID:21222186; http://dx.doi.org/10.1007/s12576-010-0130-y
- Yoshihara T, Naito H, Kakigi R, Ichinoseki-Sekine N, Ogura Y, Sugiura T, Katamoto S. Heat stress activates the Akt/mTOR signaling pathway in rat skeletal muscle. Acta Physiol 2013; 207:416-26.
- Gupte AA. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol (1985) 2011; 110:451-7; http://dx.doi.org/10.1152/japplphysiol.00849.2010
- Schliess F. Cell hydration and mTOR-dependent signalling. Acta physiologica (Oxford) 2006; 187:223-9; http://dx.doi.org/10.1111/j.1748-1716.2006.01547.x
- Aoyagi Y, McLellan TM, Shephard RJ. Interactions of physical training and heat acclimation. The thermophysiology of exercising in a hot climate. Sports Med 1997; 23:173-210; PMID:9108637; http://dx.doi.org/10.2165/00007256-199723030-00004
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol Lond 1993; 460:467-85; PMID:8487204; http://dx.doi.org/10.1113/jphysiol.1993.sp019482
- Maher JT, Bass DE, Heistad DD, Angelakos ET, Hartley LH. Effect of posture on heat acclimatization in man. J Appl Physiol 1972; 33:8-13; PMID:5037414
- Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J Appl Physiol 1998; 84:465-70; PMID:9475854
- Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol (1985) 1994; 76:589-97; PMID:8175568
- Cohen JS, Gisolfi CV. Effects of interval training on work-heat tolerance of young women. Med Sci Sports Exerc 1982; 14:46-52; PMID:7070257; http://dx.doi.org/10.1249/00005768-198201000-00009
- Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol 2011; 1:1883-928; PMID:23733692
- Horowitz M, Kodesh E. Molecular signals that shape the integrative responses of the heat-acclimated phenotype. Med Sci Sport Exer 2010; 42:2164-72; http://dx.doi.org/10.1249/MSS.0b013e3181e303b0
- Ruell PA, Hoffman KM, Chow CM, Thompson MW. Effect of temperature and duration of hyperthermia on HSP72 induction in rat tissues. Mol Cell Biochem 2004; 267:187-94; PMID:15663200; http://dx.doi.org/10.1023/B:MCBI.0000049382.63841.e4
- Lorenzo S, Minson CT. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J Appl Physiol 2010; 109:1736-43; PMID:20864556; http://dx.doi.org/10.1152/japplphysiol.007-25.2010
- Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol 1974; 37:515-20; PMID:4414615
- Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness, and blood pressure in sedentary humans. J Physiol 2016; PMID:27270841
- Bailey TG, Cable NT, Miller GD, Sprung VS, Low DA, Jones H. Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int J Sports Med 2016; PMID:27286178
- Thomas KN, van Rij AM, Lucas SJE, Gray AR, Cotter JD. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature 2016; 3:286-97; http://dx.doi.org/10.1080/23328940.2016.1156215
- Kihara T, Biro S, Ikeda Y, Fukudome T, Shinsato T, Masuda A, Miyata M, Hamasaki S, Otsuji Y, Minagoe S, Akiba S, Tei C. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circulation J official J Japanese Circulation Soc 2004; 68:1146-51; PMID:15564698
- Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am College Cardiol 2002; 39:754-9; PMID:11869837; http://dx.doi.org/10.1016/S0735-1097(01)01824-1
- Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 2012; 109:100-4; PMID:21944673; http://dx.doi.org/10.1016/j.amjcard.2011.08.014
- Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Internal Med 2015; 175:542-8; PMID:25705824; http://dx.doi.org/10.1001/jamainternmed.2014.8187
- Miyamoto H, Kai H, Nakaura H, Osada K, Mizuta Y, Matsumoto A, Imaizumi T. Safety and efficacy of repeated sauna bathing in patients with chronic systolic heart failure: a preliminary report. J Card Fail 2005; 11:432-6; PMID:16105634; http://dx.doi.org/10.1016/j.cardfail.2005.03.004
- Buono MJ, Heaney JH, Canine KM. Acclimation to humid heat lowers resting core temperature. Am J Physiol 1998; 274:R1295-R9; PMID:9644042
- Shido O, Sugimoto N, Tanabe M, Sakurada S. Core temperature and sweating onset in humans acclimated to heat given at a fixed daily time. Am J Physiol Regul Integr Comp Physiol 1999; 276:R1095-101.
- Sawka MN, Toner MM, Francesconi RP, Pandolf KB. Hypohydration and exercise - effects of heat acclimation, gender, and environment. J Appl Physiol 1983; 55:1147-53.
- Cheung SS, McLellan TM. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol 1998; 84:1731-9; PMID:9572824
- Kuno Y. Human perspiration. Pitts RF, editor. Springfield: Charles C. Thomas, 1956, 1956:417.
- Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010; 55:312-8; PMID:20048193; http://dx.doi.org/10.1161/HYPERTENSIONAHA.109.146282
- Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 2010; 588:1571-7; PMID:20211982; http://dx.doi.org/10.1113/jphysiol.2010.186965
- Schwimmer H, Eli-Berchoer L, Horowitz M. Acclimatory-phase specificity of gene expression during the course of heat acclimation and superimposed hypohydration in the rat hypothalamus. J Appl Physiol 2006; 100:1992-2003; PMID:16469936; http://dx.doi.org/10.1152/japplphysiol.00850.2005
- Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol 2016; PMID:27418688
- Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sport Exer 1991; 23:1338; http://dx.doi.org/10.1249/00005768-199112000-00004
- Convertino VA, Greenleaf JE, Bernauer EM. Role of thermal and exercise factors in the mechanism of hypervolemia. J Appl Physiol Respir Environ Exerc Physiol 1980; 48:657-64; PMID:6991462
- Nagashima K, Wu J, Kavouras SA, Mack GW. Increased renal tubular sodium reabsorption during exercise-induced hypervolemia in humans. J Appl Physiol 2001; 91:1229-36; PMID:11509520
- Nagashima K, Cline GW, Mack GW, Shulman GI, Nadel ER. Intense exercise stimulates albumin synthesis in the upright posture. J Appl Physiol 2000; 88:41-6; PMID:10642360
- Nagashima K, Mack GW, Haskell A, Nishiyasu T, Nadel ER. Mechanism for the posture-specific plasma volume increase after a single intense exercise protocol. J Appl Physiol 1999; 86:867-73; PMID:10066698
- Kirsch KA, Schlemmer M, De Santo NG, Cirillo M, Perna A, Gunga HC. Erythropoietin as a volume-regulating hormone: An integrated view. Semin Nephrol 2005; 25:388-91; PMID:16298260; http://dx.doi.org/10.1016/j.semnephrol.2005.05.007
- Kay D, Marino FE. Fluid ingestion and exercise hyperthermia: implications for performance, thermoregulation, metabolism and the development of fatigue. J Sports Sci 2000; 18:71-82; PMID:10718562; http://dx.doi.org/10.1080/026404100365135
- McLellan TM, Cheung SS. Impact of fluid replacement on heat storage while wearing protective clothing. Ergonomics 2000; 43:2020-30; PMID:11191783; http://dx.doi.org/10.1080/00140130050201454
- Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmidt A, Huber G, Friedmann B, Schmidt W. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med 2001; 22:504-12; PMID:11590477; http://dx.doi.org/10.1055/s-2001-17613
- Hopper MK, Coggan AR, Coyle EF. Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol 1988; 64:404-8; PMID:2451658
- Keiser S, Fluck D, Huppin F, Stravs A, Hilty MP, Lundby C. Heat training increases exercise capacity in hot but not in temperate conditions: a mechanistic counter-balanced cross-over study. Am J Physiol Heart Circ Physiol 2015; 309:H750-61; ajpheart 00138 2015; PMID:26150574
- Lorenzo S, Halliwill JR, Sawka MN, Minson CT. Heat acclimation improves exercise performance. J Appl Physiol (1985) 2010; 109:1140-7; PMID:20724560; http://dx.doi.org/10.1152/japplphysiol.00495.2010
- Garrett AT, Creasy R, Rehrer NJ, Patterson MJ, Cotter JD. Effectiveness of short-term heat acclimation for highly trained athletes. Eur J Appl Physiol 2012; 112:1827-37; PMID:21915701; http://dx.doi.org/10.1007/s00421-011-2153-3
- Morrison J, Hopkins W, Sleivert G. Little effect of training in the heat on cycling performance at normal temperature. Sport Sci 2002 July. Available from: http://www.sportsci.org/jour/0201/jpm.htm.
- Racinais S, Mohr M, Buchheit M, Voss SC, Gaoua N, Grantham J, Nybo L. Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. Br J Sports Med 2012; 46:810-5; PMID:22797527; http://dx.doi.org/10.1136/bjsports-2012-091227
- Patterson MJ, Stocks JM, Taylor NA. Sustained and generalized extracellular fluid expansion following heat acclimation. J Physiol-London 2004; 559:327-34; PMID:15218070
- Scoon GS, Hopkins WG, Mayhew S, Cotter JD. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J Sci Med Sport 2007; 10:259-62; PMID:16877041; http://dx.doi.org/10.1016/j.jsams.2006.06.009
- Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol 2000; 88:359-63; PMID:10642402
- Goto K. Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol 2011; 111:17-27; PMID:20803152; http://dx.doi.org/10.1007/s00421-010-1617-1
- Nosaka K, Muthalib M, Lavender A, Laursen PB. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol 2007; 99:183-92; PMID:17089155; http://dx.doi.org/10.1007/s00421-006-0331-5
- Frier BC, Locke M. Heat stress inhibits skeletal muscle hypertrophy. Cell Stress Chaperones 2007; 12:132-41; PMID:17688192
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol Regulatory, Integrative Comparative Physiol 1999; 276:R1506-R15; PMID:10233045
- Periard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones 2012; 17:375-83; PMID:22222935
- Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1 α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiological Genomics 2005; 23:79-88; PMID:16046617; http://dx.doi.org/10.1152/physiolgenomi-cs.00279.2004
- Staib JL, Quindry JC, French JP, Criswell DS, Powers SK. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am J Physiol Regul Integr Comp Physiol 2007; 292:R432-9; PMID:16990482; http://dx.doi.org/10.1152/ajpregu.00895.2005
- Moseley PL. Heat shock Proteins and heat adaptation of the whole organism. J Appl Physiol 1997; 83:1413-7; PMID:9375300
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Blood flow and other thermoregulatory changes with acclimatization to heat. J Physiol 1963; 166:548-62; http://dx.doi.org/10.1113/jphysiol.1963.sp007122
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol 1963; 166:530-47; http://dx.doi.org/10.1113/jphysiol.1963.sp007121
- Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol 2014; 4:199-230; PMID:24692139; http://dx.doi.org/10.1002/cphy.c130025
- Horowitz M, Kaspler P, Simon E, Gerstberger R. Heat acclimation and hypohydration: involvement of central angiotensin II receptors in thermoregulation. Am J Physiol Regulatory Integrative Comparative Physiol 1999; 277:R47-R55.
- Schwimmer H, Gerstberger R, Horowitz M. Heat acclimation affects the neuromodulatory role of AngII and nitric oxide during combined heat and hypohydration stress. Mol Brain Res 2004; 130:95-108; http://dx.doi.org/10.1016/j.molbrainres.2004.07.011
- Corbett J, Neal RA, Lunt HC, Tipton MJ. Adaptation to heat and exercise performance under cooler conditions: a new hot topic. Sports Med 2014; 44:1323-31; PMID:24943043
- Chalmers S, Esterman A, Eston R, Bowering KJ, Norton K. Short-Term heat acclimation training improves physical performance: A systematic review, and exploration of physiological adaptations and application for team sports. Sports Med 2014; 44:971-88; PMID:24817609
- Nybo L, Lundby C. CrossTalk opposing view: Heat acclimatization does not improve exercise performance in a cool condition. J Physiol 2016; 594:245-7; PMID:26667955; http://dx.doi.org/10.1113/JP270880
- Minson CT, Cotter JD. CrossTalk proposal: Heat acclimatization does improve performance in a cool condition. J Physiol 2016; 594:241-3; PMID:26668072; http://dx.doi.org/10.1113/JP270879
- Shvartz E, Shapiro Y, Magazanik A, Meroz A, Birnfeld H, Mechtinger A, Shibolet S. Heat acclimation, physical fitness, and responses to exercise in temperate and hot environments. J Appl Physiol Respir Environ Exerc Physiol 1977; 43:678-83; PMID:908683
- Takeno Y, Kamijo Y-I, Nose H. Thermoregulatory and aerobic changes after endurance training in a hypobaric hypoxic and warm environment. J Appl Physiol 2001; 91:1520-8; PMID:11568132
- Sawka MN, Young AJ, Cadarette BS, Levine L, Pandolf KB. Influence of heat stress and acclimation on maximal aerobic power. Eur J Appl Physiol Occupational Physiol 1985; 53:294-8; PMID:4039255; http://dx.doi.org/10.1007/BF00422841
- Creasy RH, Hopkins WG, Scoon G, Mayhew S, Cotter JD. Effects of post-exercise sauna bathing on 2000-M rowing performance and blood volumes. Med Sci Sports Exerc 2003; 35:S35.
- Zurawlew MJ, Walsh NP, Fortes MB, Potter C. Post-exercise hot water immersion induces heat acclimation and improves endurance exercise performance in the heat. Scand J Med Sci Sports 2015; PMID:26661992
- Neal RA, Corbett J, Massey HC, Tipton MJ. Effect of short-term heat acclimation with permissive dehydration on thermoregulation and temperate exercise performance. Scandinavian Med Sci Sports 2015.
- Karlsen A, Nybo L, Norgaard SJ, Jensen MV, Bonne T, Racinais S. Time course of natural heat acclimatization in well-trained cyclists during a 2-week training camp in the heat. Scand J Med Sci Sports 2015; 25(Suppl 1):240-9; http://dx.doi.org/10.1111/sms.12449
- Karlsen A, Racinais S, Jensen MV, Norgaard SJ, Bonne T, Nybo L. Heat Acclimatization does not improve VO2max or cycling performance in a cool climate in tained cyclists. Scandinavian J Med Sci Sports 2015; 25(Suppl 1):269-76; PMID:25943678; http://dx.doi.org/10.1111/sms.12409
- Bradford CD, Lucas SJ, Gerrard DF, Cotter JD. Swimming in warm water is ineffective in heat acclimation and is non-ergogenic for swimmers. Scand J Med Sci Spor 2015; 25:277-86; http://dx.doi.org/10.1111/sms.12351
- Hue O, Antoine-Jonville S, Sara F. The effect of 8 days of training in tropical environment on performance in neutral climate in swimmers. Int J Sports Med 2007; 28:48-52; PMID:16761217; http://dx.doi.org/10.1055/s-2006-923958
- Buchheit M, Voss SC, Nybo L, Mohr M, Racinais S. Physiological and performance adaptations to an in-season soccer camp in the heat: associations with heart rate and heart rate variability. Scandinavian J Med Sci Sports 2011; 21:e477-85; PMID:22092960
- Buchheit M, Racinais S, Bilsborough J, Hocking J, Mendez-Villanueva A, Bourdon PC, Voss S, Livingston S, Christian R, Périard J, et al. Adding heat to the live-high train-low altitude model: a practical insight from professional football. Br J Sports Med 2013; 47(Suppl 1):i59-i69; PMID:24282209; http://dx.doi.org/10.1136/bjsports-2013-092559
- Chen TI, Tsai PH, Lin JH, Lee NY, Liang MT. Effect of short-term heat acclimation on endurance time and skin blood flow in trained athletes. Open Access J Sports Med 2013; 4:161-70; PMID:24379721
- Gill N, Sleivert G. Effect of daily versus intermittent exposure on heat acclimation. Aviat Space Environ Med 2001; 72:385-90; PMID:11318020
- Gibson OR, Mee JA, Taylor L, Tuttle JA, Watt PW, Maxwell NS. Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 mRNA. Scandinavian J Med Sci Sports 2015; 25(Suppl 1):259-68; PMID:25943677; http://dx.doi.org/10.1111/sms.12-430
- Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol 2015; 49-50:55-65; PMID:25774027; http://dx.doi.org/10.1016/j.jtherbio.2015.02.005
- Heled Y, Peled A, Yanovich R, Shargal E, Pilz-Burstein R, Epstein Y, Moran DS. Heat acclimation and performance in hypoxic conditions. Aviation Space Environmental Med 2012; 83:649-53; PMID:22779306
- White AC, Salgado RM, Astorino TA, Loeppky JA, Schneider SM, McCormick JJ, McLain TA, Kravitz L, Mermier CM. The effect of ten days of heat acclimation on exercise performance in acute hypobaric hypoxia (4350 m). Temperature 2015; 3:176-85; http://dx.doi.org/10.1080/23328940.2015.1072659
- Daanen HA, Jonkhoff W, Castellani J, editors. Costs and benefits of training to prevent heat and cold injuries. SAS-095 Workshop on Cost/Benefit analysis of military training Amsterdam June 2012; 2012.
- White AC, Salgado RM, Astorino TA, Loeppky JA, Schneider SM, McCormick JJ, McLain TA, Kravitz L, Mermier CM. The effect of 10 days of heat acclimation on exercise performance in acute hypobaric hypoxia (4350 m). Temperature 2016; 3:176-85; PMID:27227084; http://dx.doi.org/10.1080/23328940.2015.1072659
- Ely BR, Lovering AT, Horowitz M, Minson CT. Heat acclimation and cross tolerance to hypoxia. Temperature 2014; 1:107-14; http://dx.doi.org/10.4161/temp.29800
- Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 2000; 30:1-15; PMID:10907753; http://dx.doi.org/10.2165/00007256-200030010-00001
- Garrett AT, Rehrer NJ, Patterson MJ. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med 2011; 41:757-71; PMID:21846164; http://dx.doi.org/10.2165/11587320-000000000-00000
- Rehrer NJ, Burke LM. Sweat losses during various sports. Australian J Nutri Dietetics 1996; 53:S13.
- Rolls BJ, Wood RJ, Rolls ET, Lind H, Lind W, Ledingham JG. Thirst following water deprivation in humans. Am J Physiol 1980; 239:R476-82; PMID:7001928
- Phillips PA, Rolls BJ, Ledingham JG, Morton JJ. Body fluid changes, thirst and drinking in man during free access to water. Physiol Behavior 1984; 33:357-63; PMID:6514825; http://dx.doi.org/10.1016/0031-9384(84)90154-9
- Adolph EF, editor. Physiology of Man in the Desert New York: Interscience, 1947.
- Meiri U, Shochina M, Horowitz M. Heat acclimated hypohydrated rats: Age dependent vasomotor and plasma volume responses to heat stress. J Therm Biol 1991; 16:241-7; http://dx.doi.org/10.1016/0306-4565(91)90032-W
- Buskirk ER, Iampietro PF, Bass DE. Work performance after dehydration: effects of physical conditioning and heat acclimatization. J Appl Physiol 1958; 12:189-94; PMID:13525259
- American College of Sports M, Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand Exercise and fluid replacement. Med Sci Sports Exerc 2007; 39:377-90; PMID:17277604; http://dx.doi.org/10.1249/mss.0b013e31802ca597
- Convertino VA, Armstrong LE, Coyle EF, Mack GW, Sawka MN, Senay LC, Jr, Sherman WM. American college of sports medicine position stand exercise and fluid replacement. Med Sci Sports Exerc 1996; 28:i-vii.
- Periard JD, Racinais S, Sawka MN. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scandinavian J Med Sci Sports 2015; 25(Suppl 1):20-38; PMID:25943654; http://dx.doi.org/10.1111/sms.12408
- Racinais S, Alonso JM, Coutts AJ, Flouris AD, Girard O, Gonzalez-Alonso J, Hausswirth C, Jay O, Lee JK, Mitchell N, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports 2015; 25(Suppl 1):6-19; PMID:25943653; http://dx.doi.org/10.1111/sms.12467
- Sawka MN, Young AJ, Latzka WA, Neufer PD, Quigley MD, Pandolf KB. Human tolerance to heat strain during exercise: influence of hydration. J Appl Physiol 1992; 73:368-75; PMID:1506393
- Noakes TD. Hydration in the marathon: using thirst to gauge safe fluid replacement. Sports Med 2007; 37:463-6; PMID:17465636; http://dx.doi.org/10.2165/0000-7256-200737040-00050
- Noakes TD. Is drinking to thirst optimum? Annals Nutri Metab 2010; 57(Suppl 2):9-17; PMID:21346332; http://dx.doi.org/10.1159/000322697
- Adams WC, Mack GW, Langhans GW, Nadel ER. Effects of varied air velocity on sweating and evaporative rates during exercise. J Appl Physiol 1992; 73:2668-74; PMID:1490985
- Saunders AG, Dugas JP, Tucker R, Lambert MI, Noakes TD. The effects of different air velocities on heat storage and body temperature in humans cycling in a hot, humid environment. Acta Physiologica Scandinavica 2005; 183:241-55; PMID:15743384; http://dx.doi.org/10.1111/j.1365-201X.2004.01400.x
- Dion T, Savoie F, Asselin A, Gariepy C, Goulet EB. Half-marathon running performance is not improved by a rate of fluid intake above that dictated by thirst sensation in trained distance runners. Eur J Appl Physiol 2013; 13:1-10.
- Goulet ED. Effect of exercise-induced dehydration on endurance performance: evaluating the impact of exercise protocols on outcomes using a meta-analytic procedure. Br J Sports Med 2012; 47:679-86; PMID:22763119; http://dx.doi.org/10.1136/bjsports-2012-090-958
- Goulet ED. Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis. Br J Sports Med 2011; 45:1149-56; PMID:21454440; http://dx.doi.org/10.1136/bjsm.2010.077966
- Wall BA, Watson G, Peiffer JJ, Abbiss CR, Siegel R, Laursen PB. Current hydration guidelines are erroneous: dehydration does not impair exercise performance in the heat. Br J Sports Med 2013; 49:1077–83; PMID:24055782
- Lee MJ, Hammond KM, Vasdev A, Poole KL, Impey SG, Close GL, Morton JP. Self-selecting fluid intake while maintaining high carbohydrate availability does not impair half-marathon performance. Int J Sports Med 2014; 35:1216-22; PMID:25144431; http://dx.doi.org/10.1055/s-0034-1375635
- Fleming J, James LJ. Repeated familiarisation with hypohydration attenuates the performance decrement caused by hypohydration during treadmill running. Appl Physiol Nutr Metab 2013; 39:124-9; PMID:24476466
- Cotter JD, Thornton SN, Lee JK, Laursen PB. Are we being drowned in hydration advice? Thirsty for more? Extreme Physiol Med 2014; 3:18; PMID:25356197
- Merry TL, Ainslie PN, Cotter JD. Effects of aerobic fitness on hypohydration-induced physiological strain and exercise impairment. Acta Physiologica 2009; 198:179-90.
- Merry TL, Ainslie PN, Cotter JD. Effects of aerobic fitness on hypohydration-induced physiological strain and exercise impairment. Acta Physiologica 2010; 198:179-90; http://dx.doi.org/10.1111/j.1748-1716.2009.02051.x
- Dewardener HE, Herxheimer A. The Effect of a high water intake on the kidneys ability to concentrate the urine in man. J Physiol-London 1957; 139:42-52; PMID:13481895
- Epstein FH, Kleeman CR, Hendrikx A. The influence of bodily hydration on the renal concentrating process. J Clin Invest 1957; 36:629-34; PMID:13428850; http://dx.doi.org/10.1172/JCI103462
- Schliess F, Häussinger D. Cell volume and insulin signaling. In: Kwang WJ, editor. International Review of Cytology Volume 225, Academic Press, 2003, p. 187-228.
- Horowitz M, Borut A. Plasma volume regulation in rodents: comparison of heat and dehydration effefts. Isr J Med Sci 1976; 12:864-7; PMID:977303
- Horowitz M, Samueloff S. Plasma water shifts during thermal dehydration. Journal of applied physiology: respiratory, environmental and exercise physiology 1979; 47:738-44; PMID:511680
- Sawka MN, Convertino VA, Eichner EW, Schneider SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 2000; 32:332-48; PMID:10694114; http://dx.doi.org/10.1097/00005768-200002000-00012
- Kirsch KA, von Ameln H, Wicke HJ. Fluid control mechanisms after exercise dehydration. Eur J Appl Physiol Occupational physiol 1981; 47:191-6; PMID:7026232; http://dx.doi.org/
- Merry TL, Ainslie PN, Walker R, Cotter JD. Fitness alters fluid regulatory but not behavioural responses to hypohydrated exercise. Physiol Behav 2008; 95:348-52; PMID:18644399; http://dx.doi.org/10.1016/j.physbeh.2008.06.015
- Mora-Rodriguez R, Hamouti N, Del Coso J, Ortega JF. Fluid ingestion is more effective in preventing hyperthermia in aerobically trained than untrained individuals during exercise in the heat. Appl Physiol Nutr Metab 2012; 38:73-80; PMID:23368831; http://dx.doi.org/10.1139/apnm-2012-0174
- Greenleaf JE, Brock PJ, Keil LC, Morse JT. Drinking and water balance during exercise and heat acclimation. J Applied Physiol 1983; 54:414-9; PMID:6833039
- Garrett AT, Goosens NG, Rehrer NJ, Patterson MJ, Harrison J, Sammut I, Cotter JD. Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am J Hum Biol 2014; 26:311-20; PMID:24469986; http://dx.doi.org/10.1002/ajhb.22509
- Goto M, Okazaki K, Kamijo Y, Ikegawa S, Masuki S, Miyagawa K, Nose H. Protein and carbohydrate supplementation during 5-day aerobic training enhanced plasma volume expansion and thermoregulatory adaptation in young men. J Appl Physiol 2010; 109:1247-55; PMID:20689095; http://dx.doi.org/10.1152/japplphy-siol.00577.2010
- Okazaki K, Hayase H, Ichinose T, Mitono H, Doi T, Nose H. Protein and carbohydrate supplementation after exercise increases plasma volume and albumin content in older and young men. J Appl Physiol 2009; 107:770-9; PMID:19589953; http://dx.doi.org/10.1152/japplphysiol.91264.2008
- Okazaki K, Ichinose T, Mitono H, Chen M, Masuki S, Endoh H, Hayase H, Doi T, Nose H. Impact of protein and carbohydrate supplementation on plasma volume expansion and thermoregulatory adaptation by aerobic training in older men. J Appl Physiol 2009; 107:725-33; PMID:19608927; http://dx.doi.org/10.1152/japplphys-iol.91265.2008
- Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc 2001; 33:1524-32; PMID:11528342; http://dx.doi.org/10.1097/00005768-200109000-00016
- Armstrong LE, Ganio MS, Casa DJ, Lee EC, McDermott BP, Klau JF, Jimenez L, Le Bellego L, Chevillotte E, Lieberman HR. Mild dehydration affects mood in healthy young women. J Nutr 2012; 142:382-8; PMID:22190027; http://dx.doi.org/10.3945/jn.111.142000
- Ganio MS, Armstrong LE, Casa DJ, McDermott BP, Lee EC, Yamamoto LM, Marzano S, Lopez RM, Jimenez L, Le Bellego L, et al. Mild dehydration impairs cognitive performance and mood of men. Br J Nutr. 2011; 106:1535-43; PMID:21736786; http://dx.doi.org/10.1017/S0007114511002005
- Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health 1988; 43:15-7; PMID:3355239; http://dx.doi.org/10.1080/00039896.19-88.9934367
- Yang RC, Mack GW, Wolfe RR, Nadel ER. Albumin synthesis after intense intermittent exercise in human subjects. J Appl Physiol 1998; 84:584-92; PMID:9475869
- Nose H, Mack GW, Shi XR, Nadel ER. Shift in body fluid compartments after dehydration in humans. J Appl Physiol (1985) 1988; 65:318-24; PMID:3403475
- Haskell A, Nadel ER, Stachenfeld NS, Nagashima K, Mack GW. Transcapillary escape rate of albumin in humans during exercise-induced hypervolemia. J Appl Physiol 1997; 83:407-13; PMID:9262434
- Mack GW, Yang R, Hargens AR, Nagashima K, Haskell A. Influence of hydrostatic pressure gradients on regulation of plasma volume after exercise. J Appl Physiol 1998; 85:667-75; PMID:9688745
- Costill DL, Branam G, Fink W, Nelson R. Exercise induced sodium conservation: changes in plasma renin and aldosterone. Med Sci Sports 1976; 8:209-13; PMID:1011955
- Costill D, Cote R, Miller E, Miller T, Wynder S. Water and electrolyte replacement during repeated days of work in the heat. Aviation Space Environ Med 1975; 46:795-800; PMID:1156286
- Zerbe RL, Miller JZ, Robertson GL. The reproducibility and heritability of individual differences in osmoregulatory function in normal human subjects. J Lab Clin Med 1991; 117:51-9; PMID:1987308
- Szlyk PC, Sils IV, Francesconi RP, Hubbard RW, Armstrong LE. Effects of water temperature and flavoring on voluntary dehydration in men. Physiol Behav 1989; 45:639-47; PMID:2756057; http://dx.doi.org/10.1016/0031-9384(89)90085-1
- Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutri 2010; 92:565-73; PMID:20631205; http://dx.doi.org/10.3945/ajcn.20-10.29490
- Armstrong LE, Maresh CM, Keith NR, Elliott TA, Vanheest JL, Scheett TP, Stoppani J, Judelson DA, De Souza MJ. Heat acclimation and physical training adaptations of young women using different contraceptive hormones. Am J Physiol Endocrinol Metab 2005; 288:E868-75; PMID:15598669; http://dx.doi.org/10.1152/ajpendo.00434.2004
- Mee JA, Gibson OR, Doust J, Maxwell NS. A comparison of males and females' temporal patterning to short- and long-term heat acclimation. Scandinavian J Med Sci Sports 2015; 25(Suppl 1):250-8; PMID:25943676
- Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol: Respirat Environ Exerc Physiol 1980; 49:254-61; PMID:7400008
- Shinohara T, Takahashi N, Ooie T, Ichinose M, Hara M, Yonemochi H, Saikawa T, Yoshimatsu H. Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. J Mol Cell Cardiol 2004; 37:1053-61; PMID:15522282; http://dx.doi.org/10.1016/j.yjmcc.2004.09.006
- Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, et al. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Applied Physiol 2013; 115:937-48; PMID:23681911; http://dx.doi.org/10.1152/japplphysiol.00019.2013
- Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Applied Physiol 2007; 102:1702-7; PMID:17110516; http://dx.doi.org/10.1152/japplp-hysiol.00722.2006
- Luttrell MJ, Halliwill JR. Recovery from exercise: vulnerable state, window of opportunity, or crystal ball? Front Physiol 2015; 6:204; PMID:26257656; http://dx.doi.org/10.3389/fphys.2015.00204
