ABSTRACT
Thermal perception is an essential sensory system for survival since temperature fluctuations affect various biologic processes. Therefore, evolutionary changes in thermosensory systems may have played important roles in adaptation processes. Comparative analyses of sensory receptors among different species can provide us with important clues to understand the molecular basis for adaptation. Several ion channels belonging to the transient receptor potential (TRP) superfamily serve as thermal sensors in a wide variety of animal species. These TRP proteins are multimodal receptors that are activated by temperature as well as other sensory stimuli. Among them TRPV1 and TRPA1 are activated by noxious ranges of thermal stimuli and irritating chemicals, and are mainly expressed in nociceptive sensory neurons. Comparative analyses of TRPV1 and TRPA1 among various vertebrate species revealed evolutionary changes that likely contributed to diversification of sensory perception. Whereas heat-induced TRPV1 responses have been conserved across many vertebrates, TRPA1 varied among species. Mutagenesis experiments using these two channels from various species also helped characterize the molecular basis for their activation and inhibition. Meanwhile, recent detailed comparative analyses using closely related species showed shifts in TRPV1 and TRPA1 thermal sensitivity that allowed adaptation to different thermal environments. Changes in TRPV1 heat responses appear to arise from just a few amino acid differences among species. These observations suggest that evolutionary changes in peripheral sensors are likely driving force for shifting thermal perception in adaptation processes.
Introduction
Ambient temperature is a critical environmental factor for most organisms because it affects various biologic processes that are essential for maintaining homeostasis. Thus, examination of how species have adapted to a diverse range of thermal environments in the biosphere during evolutionary processes is of significant interest. Species inhabiting different thermal niches have acquired physiologic and ecological traits that fit with the environmental conditions of their habitats. Thermosensory systems must also have changed during adaptation processes in concert with other physiologic systems, since the ability to sense the environment and body temperatures are necessary to respond to fluctuations in ambient temperature.
In most animal species, temperature stimuli are perceived by peripheral sensory neurons. Thermal sensors expressed by peripheral sensory neurons are key players that transform temperature changes into electric signals.Citation1 The first discovery of a thermal sensor in rats facilitated investigations that focused on signal transduction pathways,Citation2 and more in-depth characterization of these sensors and pathways revealed the molecular mechanisms of thermal perception in model animals such as rodents and Drosophila.Citation1,Citation3-5 Identification of thermal sensors also enabled the comparison of thermosensory systems among different animal species at a molecular level through characterization of their functional properties. These comparative analyses of thermal sensors in diverse animal species revealed the conservation and also the diversification of the functional properties involved in temperature and chemical sensitivity.Citation6
In recent years, our understanding of thermal sensors in various animal species has rapidly increased. Comparative analyses among different animal species showed that functional changes in thermal sensors contribute to the diversification in sensory perception among species. However, the relationships between evolutionary changes in thermal sensors and evolutionary adaptation are poorly understood. Recent comparative studies that examined closely related animal species adapted to different thermal environments, clarified how evolutionary changes in thermal sensors contribute to niche selection.Citation7,Citation8 This review focuses on the evolution of thermal sensors, especially changes that may be involved in adaptation processes. In addition, we will also discuss how diversity of functional properties among species offers the opportunity to identify the structural bases for activation/inhibition mechanisms of thermal sensors.
Thermosensitive transient receptor potential channels as thermal sensors
Environmental temperatures are detected as changes in skin temperature. Free nerve endings of primary sensory neurons, that lie just beneath the outer surface of the skin, mainly perceive temperature changes as thermal stimuli.Citation1 Several temperature-sensitive ion channels belonging to the transient receptor potential (TRP) superfamily called thermoTRP channels act as thermal sensors.Citation1,Citation3,Citation4 To date, ten thermoTRP channels have been identified in mammals, and they belong to three subfamilies: TRPV (TRPV1, TRPV2, TRPV3, and TRPV4), TRPM (TRPM2, TRPM3, TRPM4, TRPM5, and TRPM8), and TRPA (TRPA1). Many TRP channels are non-selective cation channels that have a tetrameric structure wherein each subunit is composed of six transmembrane domains (). An ion permeable pore is formed by the pore loop domain between the fifth and sixth transmembrane domains.Citation9-11 The N- and C-termini are located intracellularly, and their structural features vary among channels belonging to different subfamilies. For example, the N-terminal region of TRPV and TRPA subfamily channels contain several ankyrin repeat domains.Citation9,Citation11
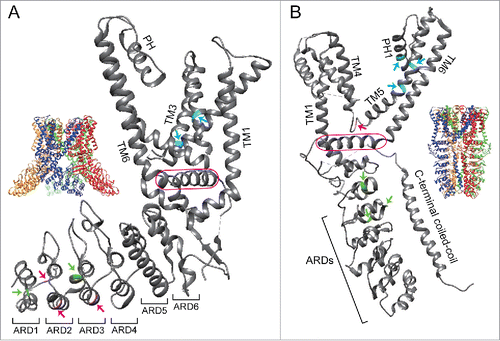
Figure 1. Positions of amino acids involved in the responses of TRPV1 or TRPA1 to chemical and thermal stimuli. (A) Structures of a tetrameric (inset) or a single subunit of rat TRPV1 are shown with critical amino acids involved in its responses to capsaicin (cyan) and heat (magenta or green).Citation9 Magenta and green arrows indicate amino acids that are involved in the responses of TRPV1 to heat in Xenopus and squirrels/camels, respectively.Citation7,Citation8 (B) Structures of a tetrameric (inset) or a single subunit of human TRPA1 are shown with critical amino acids involved in activation or inhibition by A-967079, HC-030031, and methyl anthranilate highlighted in cyan, magenta, and green, respectively.Citation11,Citation52,Citation63,Citation69,Citation70,Citation72 Critical amino acids involved in TRPA1 sensitivity to MA were identified using chicken TRPA1.Citation52 TM, transmembrane domain; PH, pore helix; ARD, ankyrin repeat domain. TRP domain in TRPV1 and TRP-like domain in TRPA1 are enclosed by a magenta line. All figures were prepared using UCSF chimera.Citation84 PBD ID for rat TRPV1 and human TRPA1 are 3J5R and 3J9P, respectively.
Each thermoTRP channel has a distinct range of thermal sensitivity. In rodents, TRPV1, TRPV2, and TRPM3 are activated by noxious heat, whereas TRPM8 is activated by innocuous cold.Citation2,Citation12-15 TRPA1 is reported to be activated by cold in rodents.Citation16 Meanwhile, other thermoTRP channels (TRPV3, TRPV4, TRPM2, TRPM4, and TRPM5) are activated by warmth.Citation1,Citation3,Citation4 Contradictory observations regarding TRPA1 thermal sensitivity have been reported in mammalian species. TRPA1 from mice was first reported to be activated by cold stimulation when heterologously expressed in cultured cells.Citation16 However, later study contended that TRPA1 was not temperature sensitive channel.Citation17 Contradictory results have also been reported in behavioral assays using TRPA1 knockout mice.Citation18-20 Thus, the thermal sensitivity of TRPA1 from both humans and rodents remains a subject of debate.Citation21,Citation22
A peculiar feature of thermoTRP channels is that they are also activated by non-thermal stimulation. For example, TRPV4 is activated by hypotonic and mechanical stimulation.Citation23,Citation24 On the other hand, TRPV1 is activated by capsaicin, contained in chili pepper, and also by extracellular acidic stimulation.Citation2,Citation25 TRPA1 is activated by various irritating chemical compounds contained in plants, including allyl isothiocyanate (AITC), cinnamaldehyde, and carvacrol, which are agents present in wasabi, cinnamon, and oregano, respectively.Citation16,Citation17,Citation21,Citation26 TRPA1 is also activated by environmental irritants such as acrolein contained in exhaust gas and cigarette smoke.Citation20
The tissue distribution of thermoTRP channels varies.Citation1,Citation3,Citation4 TRPV1, TRPA1, and TRPM8 are mainly expressed in dorsal root ganglia (DRG) and trigeminal ganglia (TG) where they serve as thermal sensors in primary sensory neurons. Both TRPA1 and TRPV1 are expressed in nociceptors in DRG and TG, and act as sensors for noxious thermal and chemical perception.Citation1,Citation3,Citation4 TRPV3 and TRPV4 are expressed in keratinocytes, where responses to thermal stimulation are reportedly transmitted to primary sensory neurons.Citation1,Citation27 ThermoTRP channels are also expressed in various tissues and are reported to have diverse physiologic roles, which have been extensively discussed in previous reviews.Citation1,Citation3,Citation4,Citation28-32
Since capsaicin is known to affect core body temperatures in mammals, the involvement of capsaicin-sensitive TRPV1 in thermoregulation was extensively investigated after its discovery. Systemic application of capsaicin is known to cause an acute decline in core body temperature in several mammalian species by increasing heat loss such as peripheral vasodilation.Citation33 This thermoregulatory effect of capsaicin is clearly diminished in TRPV1 knockout mice.Citation34 In addition, systemic application of TRPV1 antagonists causes hyperthermia in several mammalian species including rodents and humans,Citation35 but this effect is completely absent in TRPV1 knockout mice.Citation36 Although TRPV1 knockout mice show normal core body temperature,Citation34 they have increased vasoconstriction and locomotion activity.Citation37 Together these observations support a role for TRPV1 in regulating core body temperatures in mammals. However, mammalian TRPV1 that is heterologously expressed in mammalian cultured cells is activated by noxious temperatures (> 43 °C) that are several degrees higher than typical mammalian core body temperatures, suggesting that the thermal sensitivity of this channel is modulated by the presence of other activators such extracellular protons.Citation25 In addition, TRPV1 phosphorylation mediated by protein kinases alters its activity and sensitivity.Citation38 In contrast to TRPV1 antagonists, systemic application of a TRPM8 antagonist resulted in a decline of core body temperatures in mammals, indicating that TRPM8 is also involved in thermoregulation.Citation39 On the other hand, TRPA1 is not involved in thermoregulation in rodents.Citation40 The physiologic roles of thermoTRP channels in thermoregulation have been summarized in several comprehensive reviews.Citation33,Citation41,Citation42
Species diversity of TRPV1 and TRPA1 in vertebrates
ThermoTRP channels are found in diverse animal species, including vertebrates and invertebrates, in which they serve as thermal sensors. Among the thermoTRP channels, TRPV1 and TRPA1 have been extensively compared across several species since these two channels are involved in nociception that is required to avoid harmful stimulations and are crucial for survival in most animal species.Citation6,Citation43,Citation44 Thus, it is interesting to examine whether or not the functional properties of sensors involved in nociception are conserved through evolutionary processes. In addition, these two channels appear to have functional coordination since they are highly co-expressed in subsets of DRG neurons in rodents.Citation16 Thus, the genes encoding TRPV1 and TRPA1 would be suitable for the investigation of evolutionary relationships involved in similar physiologic roles such as nociception.
Comparison of TRPV1 channel properties revealed that TRPV1 from mammals, birds (chickens), amphibians (tropical clawed frogs), and teleost fishes (zebrafish) is activated by heat.Citation2,Citation45-47 Thus, heat-evoked activity of TRPV1 is generally a conserved trait across a wide variety of vertebrate species, although a recent study showed that its heat activity is diminished in ground squirrels and camels (see below).Citation7 On the other hand, TRPV1 capsaicin sensitivity varies among vertebrate species.,Citation6,Citation8,Citation46-48 TRPV1 from several mammalian species including humans, rodents, and dogs is highly sensitive to capsaicin, whereas TRPV1 from rabbits, chickens, tropical clawed frogs, and zebrafish has considerably lower sensitivity to capsaicin.Citation45-50
Comparison of TRPA1 channel properties among vertebrate species showed significant variations in thermal sensitivity (). As mentioned above, TRPA1 from humans and rodents can be activated by cold.Citation3,Citation16,Citation18,Citation22 In contrast, heterologously expressed TRPA1 from chickens, reptiles (several snake species and green anole lizards), and tropical clawed frogs is activated by heat stimulation.Citation51-53 TRPA1 from dipteran insects is also reported to be activated by warmth and is known to be involved in negative thermotaxis.Citation54-57 Heterologously expressed zebrafish TRPA1 was recently shown to be activated by both heat and cold,Citation58 but zebrafish with TRPA1 knockout had no behavioral abnormalities in response to either cold or heat stimulation,Citation59 suggesting that its physiologic roles in thermal sensitivity await further analysis. Although the thermal sensitivity of TRPA1 from distantly related animal species is likely to differ, chemical sensitivity to electrophilic compounds such as AITC and cinnamaldehyde is generally conserved.Citation6,Citation51-53,Citation58,Citation59 Therefore, a physiologic role of TRPA1 in detecting irritating chemicals seems to be maintained through animal evolutionary processes.
Figure 2. Evolutionary scheme for TRPA1 and TRPV1 in vertebrates. TRPA1 was likely acquired as a sensor for heat and noxious chemicals in ancestral animal species. Subsequently, TRPV1 emerged by gene duplication in ancestral vertebrates and was likely co-expressed with TRPA1 in sensory neurons. TRPV1 retained heat sensitivity, whereas thermal sensitivity of TRPA1 may have changed during vertebrate evolution. MYA: million years ago.
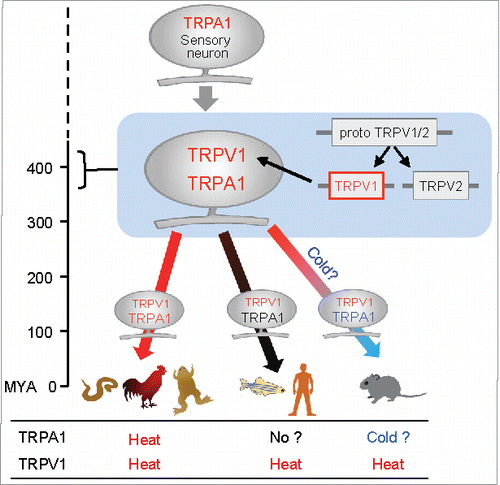
Phylogenetic analysis revealed that TRPA1 orthologs are shared between vertebrates and invertebrates, suggesting that TRPA1 emerged during the early stages of animal evolution ().Citation6,Citation60,Citation61 On the other hand, TRPV1 appear to have emerged later in ancestral vertebrates by a gene duplication event.Citation6,Citation61,Citation62 Furthermore, TRPV1 and TRPA1 are known to be highly co-expressed in DRG neurons in a wide range of vertebrate species, including zebrafish, tropical clawed frogs, chickens, and rodents.Citation16,Citation52,Citation53,Citation59 All of these observations suggest that heat-activated TRPV1 emerged more recently and has become co-expressed with pre-existing TRPA1 in the subset of nociceptive neurons in ancestral vertebrates ().Citation6,Citation52,Citation53 Acquisition of TRPV1 as a novel heat sensor in ancestral vertebrates could have reduced functional constraints of TRPA1 and led to changes in its thermal sensitivity in several vertebrate lineages. Based on its ability to detect irritating chemicals and its physiologically conserved roles in each of the species examined, evolutionary pressure to retain TRPA1 gene was likely higher throughout vertebrate evolutionary processes.
Using species-specific differences to identify the molecular basis for TRPV1 and TRPA1 activation/inhibition
Comparative studies of thermoTRP channels among different vertebrate species revealed evolutionary diversification of channel properties. These species-specific differences in thermoTRP channels can provide clues for identifying the structural basis for activation or inhibitory mechanisms.Citation22,Citation63,Citation64 Comparative analyses and mutagenesis experiments examining TRPV1 among different vertebrate species revealed that two amino acids located in the third and fourth transmembrane domains play a key role in determining sensitivity to capsaicin (A).Citation46-48 For example, serine and threonine residues at positions 512 (S512) and 550 (T550) in rat TRPV1 are substituted with tyrosine (Y523) and alanine (A561) in the corresponding positions of tropical clawed frog TRPV1, which shows reduced sensitivity to capsaicin.Citation6,Citation47 Recently, a detailed 3D structure of rat TRPV1 in complex with capsaicin (and related vanilloid compounds) showed that critical amino acids identified in previous studies were located within the capsaicin binding pocket (A).Citation9,Citation65,Citation66 Moreover, S512 and T550 form a hydrogen bond with moieties within and near the vanilloid ring, respectively.Citation66 Thus, these two residues play a critical role in the binding of vanilloid compounds to TRPV1.Citation65,Citation66 In tropical clawed frog TRPV1, interactions between the side chains of two critical amino acids (Y523 and A561) and vanilloid compounds likely interfere because of difference in side chain sizes (tyrosine and alanine possess a larger and smaller side chains compared with those of serine and threonine, respectively). Interestingly, African clawed frog TRPV1 has a cysteine residue (C521) at the corresponding position of 512 in rat TRPV1 (S512) and side changes of these two amino acids have relatively similar sizes. Accordingly, African clawed frog TRPV1 has higher sensitivity to capsaicin than the tropical clawed frog TRPV1, and mutating C521 to tyrosine (C521Y) leads to a marked reduction in TRPV1 sensitivity to capsaicin.Citation8 It has been proposed that phosphatidylinositides bind to rat TRPV1 in the closed state at the same binding site as that for vanilloid compounds, and replacement of phosphatidylinositides with vanilloid compounds leads to TRPV1 activation.Citation66
Similar to TRPV1, sensitivity of TRPA1 to several non-electrophilic chemicals differs among species, although sensitivities of TRPA1s to electrophilic chemicals are well conserved. Methyl anthranilate (MA) is known as a repellent for birds and TRPA1 from chickens is activated by MA.Citation52,Citation67 TRPA1 from humans and mice is also activated by MA, whereas TRPA1 from green anole lizards and tropical clawed frogs showed reduced responses to it. A comparison of amino acid sequences among TRPA1s from these five species highlighted several candidate amino acids sites that could be involved in species-specific differences. The potential amino acid sites were further screened by assessing chicken TRPA1 point mutants, which showed that the three critical amino acids involved in TRPA1 activity to MA lie close to the linker between the ankyrin repeat domains and transmembrane domains ().Citation52
A similar strategy led to the identification of critical amino acids in TRPA1 inhibition. Several TRPA1 antagonists have been developed for use as potential analgesics. As these antagonists were developed against mammalian TRPA1, their antagonistic effects differ for TRPA1s from non-mammalian species. For example, a potent mammalian TRPA1 antagonist A-967079 does not inhibit TRPA1 from non-mammalian species, such as chickens and tropical clawed frogs.Citation68-70 Detailed comparative analyses revealed that several amino acid residues located within the fifth transmembrane domain of mammalian TRPA1 are important for the inhibitory effect of A-967079.Citation63,Citation69,Citation70 A subsequent 3D structure of human TRPA1 in complex with A-967079 showed that amino acid residues reported to be involved in TRPA1 inhibitions are located near the binding sites of A-967079 ().Citation11 Two amino acid residues (S873 and T874) located in the fifth transmembrane domain lie at the bottom of the A-967079 binding pocket.Citation11 Non-mammalian vertebrate TRPA1s are not inhibited by A-967079, which is consistent with different amino acids in the corresponding region that may be responsible for the lack of inhibitory effect.Citation6,Citation69,Citation70 A-967079 also interacts with F909, which is conserved among vertebrate TRPA1 in pore-helix 1 and proposed to act as a molecular wedge to inhibit opening of the lower gate of the channel.Citation11
Interestingly, A-967079 acts as an agonist to TRPA1 from chickens and tropical clawed frogs, and detailed comparative analyses showed that a single amino acid mutation (L881I) in human TRPA1 resulted in activation by A-967079 rather than inhibition.Citation69 L881 is in the fifth transmembrane domain and lies close to A-967079 and F909 in the 3D structure model of human TRPA1 ().Citation11 Notably, leucine and isoleucine are structurally similar, suggesting that slight conformational changes in chemical binding pockets can drastically change channel properties.
Species differences are also seen for the another TRPA1 antagonist HC-030031, which has a different structure from A-967079 and was first developed as a mammalian TRPA1 antagonist.Citation71 HC-030031 was subsequently shown to inhibit chicken and green anole TRPA1 activity, but it had no inhibitory effect on TRPA1 from tropical clawed frogs and zebrafish.Citation52,Citation53,Citation70 Examination of TRPA1 chimeric channels between humans and tropical clawed frogs and subsequent point mutant analysis revealed that a single amino acid residue (N855) located in the linker between the fourth and fifth transmembrane domains plays a critical role in the inhibitory effect of HC-030031 on human TRPA1 ().Citation72 Furthermore, a molecular dynamics simulation showed that N855 could interact with HC-030031. In the 3D structure of human TRPA1, N855 is located close to the TRP-like domain that is situated below the transmembrane domains, and can interact with the intracellular N- and C-terminal regions ().Citation11 A detailed chimeric and mutagenesis analysis between TRPA1 from human and tropical clawed frogs indicated that the C-terminal region that include a TRP-like domain is also involved in HC-030031-mediated inhibition of TRPA1.Citation72
On the other hand, green anole TRPA1 requires the presence of extracellular calcium ions for its heat-evoked activation.Citation73 Furthermore, comparative and mutagenesis analyses using TRPA1s from green anoles, rat snakes, and chickens showed that three negatively charged amino acids near the outer pore vestibule are involved in heat-evoked activation of TRPA1.Citation74 Presumably, calcium ions would neutralize these negatively charged amino acids to allow heat-induced opening of the channel pore. Thus, species diversity of thermoTRP channels provides informative clues for identifying critical amino acids involved in the activation and inhibitory action of these channels. Moreover, structural information for thermoTRP channels helps clarify the molecular mechanism for regulating channel activity, and provides mechanistic insights into evolutionary changes of channel function
Evolutionary changes in thermal sensors among species adapted to different environmental niches
One intriguing topic in thermal biology is molecular basis for adaptation. Given that two different species adapted to different thermal niches in the course of evolution, temperature sensitivity would be expected to diverge between the two species. To elucidate the evolutionary changes in thermal perception, comparative analyses among closely related species inhabiting different niches are needed. For this purpose, thermal responses were compared between two closely related clawed frog species, the tropical clawed frog, Xenopus tropicalis and the African clawed frog, Xenopus laevis ().Citation8 The distributions of these two species are different in Africa. X. tropicalis inhabits a hotter environment and thus has a higher optimal temperature range than X. laevis ().Citation75 A comparison of nocifensive responses (jumping) to heat stimulation revealed, as expected, that X. laevis has higher sensitivity to heat than X. tropicalis (). In addition, the thermal sensitivity of dissociated DRG neurons also differed between the two Xenopus species, suggesting that heat sensitivity differs both at behavioral and neural levels.
Figure 3. (A) Species differences in heat responses between X. laevis and X. tropicalis. Optimal temperatures are indicated beside the images of the two Xenopus species. (B) Species differences in heat responses between the two species of clawed frogs are summarized.
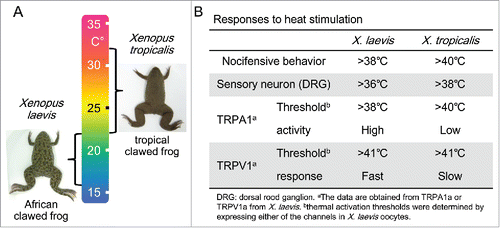
Then, functional properties of TRPA1 and TRPV1 were compared. X. laevis carries two TRPA1 gene copies (TRPA1a and TRPA1b) since they are allotetraploid species,Citation76 whereas X. tropicalis has only one copy of the TRPA1 gene. Expression of X. laevis TRPA1b is confined to the brain, whereas X. laevis TRPA1a is widely expressed in peripheral neurons, spinal cord, and brain,Citation8 suggesting that TRPA1a potentially plays a major role in thermal perception. The amplitudes of heat-evoked currents of X. laevis TRPA1a are much larger than those of X. tropicalis TRPA1 when both were heterologously expressed. Moreover, thermal activation thresholds for X. laevis TRPA1a were lower than those of X. tropicalis TRPA1. These observations indicate that the activities and sensitivities of TRPA1 to heat stimulation are significantly higher in X. laevis compared with X. tropicalis, which is consistent with the finding that X. laevis is behaviorally much more sensitive to heat than X. tropicalis ().
Whereas the thermal activation thresholds for TRPV1 are similar between the two Xenopus species, a species difference in TRPV1 was observed upon application of repeated heat stimulation. As with TRPA1, X. laevis also carries two TRPV1 genes (TRPV1a and TRPV1b). Both copies of X. laevis TRPV1 are widely expressed in neural tissues; however, current amplitudes elicited by X. laevis TRPV1b are much smaller than those seen for X. laevis TRPV1a. Meanwhile, X. tropicalis TRPV1 channel activity gradually increased with repeated heat stimulation, whereas that of X. laevis TRPV1a peaked during the first heat stimulation and became desensitized to repeated heat stimulations when it was heterologously expressed (). Thermal activation thresholds of TRPV1 for the two species were nearly the same. Therefore, X. laevis TRPV1a is activated much faster than X. tropicalis TRPV1 upon exposure to temperatures that exceed their activation thresholds. All of these observations are consistent with the greater heat sensitivity of X. laevis compared with X. tropicalis. The species differences of Xenopus TRPV1 heat responses can be partly explained by the three amino acid substitutions that occur in ankyrin repeat domains 1–3 ().Citation8 In rats, several molecules such as ATP and calmodulins are known to bind to the ankyrin repeat domains and modulate TRPV1 activity.Citation77-79 Single critical amino acid located within ankyrin repeat domain 3 overlaps with the calmodulin binding region in rat TRPV1. Future biochemical analyses will be needed to clarify the molecular mechanisms responsible for the species differences of Xenopus TRPV1.
As discussed above, species differences in both TRPV1 and TRPA1 can be linked to differences in neural and behavioral responses between Xenopus species adapted to different thermal niches. However, the degree of these differences was relatively small in the context of the optimal temperature ranges for the two species (). This small difference might be due to the experimental conditions used since rapid heat stimulation was adopted throughout the studies. Thus, comparison of behavioral traits using other experimental conditions and approaches (e.g., thermal preference test) should reveal different aspects of species differences.
Striking differences in TRPV1 heat responses were also found among mammalian species that are adapted to extremely hot environments and acquired heat tolerances such as the thirteen-lined ground squirrel, Ictidomys tridecemlineatus and the Bactrian camels, Camelus ferus.Citation7 I. tridecemlineatus showed reduced heat avoidance relative to mice and dissociated DRG neurons from these squirrels also showed reduced heat sensitivity. Characterization of TRPV1 from I. tridecemlineatus revealed that its heat-evoked activity was severely suppressed, although its responses to capsaicin and acid stimulation were maintained, suggesting that TRPV1 in this species specifically lost heat sensitivity. C. ferus TRPV1 also showed a specific loss of heat sensitivity, suggesting that convergent evolution could have occurred in different mammalian lineages.Citation7 Detailed mutagenesis analysis revealed that mutation of two amino acid residues located within the ankyrin repeat domains resulted in the gain of heat-evoked activity in TRPV1 from I. tridecemlineatus and C. ferus (), although these amino acid changes likely did not occur in the lineages leading to each species.
As species shift among thermal niches, various kinds of genes that are expressed in tissues, including those from peripheral to the central nervous systems, are potentially involved in evolutionary changes in thermal perception. Nevertheless, peripheral sensors such as thermoTRP channels underwent tuning of functional properties to fit with environmental niches during the adaptation process.Citation7,Citation8 For Xenopus species, relatively small functional shifts in thermal sensors occurred. Such small and gradual changes in sensory receptors may be frequent events in the evolutionary adaptation to different thermal niches. On the other hand, for I. tridecemlineatus and C. ferus, heat sensitivity of TRPV1 was specifically lost even though it is involved in noxious heat perception and is generally thought to be required to escape harmful stimuli. This loss may imply that to adapt to extreme environments, even noxious signal transduction pathways can degenerate if the overall effect is beneficial. Functional changes in thermoTRP channels can likely be achieved through a small number of amino acid substitutions ().Citation7,Citation8 In this way, thermoTRP channels can retain functional flexibility during evolutionary processes and might serve as one example of how thermoTRP channels could participate in evolutionary tuning of thermal sensing adaptations.
Future Perspectives
Almost two decades have passed since the discovery of the first thermoTRP channels.Citation2 Since then, numerous studies have been conducted to examine the physiologic role of these channels in model animals such as Drosophila and rodents. In contrast, investigations that explored the relationships between thermoTRP channels from different species and evolutionary adaptation have just begun to accumulate. Recent comparative studies in clawed frogs and mammals revealed clear species differences that likely contribute to adaptations to thermal niches.Citation7,Citation8 In these studies, however, experimental evidence showing the in vivo roles of thermoTRP channels is lacking, and what extent thermoTRP channels affect sensory perception and behavioral responses remain unclear. TRPV1 knockout mice have deficits in perception in a noxious temperature range (> 50 °C),Citation34 even though TRPV1 is activated above 43 °C in heterologous expression systems.Citation25 This finding implies that TRPV1 plays only partial roles in nociceptive heat responses in mice. Therefore, assessment of the in vivo roles of thermoTRP channels among species will be important to investigate in the future studies. In this respect, recently developed genome editing technologies can provide opportunities to examine the physiologic roles of thermoTRP channels in species for which generation of genetically modified animals was previously difficult.Citation80,Citation81
Another intriguing area of investigation is the molecular basis for evolutionary changes in thermoTRP channels. Comparative analyses of thermoTRP channels in clawed frogs and mammals identified critical amino acid changes in the ankyrin repeat domains that altered thermal responses of TRPV1 ().Citation7,Citation8 These observations suggest that ankyrin repeat domains could be target regions for functional tuning in an evolutionary context. However, whether the currently identified amino acids actually play critical roles in evolutionary processes is unclear, since both studies only compared TRPV1 from extant species for chimeric and mutagenesis analyses. The same amino acid mutations are known to have the possibility to induce inconsistent functional changes in different protein backgrounds due to epistatic interactions among amino acids.Citation82,Citation83 Thus, in some case, mutagenesis analyses using only extant species can lead to inaccurate conclusions. Evolutionary trajectories of protein sequences can be recapitulated by inferring ancestral states, and the effects of amino acid changes can also be examined by introducing mutations in ancestral protein backgrounds to identify critical changes that may have caused functional shifts in an evolutionary context.Citation82,Citation83 The future challenge will be to identify critical amino acid changes that caused the functional shifts in thermoTRP channels in an evolutionary context, and introducing these mutations in species under investigation using genome editing technologies to assess their effects on neural and behavioral responses. Moreover, additional evidence from various groups of animal species will be required for a better understanding of the general molecular mechanisms that are associated with adaptive evolutionary changes in thermal perception.
Abbreviations
| AITC | = | allyl isothiocyanate |
| DRG | = | dorsal root ganglia |
| MA | = | methyl anthranilate |
| TG | = | trigeminal ganglia |
| TRP | = | transient receptor potential |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Claire T. Saito for critical reading of this manuscript.
This work was supported by Grants-in-aid for Scientific Research 15H05927 (to M.T.) and 16K07470 (to S.S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Funding
References
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4(7):529–539. PMID:12838328; doi:10.1038/nrn1141.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. PMID:9349813; doi:10.1038/39807.
- Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17(4):490–497. PMID: 17706410; doi:10.1016/j.conb.2007.07.014.
- Tominaga M. The Role of TRP Channels in Thermosensation. In: Liedtke WB, Heller S, editors. TRP ion channel function in sensory transduction and cellular signaling cascades. Frontiers in neuroscience; 2007. Boca Raton, FL: CRC Press. PMID: 21204494.
- Barbagallo B, Garrity PA Temperature sensation in Drosophila. Curr Opin Neurobiol. 2015;34:8–13. PMID:25616212; doi:10.1016/j.conb.2015.01.002.
- Saito S, Tominaga M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium. 2015;57(3):214–221. PMID:25533790; doi:10.1016/j.ceca.2014.12.001.
- Laursen WJ, Schneider ER, Merriman DK, Bagriantsev SN, Gracheva EO. Low-cost functional plasticity of TRPV1 supports heat tolerance in squirrels and camels. Proc Natl Acad Sci USA. 2016;113(40):11342–11347. PMID:27638213; doi:10.1073/pnas.1604269113.
- Saito S, Ohkita M, Saito CT, Takahashi K, Tominaga M, Ohta T. Evolution of heat sensors drove shifts in thermosensation between Xenopus species adapted to different thermal niches. J Biol Chem. 2016;291(21):11446–11459. PMID:27022021; doi:10.1074/jbc.M115.702498.
- Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. PMID:24305160; doi:10.1038/nature12822.
- Saotome K, Singh AK, Yelshanskaya MV, Sobolevsky AI. Crystal structure of the epithelial calcium channel TRPV6. Nature. 2016;534(7608):506–511. PMID:27296226; doi:10.1038/nature17975.
- Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–517. PMID:25855297; doi:10.1038/nature14367.
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. PMID:10201375; doi:10.1038/18906.
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. PMID:11893340; doi:10.1016/S0092-8674(02)00652-9.
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. PMID:11882888; doi:10.1038/nature719.
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen XD, Benoit M, Xue FQ, Janssens A, Kerselaers S, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70(3):482–494. PMID: 21555074; doi:10.1016/j.neuron.2011.02.051.
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. PMID:12654248; doi:10.1016/S0092-8674(03)00158-2.
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. PMID:14712238; doi:10.1038/nature02282.
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natil Acad Sci U S A. 2009;106(4):1273–1278. PMID:19144922; doi:10.1073/pnas.0808487106.
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. PMID: 16630838; doi:10.1016/j.neuron.2006.03.042.
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. PMID: 16564016; doi:10.1016/j.cell.2006.02.023.
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. PMID:15046718; doi:10.1016/S0896-6273(04)00150-3.
- Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013;4:2501. PMID: 24071625; doi:10.1038/ncomms3501.
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–535.
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. PMID:11025659; doi:10.1038/35036318.
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. PMID:9768840; doi:10.1016/S0896-6273(00)80564-4.
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9(5):628–635. PMID:16617338; doi:10.1038/nn1692.
- Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451(1):160–167. PMID:15952037; doi:10.1007/s00424-005-1438-y.
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464(5):425–458. PMID:23001121; doi:10.1007/s00424-012-1158-z.
- Laing RJ, Dhaka A. ThermoTRPs and Pain. Neuroscientist. 2016;22(2):171–187. PMID:25608689; doi:10.1177/1073858414567884.
- Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. PMID:24099085; doi:10.1146/annurev-cellbio-101011-155833.
- Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol. 2012;2(1):563–608. PMID:23728980; doi:10.1002/cphy.c110026.
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61(1):3–12. PMID:15362149; doi:10.1002/neu.20079.
- Wang H, Siemens J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature. 2015;2(2):178–187. PMID:27227022; doi:10.1080/23328940.2015.1040604.
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. PMID:10764638; doi:10.1126/science.288.5464.306.
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–210. PMID: 18337008; doi:10.1016/j.pain.2008.01.024.
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27(28):7459–7468. PMID:17626206; doi:10.1523/JNEUROSCI.1483-07.2007.
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31(5):1721–1733. PMID:21289181; doi:10.1523/JNEUROSCI.4671-10.2011.
- Li L, Hasan R, Zhang X. The basal thermal sensitivity of the TRPV1 ion channel is determined by PKCbetaII. J Neurosci. 2014;34(24):8246–8258. PMID:24920628; doi:10.1523/JNEUROSCI.0278-14.2014.
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32(6):2086–2099. PMID:22323721; doi:10.1523/JNEUROSCI.5606-11.2012.
- de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR, et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci. 2014;34(13):4445–4452. PMID:24671991; doi:10.1523/JNEUROSCI.5387-13.2014.
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61(3):228–261. PMID: 19749171; doi:10.1124/pr.109.001263.
- Szolcsanyi J. Effect of capsaicin on thermoregulation: an update with new aspects. Temperature. 2015;2(2):277–296. PMID:27227029; doi:10.1080/23328940.2015.1048928.
- Bagriantsev SN, Gracheva EO. Molecular mechanisms of temperature adaptation. J Physiol. 2015;593(16):3483–3491. PMID:25433072; doi:10.1113/jphysiol.2014.280446.
- Laursen WJ, Anderson EO, Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. Species-specific temperature sensitivity of TRPA1. Temperature. 2015;2(2):214–226. PMID:27227025; doi:10.1080/23328940.2014.1000702.
- Gau P, Poon J, Ufret-Vincenty C, Snelson CD, Gordon SE, Raible DW, Dhaka A. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J Neurosci. 2013;33(12):5249–5260. PMID:23516290; doi:10.1523/JNEUROSCI.5403-12.2013.
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell. 2002;108(3):421–430. PMID:11853675; doi:10.1016/S0092-8674(02)00637-2.
- Ohkita M, Saito S, Imagawa T, Takahashi K, Tominaga M, Ohta T. Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates. J Biol Chem. 2012;287(4):2388–2397. PMID:22130664; doi:10.1074/jbc.M111.305698.
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279(19):20283–20295. PMID:14996838; doi:10.1074/jbc.M312577200.
- Phelps PT, Anthes JC, Correll CC. Cloning and functional characterization of dog transient receptor potential vanilloid receptor-1 (TRPV1). Eur J Pharmacol. 2005;513(1–2):57–66. PMID: 15878709; doi:10.1016/j.ejphar.2005.02.045.
- McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Brit J Pharmacol. 2001;132(5):1084–1094. PMID: 11226139; doi:10.1038/sj.bjp.0703918.
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010;464(7291):1006–1011. PMID:20228791; doi:10.1038/nature08943.
- Saito S, Banzawa N, Fukuta N, Saito CT, Takahashi K, Imagawa T, Ohta T, Tominaga M. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol Biol Evol. 2014;31(3):708–722. PMID:24398321; doi:10.1093/molbev/msu001.
- Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem. 2012;287(36):30743–30754. PMID:22791718; doi:10.1074/jbc.M112.362194.
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423(6942):822–823. PMID:12815418; doi:10.1038/423822a.
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19(4):419–424. PMID:15681611; doi:10.1101/gad.1278205.
- Kang KJ, Panzano VC, Chang EC, Ni LN, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481(7379):76–U82. PMID: 22139422; doi:10.1038/nature10715.
- Bellemer A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature. 2015;2(2):227–243. PMID:27227026; doi:10.1080/23328940.2015.1004972.
- Oda M, Kurogi M, Kubo Y, Saitoh O. Sensitivities of two Zebrafish TRPA1 paralogs to chemical and thermal stimuli analyzed in heterologous expression systems. Chem Senses. 2016;41(3):261–272. PMID:26826723; doi:10.1093/chemse/bjv091.
- Prober DA, Zimmerman S, Myers BR, McDermott BM, Jr, Kim SH, Caron S, Rihel J, Solnica-Krezel L, Julius D, Hudspeth AJ, et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28(40):10102–10110. PMID:18829968; doi:10.1523/JNEUROSCI.2740-08.2008.
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464(7288):597–600. PMID:20237474; doi:10.1038/nature08848.
- Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics. 2006;27(3):219–230. PMID:16926268; doi:10.1152/physiolgenomics.00322.2005.
- Saito S, Fukuta N, Shingai R, Tominaga M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011;7(4):e1002041. PMID:21490957; doi:10.1371/journal.pgen.1002041.
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28(39):9640–9651. PMID:18815250; doi:10.1523/JNEUROSCI.2772-08.2008.
- Nagatomo K, Ishii H, Yamamoto T, Nakajo K, Kubo Y. The Met268Pro mutation of mouse TRPA1 changes the effect of caffeine from activation to suppression. Biophys J. 2010;99(11):3609–3618. PMID: 21112285; doi:10.1016/j.bpj.2010.10.014.
- Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504(7478):113–118. PMID:24305161; doi:10.1038/nature12823.
- Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534(7607):347–351. PMID:27281200; doi:10.1038/nature17964.
- Mason JR, Adams MA, Clark L. Anthranilate repellency to starlings – chemical correlates and sensory perception. J Wildlife Manage. 1989;53(1):55–64. doi:10.2307/3801306.
- Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152(5):1165–1172. PMID: 21402443; doi:10.1016/j.pain.2011.01.049.
- Banzawa N, Saito S, Imagawa T, Kashio M, Takahashi K, Tominaga M, Ohta T. Molecular basis determining inhibition/activation of nociceptive receptor TRPA1: a single amino acid dictates species-specific actions of the most potent mammalian TRPA1 antagonists. J Biol Chem. 2014;289(46):31927–31939. PMID:25271161; doi:10.1074/jbc.M114.586891.
- Nakatsuka K, Gupta R, Saito S, Banzawa N, Takahashi K, Tominaga M, Ohta T. Identification of molecular determinants for a potent mammalian TRPA1 antagonist by utilizing species differences. J Mol Neurosci. 2013;51(3):754–762. PMID:23872983; doi:10.1007/s12031-013-0060-2.
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104(33):13525–13530. PMID:17686976; doi:10.1073/pnas.0705924104.
- Gupta R, Saito S, Mori Y, Itoh SG, Okumura H, Tominaga M. Structural basis of TRPA1 inhibition by HC-030031 utilizing species-specific differences. Sci Rep. 2016;6:37460. PMID:27874100; doi:10.1038/srep37460.
- Kurganov E, Zhou Y, Saito S, Tominaga M. Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflugers Arch. 2014;466(10):1873–1884. PMID:24385018; doi:10.1007/s00424-013-1420-z.
- Kurganov E, Saito S, Saito CT, Tominaga M. Requirement of extracellular Ca2+ binding to specific amino acids for heat-evoked activation of TRPA1. J Physiol. 2017. PMID:28194754; doi:10.1113/JP274083.
- Tinsley RC, Kobel HR, The biology of Xenopus. London: Zoological Society of London; 1996. p. 35–44.
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538(7625):336–343. PMID:27762356; doi:10.1038/nature19840.
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. PMID: 17582331; doi:10.1016/j.neuron.2007.05.027.
- Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285(1):731–740. PMID:19864432; doi:10.1074/jbc.M109.052548.
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62. PMID:14699077; doi:10.1085/jgp.200308906.
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. PMID:22745249; doi:10.1126/science.1225829.
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–355. PMID:24584096; doi:10.1038/nbt.2842.
- Dean AM, Thornton JW. Mechanistic approaches to the study of evolution: the functional synthesis. Nat Rev Genet. 2007;8(9):675–688. PMID:17703238; doi:10.1038/nrg2160.
- Yokoyama S. Synthetic biology of phenotypic adaptation in vertebrates: the next frontier. Mol Biol Evol. 2013;30(7):1495–1499. PMID:23603936; doi:10.1093/molbev/mst075.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF chimera – a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. PMID:15264254; doi:10.1002/jcc.20084.
