In nature, there are homoeothermic (“warm-blooded,” such as mammals) and poikilothermic (“cold-blooded,” such as lizards) animals, depending on their metabolic activity and thermodynamic efficiency.Citation2 At thermoneutrality, physiological basal thermogenesis is enough to maintain body temperature without the involvement of any other homeostatic regulatory mechanism.Citation2 Below a species-dependent threshold in environmental temperature, mammals activate heat-saving mechanisms such as vasoconstriction, piloerection, rounded positions and reduction of mobility. When these mechanisms are not enough to maintain body temperature within a physiological range, several thermogenic pathways are triggered such as shivering and non-shivering facultative thermogenesis in brown adipose tissue (BAT). At molecular level this effect is mediated by a BAT protein named uncoupling protein 1 (UCP1), which disrupts the proton electrochemical gradient in the mitochondria from ATP production, to release heat.Citation2 BAT activity is strongly regulated by several brain areas, among them the hypothalamus playing a key role.Citation5
Thyroid hormones (THs) are the main endocrine factors controlling heat production and body temperature.Citation3 The effect of thyroid hormones on thermogenesis and energy expenditure is mediated via 2 compatible non-excluding pathways: “the classical view” and “the central view.”Citation3 In the first one, thyroid hormones directly act on target organs such as liver, white adipose tissue (WAT), BAT, heart, and skeletal muscle increasing their metabolism. In the second one, thyroid hormones act in specific regions of the brain to modulate BAT. More specifically thyroid hormones act in the ventromedial nucleus of hypothalamus (VMH) to inhibit AMP-activated protein kinase (AMPK), leading to increased thermogenesis through activation of the sympathetic nervous system (SNS).Citation3,4 This “VMH AMPK-SNS-BAT axis” is a canonical mechanism modulating energy balance as it has been also described to mediate the thermogenic action of leptin, bone morphogenetic protein 8B, estradiol, glucagon like peptide-1 and nicotine.Citation3,4
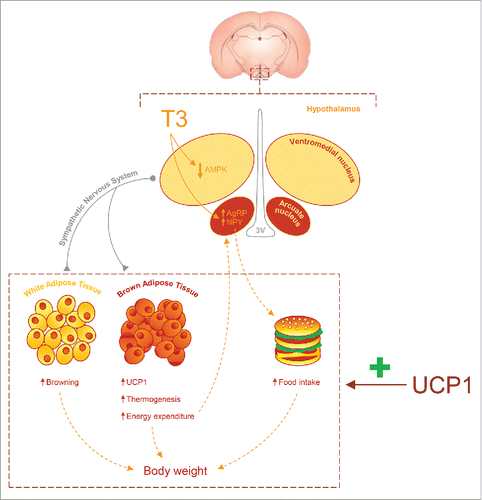
We have investigated the contribution of BAT UCP1 to the thermogenic effects of central thyroid hormones. Using Ucp1 ablated mice and wildtype littermates as controls, we demonstrated that central T3-treatment to control mice increases oxygen consumption and body temperature, leading to weight loss. Notably, those responses are not observed in UCP1 KO counterparts. Importantly, despite these data, the central response to central T3, namely decreased activation of AMPK, remains unaltered in null animals, demonstrating the lack UCP1 protein in BAT does not affect the hypothalamic mechanism, but the peripheral responses to thyroid hormones.Citation1 One important feature is that besides the BAT phenotype, central T3 also promoted browning of WAT, an effect that is also lost in the null animals. Overall these results indicate that a normal UCP1 expression is essential for the effect of thyroid hormones on both BAT and WAT function.Citation1
We also examined the effect of UCP1 ablation on the central effect of T3 on food intake. Our results showed that T3-induced hyperphagia was blunted in null mice. The physiological relevance of this effect is uncertain, but one option could be that lack of thermogenic capacity in Ucp1 null animals would account for reduced fuel demands and then, there was not a necessity for increased feeding. This idea is supported by the fact that central T3 induces a clear increase in the expression of the orexigenic factors agouti-related peptide and neuropeptide Y in the arcuate nucleus of the hypothalamus of wildtype, but not UCP1 KO mice. Overall, these findings support a model were T3 effects on food behavior are modulated by UCP1 induction.Citation1 Whether this is a direct mechanism, or it may be mediated by an indirect humoral feedback mechanism will deserve further investigation.
In summary, this study proposes for the first time how BAT and, more specifically, UCP1 are essential for the thyroid hormones central actions on energy balance (). Considering that the “VMH AMPK-SNS-BAT axis” is a central mechanism modulating energy homeostasis,Citation3,4 it does not seem adventurous to speculate that the thermogenic effect of other factors, such as leptin, bone morphogenetic protein 8B, estradiol, glucagon like peptide-1 and nicotine could be also dependent on normal UCP1 expression in brown fat.
Figure 1. UCP1 is a key factor on central actions of T3. Central T3 inhibits AMP-activated protein kinase (AMPK) in the ventromedial nucleus of the hypothalamus; this modulates brown adipose tissue thermogenesis and browning of white adipose tissue. Moreover, T3 increases food intake through an elevation of the agouti-related peptide (AgRP) and neuropeptide Y (NPY) expression in the arcuate nucleus. All this actions are dependent of UCP1 expression in brown fat and therefore absent in Ucp1 null mice. 3V: third ventricle.
So far, all the evidence on central effect of thyroid hormones on BAT and WAT has been obtained in rodents. The discovery that humans possess active BAT evidently implies that similar mechanisms may occur in humans. This is of relevance because it is known that thyroid status is related to body weight in humans, with hypothyroidism and hyperthyroidism being associated with obesity and leanness, respectively.Citation3 Thus, the targeting of central T3 actions (i.e. AMPK) may provide new strategies to ameliorate thyroid diseases.
References
- Alvarez-Crespo M, Csikasz RI, Martínez-Sánchez N, Diéguez C, Cannon B, Nedergaard J, López M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol Metab 2016; 5:271; PMID:27069867; http://dx.doi.org/10.1016/j.molmet.2016.01.008
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277; PMID:14715917; http://dx.doi.org/10.1152/physrev.00015.2003
- Lopez M, Alvarez CV, Nogueiras R, Diéguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 2013; 19:418; PMID:23707189; http://dx.doi.org/10.1016/j.molmed.2013.04.004
- Lopez M, Nogueiras R, Tena-Sempere M, Diéguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat Rev Endocrinol 2016; 12:421; PMID:27199291
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014; 19:741-56; PMID:24630813; http://dx.doi.org/10.1016/j.cmet.2014.02.007
