An animal is able to detect and interpret a wide range of temperature, while also being able to discriminate between two slightly different temperatures. Such dynamic range enables an animal not only to avoid temperature at both extremities that may cause tissue damage, but also accurately adjust its metabolism (for an endotherm) and/or behavior in response to slight ambient temperature fluctuations. How does the nervous system effectively signal this information with such a high dynamic range?
External temperature is first detected by the free nerve endings in the skin, whose cell bodies are located in the dorsal root ganglia (DRG) (). Over the last two decades, scientists have discovered various molecular detectors expressed by these primary sensory neurons that convert temperature information to electric impulses that can be transmitted through the nervous system. In in vitro systems, each TRP channel is featured by a distinct activation threshold temperature, such as 43°C for the heat/capsaicin-activated channel TRPV1, 25°C for the mild cold/menthol-activated channel TRPM8, 15°C for the putative cold pain channel TRPA1, etc.Citation1,Citation2 Upon activation, ion influx through these TRP channels leads to nerve impulses, and external temperature is detected.
Figure 1. (A) Anatomy of the thermosensory pathway in the spinal cord and periphery. Ambient temperature is first detected by the free nerve endings of primary sensory neurons in the skin. These neurons, whose cell bodies are located in the dorsal root ganglia (DRG), relay information to the dorsal horn of the spinal cord, where thermal information is further processed before being sent to the brain. (B) A schematic illustrating the spinal cord imaging preparation, modified from CitationRef. 4. After a dorsal laminectomy, the lumbar vertebrae of an anesthetized mouse were stabilized using a pair of spinal clamps. The exposed spinal cord was loaded with a fluorescent calcium indicator, allowing in vivo optical recording of neuronal activity. The hind limb was superfused with water, the temperature of which could be precisely controlled. (C) The different temperature intensity-response relationships in the cold (left) and heat (right) ranges. (D) The different kinetics of cold and heat responses. Neuronal response to cold (bottom) correlates with the change of temperature (top), whereas response to heat correlates with the absolute temperature.
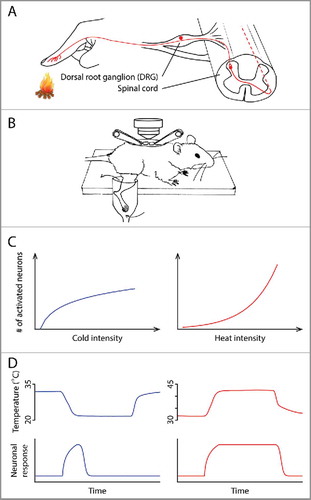
An important next step of the thermosensory field is to understand how electric signals generated at periphery are processed in the central nervous system, which ultimately leads to physiological/behavioral responses and generates our perception of temperature. The distinct activation thresholds of TRP channels imply a modality-specific code at periphery.Citation2 Namely, dedicated “labeled lines” transmit information about an innocuous temperature or a noxious temperature. However, it is not known whether the central nervous system simply retains the modality-specific coding from the peripheral nervous system, or if the representation is transformed into a different scheme. Thus, we investigated the representations of temperature in the dorsal horn of the spinal cord, which receives inputs from primary thermosensory DRG neurons (). The spinal cord is the first relay center in which thermal information is processed by a neural network before being sent to the brain ().Citation3 To study this representation, we developed an in vivo two-photon calcium imaging preparation, which permits simultaneous recording of thermal stimuli-evoked activities of hundreds of spinal neurons in one mouse ().Citation4
If each group of spinal neurons only receives inputs from a specific class of DRG neurons that expresses one type of TRP channel, these spinal neurons would simply retain the activation threshold of their DRG inputs. If so, the whole population of spinal neurons' thresholds would cluster at a few temperature points, just like their DRG partners. Surprisingly, we found that the activation thresholds of spinal neurons are widely and rather evenly distributed in both the heat and cold ranges.Citation4 No distinction between innocuous temperature-sensitive neurons and nociceptive-specific neurons was observed. Namely, the neural representation has been transformed from a modality-specific manner to a population intensity one, by which the spinal cord gradually recruits more neurons to signal increased temperature intensity. The fact that neurons have unique activation thresholds may enable the system to signal a wide spectrum of temperature with high dynamic range. We also notice that the temperature intensity-response relationships in the cold and heat ranges are different. A steep increase in the number of responsive neurons is observed when temperature rises from warmth to strong heat, whereas a more gradual increase is found in the cold range ().
Besides the absolute temperature intensity, our perception of temperature is also influenced by the rate of temperature change. We set out to test it by temporally separating temperature change from absolute temperature. We first let stimulation temperature change at different rates. Then, it enters a stable stage in which it is maintained at its target temperature for an extended period of time. In the heat range, we found neuronal responses stay high while temperature is high, like simple thermometers ().Citation4 Interestingly, it is the change of temperature, rather than the low absolute temperature, that is the effective stimulus to evoke a cold response. When the absolute temperature stays low without changing, the neuronal responses decrease to near baseline level ().Citation4 A series of experiments further confirmed this conclusion. For example, a drop of 8°C in very different absolute temperature ranges elicits similar responses, whereas responses to an increase of 8°C critically depend on the absolute temperature – the higher the absolute temperature is, the larger the responses are.
What is the evolutionary significance for animals to have different mechanisms for signaling cold and heat? First, many animals have a narrow heat tolerance range, and heat can be very detrimental to the animals. Thus, it is critical for heat responses to be non-adaptive so that it could provide a persistent warning signal for the animal. Second, temperatures above normal skin temperature (32°C) differ in salience. Warmth is often attractive to animals and is perceived to be pleasant by human, whereas noxious heat elicits pain and is avoided. By contrast, cold temperatures often do not differ in salience. Any temperatures below 32°C trigger avoidance in mice and only differ quantitatively.Citation5 (In situations that the external temperature is extremely high and cold become attractive, noxious cold usually does not exist in the same environment.) Therefore, precisely discriminating heat temperatures would require a steep intensity-response relationship and a non-adaptive feature. By contrast, the adaptive responses to cold enable the system to signal cold with the similar and relatively small population of neurons at different absolute cold temperatures, which usually carry the same aversive value for an animal after all ().
In summary, our research provides the first comprehensive investigation of the neural representations for temperature in the spinal cord. We demonstrate that the two opposite thermal modalities, cold and heat, are signaled asymmetrically. However, we have just begun to understand the neural circuit of thermosensation in the spinal cord. Future research on the roles of distinct cell types and how these representations change under chronic pain condition will further advance our understandings about this basic sense.
References
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135-161. doi:10.1146/annurev.neuro.29.051605.112958.
- Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34:14-19. doi:10.1016/j.conb.2015.01.010.
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823-836. doi:10.1038/nrn2947.
- Ran C, Hoon MA, Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci. 2016;19:1201-1209. doi: 10.1038/nn.4350.
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533-5541. doi:10.1523/JNEUROSCI.5788-12.2013.
