ABSTRACT
Brain-derived neurotrophic factor (BDNF) is a biomarker of cognitive function that is released into the blood stream following exercise, and cognitive function is impaired by environmental temperatures that are hot and cold. Purpose: To evaluate the exercise-dependent release of BDNF in different environmental temperatures. Methods: Recreationally trained males each completed three trials consisting of cycling for 1 h at 60% Wmax at three different temperatures: 33°C (hot), 7°C (cold), and 20°C (moderate room temperature). Blood was taken from the antecubital vein pre-exercise, immediately post-exercise, and 3 h post-exercise. Respiratory gases were collected periodically throughout exercise and recovery. Results: BDNF was elevated immediately following an exercise bout (1711 ± 766 pg·ml−1) regardless of temperature from pre-exercise (1257 ± 653 pg·ml−1, p = 0.001) and returned to basal levels following 3 h of recovery (1289 ± 650 pg·ml−1, p = 0.786). There was no effect (p > 0.05) of temperature on BDNF following the exercise bout. Plasma glucose was elevated in hot (6.2 ± 0.9 mmol) over cold (5.3 ± 0.6 mmol, p = 0.035) and moderate room temperature (5.2 ± 0.5, p = 0.008). VO2 was elevated during exercise in hot (3.01 ± 0.45 L·min−1) over cold (2.67 ± 0.35 L·min−1, p = 0.005) and moderate room temperature (2.80 ± 0.38 L·min−1, p = 0.001). There was no relationship between BDNF and plasma glucose (p > 0.05) or VO2 across any time point or temperature (p > 0.05). Conclusion: With aerobic exercise, BDNF is elevated; however, the release of BDNF is not impacted by different environmental temperatures during exercise.
Introduction
Aerobic fitness has been associated with higher cognitive control in children.Citation1-Citation4 Higher cognitive function with increasing aerobic function has also been observed in terms of academic performance in children.Citation5 In older adults, aerobic fitness positively correlates with cognitive functions (r > 0.4, p < 0.05),Citation6 brain volume (p = 0.025),Citation6 and cognitive control (p < 0.05).Citation7 It was previously thought that any cognitive improvement from aerobic exercise was facilitated by an increase in number of blood vessels in the brain thereby increasing oxygen delivery to the neurons.Citation8 However, cognitive function improvements via aerobic exercise can be attributed to neuronal growth and development as well.Citation8 Following aerobic training, rats demonstrated drastic improvement in memory and spatial learning.Citation9 and improved performance in a Morris water maze.Citation10 Little is known about the underlying processes that facilitate these cognitive improvements.
Improvements in spatial memory and cognitive function are likely due to neural alterations within the hippocampus. The hippocampus converts short-term memories to long-term memories and is the learning center of the brain.Citation11-Citation14 The hippocampus is the first site affected by the release of Brain-derived neurotrophic factor (BDNF), a powerful neurotrophin in neoneurogenesis.Citation15 BDNF may be a key factor in exercise related cognitive improvement, and further investigation into the nature of the control pathways of exercise-dependent BDNF response is warranted.
BDNF release also appears to be associated with metabolic processes and energy management. There is a 2- to 3-fold increase in BDNF release during exercise,Citation16 elevating from resting levels of serum BDNF that range from 0.4 ng/ml to 30 ng/ml.Citation17 Furthermore, there is an elevation in BDNF production when exogenous glucose is administered to mice in a resting state,Citation18,Citation19 showing a sensitivity of BDNF to blood glucose. These increases in BDNF release are believed to be a physiologic response to energy management and metabolism.Citation18,Citation20 Increases in serum levels of BDNF are transient, and typically return to baseline levels within 1 h.Citation16,Citation17
Exercise-dependent BDNF release may alter the metabolic response to exercise. One potential strategy to alter metabolic response to exercise is to alter the environmental temperature.Citation21 PGC-1α is a protein that has been shown to be responsive to exercise and further influenced by environmental temperature variation,Citation22 and BDNF release in the hippocampus as a result of exercise is thought to be linked to the PGC-1α pathway.Citation23 Metabolic changes that impact the PGC-1α pathway may provide a mechanism for BDNF alterations to exercise and temperature.Citation24 Thus, it is possible that Exercise-dependent BDNF response may be influenced via environmental temperature.
The increase in BDNF response to temperature may also be a compensatory response to cognitive performance in different environmental temperatures. Significant declines in functional working memory have been observed when participants are exposed to passive hyperthermia.Citation25 In addition to short-term memory effects, heat exposure also affects peripheral motor function via neural dysfunction.Citation26 Soccer referees’ decision-making cognitive function has been shown to be impeded during exposure to cold.Citation27 These cognitive effects may be the result of stress applied to the neural circuitry.Citation28 If BDNF response is linked to environmental stress on the neural system alone, the exercise-dependent release of BDNF may be elevated in both hot and cold environmental conditions.
The purpose of this study was to evaluate the effects of environmental temperature on the exercise-induced release of BDNF into the blood to determine the nature of the BDNF response to exercise. It was hypothesized that BDNF release would increase during the exercise bout, and that plasma BDNF levels would be affected by environmental temperature in one of three ways. If exercise- dependent BDNF response is positively affected by environmental temperature, it may be responding to glucose energy management strategies. If exercise-dependent BDNF response is negatively affected by environmental temperature, it is likely dependent on the PGC-1α pathway. Finally, if exercise-dependent BDNF response is elevated by both hot and cold conditions, it is likely a compensatory response to neuronal stress. Determination of an effective temperature to maximize BDNF release may allow an exercise intervention to be developed that can enhance neuronal development.
Methods
Initial visit
Twelve male participants that were considered recreationally trained completed the study. Participants completed an Institutional Review Board approved informed consent form outlining the goal of the study, experimental procedures, benefits and risks of participation, and a description of their rights as a volunteer. Ample time was permitted for participants to read and understand the consent form, as well as ask any questions they had. After informed consent was received, participants were asked to complete a brief health history form that included risk factors for participation of exercise. Each participant's health history was reviewed to ensure safe participation in the study. Upon inclusion, each participant went through a battery of descriptive exercise tests.
Participants had height, weight, body composition, and aerobic capacity assessed. Height was taken on a Seca 213 Stadiometer (United Kingdom), and weight was taken on a Befour PS-660 ST digital scale (Saukville, WI) Body fat percentage was determined using hydrostatic weighing on an electronic load cell-based system (Exertech, Dresbach, MN) and correcting for estimated residual lung volume and gastrointestinal air.Citation29 Participants completely submerged themselves in a hydrostatic weighing tank, expelled as much air from their lungs, and remained as still as possible for 3–5 sec while the Exertech system recorded the underwater weight during each trial. Six to 10 trials were recorded and the three highest masses were averaged to calculate body density and derive percent body fat using the Siri equation.Citation30 After completion of the hydrostatic weighing session, aerobic capacity was measured using a graded cycle test.
Aerobic capacity (peak VO2) was determined using a graded cycle ergometry test to volitional fatigue on a Velotron cycle ergometer (Racermate, Seattle, WA). Oxygen consumption was recorded with a flow and gas calibrated Parvo Medics True One 2400 Metabolic Measurement System (Sandy, UT), and heart rate was continuously monitored via a Polar (Lake Success, NY) heart rate monitor. The test started at 95 W and the workload was increased by 35 W every 3 min until volitional fatigue. Aerobic capacity (peak VO2) was determined by the highest recorded 15 sec average value. Maximum workload (Wpeak) was determined by the fraction of time completed in the final stage of the test multiplied by 35 W and added to the workload of the last completed stage. The intensity for the experimental trials was set at 60% Wpeak.
Experimental trials
Participants completed three experimental trials at three different environmental temperatures in a randomized and counter-balanced order at 60% relative humidity. The three temperature trials were: hot (H) at 33°C, cold (C) at 7°C, and moderate room temperature (RT) at 20°C. Participants kept a food log 24 h before and an exercise log 48 h before the first trial and replicated the same diet and exercise before their second and third trial. Trials consisted of 1 h of cycling at 60% Wpeak in a temperature and humidity controlled environmental chamber (Darwin Chambers Company, St. Louis, MO). These temperatures have been shown to be safe for extended participant exposure without unsafe changes in core body temperature.Citation22 Approximately, 1 h before experimental trials began (55 ± 6 min) 125 ml of water, a General Mills Fiber One bar (Minneapolis, MN), and a Jonah Core Body Temperature Capsule (Hidalgo Limited, Cambridge, UK) were ingested to ensure a normalized hydration and calorie intake before exercise and to monitor core body temperature throughout the trial. The core body temperature data have been previously reported.Citation31 The food bars consist of 4 g fat, 2 g protein, 29 g carbohydrates, and 9 g of dietary fiber. To ensure dietary intake was not responsible for any variation in dose response, the same bar was ingested for every bout. Gas exchange was measured using the same calibrated metabolic cart at minutes 10–15, 25–30, 40–45, and 55–60 during exercise, and minutes 25–30, 85–90, and 145–150 post-exercise. The indirect calorimetry measures were used to determine caloric expenditure and substrate use.Citation32 Participants were required to consume 125 ml of water after each gas collection for a total of 500 ml of water during each exercise trial.
Blood sample analysis
Blood samples were obtained via venipuncture to the antecubital vein before exercise, immediately following exercise, and following 3 h of recovery for each temperature trial. Blood was drawn into BD Vacutainer tubes with EDTA anticoagulant. Samples were then spun in a centrifuge at 1000 g at 4°C for 10 min. Plasma was separated and stored at −80°C for later analysis. For final analysis, plasma was further cleared of platelet rich plasma by spinning in a centrifuge at 1000 g at 4°C for 10 min. An Enzyme-Linked Immunosorbent Assay (ELISA) kit (R&D Systems, Minneapolis, MN) was used to quantify BDNF in the plasma according to the manufacturer's instructions. The manufacturer-reported sensitivity is 20 pg/ml, the intra-assay CV is 5%, and the inter-assay CV is 11.3%. Samples were analyzed in duplicate with mean concentrations calculated for statistical analysis. The plasma samples were further analyzed for glucose levels using Infinity Glucose Hexokinase reagent (Thermo Scientific, Middletown, Va., USA) that was evaluated with a Nanodrop 2000c spectrophotometer (Thermo Scientific) at a 340 nm wavelength.
Statistical analysis
A repeated measures two-way analysis of variance (time×trial) was used to analyze the effect that exercise in varying temperatures has on blood glucose levels, VO2, substrate use, and BDNF levels for each of the trials at each of the time points. If the F-ratio found a probability of type 1 error of less than 5% (p < 0.05) it was considered significant and a Fisher's protected least significant difference post hoc evaluation was used to determine where the significance occurred. Correlation analysis was performed to evaluate BDNF's relationship with VO2 and plasma glucose. Finally, linear regression analysis was completed to check for any predictive nature of the descriptive data. All statistical data were analyzed via computer using the Statistical Package for Social Sciences software (SPSS 23.0). All data are reported in the text as mean ± SD, and displayed graphically as mean ± SE.
Results
Descriptive data
Twelve participants (age: 25 ± 4 yr, height: 178 ± 5 cm; weight: 79.2 ± 12.8 kg; body fat: 14.5 ± 3.5; VO2peak: 4.29 ± 0.82 L · min−1; Wmax: 276 ± 39 W) completed the study. Multiple regression analysis was performed; however, there was no significant relationship between any of the descriptive data and BDNF concentrations at any time point during any trial (Data not shown).
Plasma BDNF
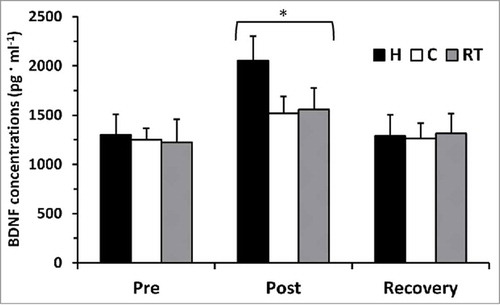
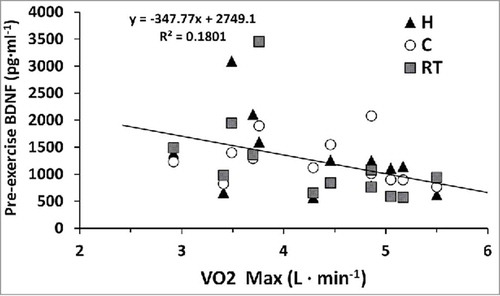
Plasma BDNF concentrations, regardless of temperature, increased from 1257 ± 653 pg·ml−1 to 1711 ± 766 pg·ml−1 immediately following an exercise bout (p = 0.001) and returned to 1289 ± 650 pg·ml−1 following 3 h of recovery time (p = 0.786). No other significant differences were noted between trials or temperatures (p > 0.05), see . There was a significant moderate relationship between participants’ VO2peak and pre-exercise BDNF concentrations (p = 0.01), see . Similar results were observed when plasma volume shifts were applied to plasma BDNF concentrations.
Plasma glucose
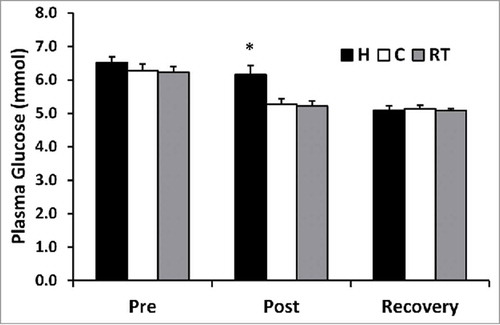
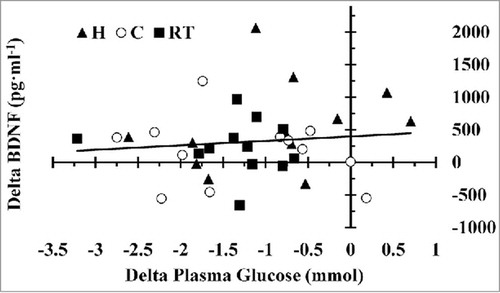
There was no difference in plasma glucose between trials before exercise (p > 0.05). However, plasma glucose was higher immediately post-exercise in H compared with C (p = 0.035) and RT (p = 0.008), see . Plasma glucose was similar immediately following the exercise bout in the C and RT trials (p > 0.05). Following 3 h of recovery, plasma glucose was not different between trials (p > 0.05). There was no relationship between plasma glucose levels and BDNF concentrations (p > 0.05), see . Similar results were observed when plasma volume shifts were applied to plasma glucose. H was greater than C (p = 0.028) and RT (p = .034) but not between C and RT (p = 0.524). However, no significance occurred with interaction effect (p = 0.079).
Oxygen utilization & substrate use
Oxygen uptake during the exercise bout was greater in H than RT (p = 0.001), and RT was greater than C (p = 0.005). Oxygen uptake was the same during recovery for all three temperature trials (p > 0.05), see .
Table 1. Participant oxygen utilization, carbohydrate oxidation, and fat oxidation during exercise and recovery.
The exercise carbohydrate oxidation rate during H was lower than RT (p = 0.023) and trending lower than the cold trial (p = 0.091). During exercise the glucose oxidation rate was not different between the cold and neutral trials (p > 0.05). Glucose oxidation rates during recovery were the same for all temperature trials (p > 0.05), see .
The exercise fat oxidation rate during H was greater than RT (p = 0.001) and C (p = 0.003). During exercise the lipid oxidation rate was the same between the cold and neutral trials (p > 0.05). Lipid oxidation rates during recovery were the same for all temperature trials (p > 0.05), see .
Discussion
BDNF concentrations increased following the exercise bout regardless of the temperature of the trial. There was no effect for different environmental temperatures on post-exercise BDNF concentrations. There was an effect of temperature on both post-exercise plasma glucose and exercise oxygen utilization, two physiologic processes that have been linked to BDNF concentrations. However, no relationship between BDNF and plasma glucose or oxygen utilization was noted in this study.
BDNF's release during exercise is important because cognitive function is improved with participation in physical activity throughout the life-span and BDNF is thought to be key to that improvement. In children, executive control of relational memory is related to aerobic fitness (p = 0.02).Citation1 Furthermore, pre-adolescent students’ aerobic fitness explained 2–5% of variance in academic performance, but was not associated with physical activity levels.Citation5 There is also a cognitive benefit to cardiovascular health on the other end of the life spectrum. Aerobic fitness has a strong relationship with the preservation of white brain matter in older adults.Citation33 Also, performance on tasks requiring greater cognitive control is improved with participation in exercise.Citation34 These improvements in cognitive function through exercise are attributed to neuronal growth and synapse improvement.Citation8 BDNF is a key protein involved in cognitive function management and synapse improvement.Citation35,Citation36 To understand the nature of the exercise related improvements in cognitive function, it is important to understand the nature of the mechanism driving cognitive improvement.
Individual aerobic exercise bouts have a positive effect on plasma BDNF concentrations,Citation16 which may indicate that aerobic exercise training may positively affect basal plasma BDNF concentrations. Blood BDNF is closely associated with brain BDNF in rats and pigs, which makes plasma BDNF a viable marker in brain function.Citation37 Previous research has had varied results when comparing basal BDNF concentrations to fitness levels. When comparing cardio-respiratory fitness (VO2peak) with resting BDNF levels an inverse relationship was found.Citation17 However, in a training protocol investigation, it was found that in older adults the basal BDNF concentrations were elevated in response to an aerobic training protocol.Citation38 Another training study found that despite an increase in VO2peak and max workload there was no effect on resting BDNF concentrations.Citation39 Additionally, long-term aerobic exercise training has also been shown to increase the acute exercise-dependent BDNF response to an exercise bout.Citation40 In the current study, no relationship was found between VO2peak and plasma BDNF concentrations at baseline, following the exercise bout, or following recovery during any temperature trial. This may be due to a higher aerobic fitness of our participants compared with the previously reported means for VO2peak in the Williams,Citation39 Leckie,Citation41 and GriffinCitation40 studies (21.5 ± 4.7 ml·kg−1·min−1, 33.8 ml·kg−1·min−1, and 39.7 ± 6.7 ml·kg−1·min−1, respectively). There may be a critical level of aerobic fitness above which BDNF response is maximized and therefore would account for the differences between studies.
Beyond the relationship to aerobic fitness, BDNF has been linked to metabolic energy management.Citation42,Citation43 Our protocol altered the metabolic and energy management responses to exercise via environmental temperature manipulation. Contrary to the proposed hypotheses, a temperature related difference in exercise-dependent BDNF release was not discovered, but changes in metabolism across temperatures did occur, as evidenced by the difference in exercise VO2 between temperature trials. Additionally, we have previously reported a significant difference in core body temperature during the final 10 min of the trial.Citation31 Although this study was unable to find statistical significance in plasma BDNF between trials, we did note an increase in plasma BDNF concentrations following exercise in the heat compared with the other temperatures. The non-statistically significant elevation in BDNF concentrations following the exercise bout in the heat may be related to metabolic energy management strategies adopted to compensate for elevated plasma glucose during exercise in the heat, and suggests that neuronal growth and synapse improvement potential was greatest following the H trial.
To investigate the relationship between BDNF and energy management strategies, the current study observed how plasma glucose levels related to the plasma BDNF concentrations. One role of BDNF in energy management is an improvement in the hypoglycemic effect of insulin.Citation18 The baseline plasma glucose levels were higher than the expected resting level of 4.0–5.0 mmol. This was likely due to the ingestion of the fiber bar approximately 45 min before the pre-exercise blood draw. In this study, there was not a relationship between plasma glucose. Carbohydrate oxidation rates were lowest in H when compared with C and RT, showing a decrease in glucose metabolism and an increase in plasma glucose levels with no alteration in plasma BDNF concentrations. The elevated plasma glucose in H trial may be due to a temperature related inhibition of glucose transport out of the blood via insulin.Citation44 It has been suggested that this inhibition is due to an increase in skin and visceral blood flow to regulate temperature that limits blood flow to tissues, such as muscle and fat, which are sensitive to insulin and are active in glucose absorption.Citation44 An investigation of BDNF concentrations and glucose metabolism in type II diabetic patients found a strong relationship between BDNF and immunoreactive insulin in females,Citation43 suggesting a relationship between plasma glucose and BDNF concentrations. The mechanism is possibly a result of BDNF assisting insulin in the glucose management process and not BDNF responding to the process of glucogenesis. However, this study could not link post-exercise BDNF concentrations to post-exercise plasma glucose levels, but this may have been prohibited by the variability of the BDNF concentration data and high control of glucose homeostasis in the current physically active sample.
The high degree of variability of plasma BDNF concentrations in our study cannot be attributed to variance in plasma glucose or aerobic fitness levels. This variability was expected as basal BDNF levels range from 400 to 30,000 pg·ml−1.Citation17 Some of the inter-investigation variability in BDNF concentrations may also stem from the blood collection and processing protocols used, as the nomenclature of serum and plasma are often used interchangeably. However, plasma BDNF concentrations are 10-fold lower in platelet-poor plasma than serum.Citation42 This is explained by platelets storing BDNF proteins to be released from an agonistic stimulation.Citation42 In the evaluation of a BDNF response, it would be undesirable to include BDNF stored within the platelets as this BDNF is not bioavailable to exert effects. If the plasma separation process was not fully completed, plasma BDNF concentration may be greatly affected. In the current investigation, whole blood samples were spun in a centrifuge at 1000 g for 10 min at 4°C. Plasma was manually separated and stored at −80°C for later analysis. Prior to final analysis, plasma was further separated from the platelet rich portion by spinning in a centrifuge at 1000 g for 10 min at 4°C to create platelet-poor plasma to be analyzed. Any further studies would benefit from a larger number of participants to increase the statistical power and the standardized use of platelet-poor plasma for evaluation.
Conclusion
This study supports previous findings of an exercise-dependent release of BDNF. Previous research suggests that cognitive function is inhibited in higher environmental temperaturesCitation25,Citation45 thought to be a result of neuronal dysfunction.Citation26 However, decreased cognitive function is not always observed depending on the subjects existing thermal conditions.Citation46 There was not an effect of temperature on BDNF in the current study. Since cognitive function is affected by the ingestion of carbohydrate rich food via an improvement in insulin sensitivity and glucose uptake in the brain,Citation47 there is a possibility that the brain follows the same heat related glucose uptake inhibition pattern as muscle and fat that has been described previously.Citation44 Thus, the temperature dependent limitations of cognitive function may be caused by the potential inhibition of glucose uptake in the brain. Further, mechanistic research is needed to determine if in fact glucose uptake by the brain is inhibited in the heat.
Abbreviations
| BDNF | = | Brain-derived neurotrophic factor |
| C | = | Cold |
| ELISA | = | Enzyme-linked immunosorbent assay |
| H | = | Hot |
| PGC-1α | = | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| RT | = | Moderate room temperature |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This publication was made possible by grants from the National Institute for General Medical Science (NIGMS; 5P20GM103427), a component of the National Institutes of Health (NIH) and its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
References
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43(2):344–9. PMID:20508533; doi:10.1249/MSS.0b013e3181e9af48.
- Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, Pontifex MB, Hillman CH, Kramer AF. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89(1):260–8. PMID:22061423; doi:10.1016/j.biopsycho.2011.10.017.
- Raine LB, Lee HK, Saliba BJ, Chaddock-Heyman L, Hillman CH, Kramer AF. The influence of childhood aerobic fitness on learning and memory. PLoS One. 2013;8(9):e72666. PMID:24039791; doi:10.1371/journal.pone.0072666.
- Wu CT, Pontifex MB, Raine LB, Chaddock L, Voss MW, Kramer AF, Hillman CH. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology. 2011;25(3):333–41. PMID:21443340; doi:10.1037/a0022167.
- Hansen DM, Herrmann SD, Lambourne K, Lee J, Donnelly JE. Linear/nonlinear relations of activity and fitness with children's academic achievement. Med Sci Sports Exerc. 2014;46(12):2279–85. PMID:24781896; doi:10.1249/MSS.0000000000000362.
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. PMID:17167157; doi:10.1093/gerona/61.11.1166.
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum Brain Mapp. 2013;34(11):2972–85. PMID:22674729; doi:10.1002/hbm.22119.
- Erickson KI, Weinstein AM, Sutton BP, Prakash RS, Voss MW, Chaddock L, Szabo AN, Mailey EL, White SM, Wojcicki TR, et al. Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. 2012;2(1):32–41. PMID:22574272; doi:10.1002/brb3.30.
- Ahmadiasl N, Alaei H, Hänninen O. Effect of exercise on learning, memory and levels of epinephrine in rats’ hippocampus. J Sports Sci Med. 2003;2(3):106–9. PMID:24627662; doi: PMC3942636.
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in and enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. PMID:22698691; doi:10.1016/j.neuroscience.2012.06.007.
- Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2(2):151–63. PMID:1308180; doi:10.1002/hipo.450020207.
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15(2):260–72. PMID:15523608; doi:10.1002/hipo.20056.
- King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12(6):811–20. PMID:12542232; doi:10.1002/hipo.10070.
- Luo J, Niki K. Function of hippocampus in ?insight? of problem solving. Hippocampus. 2003;13(3):316–23; PMID:12722972; doi:10.1002/hipo.10069.
- Cotman C.W., Cotman CW, Berchtold N.C.. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. PMID:12086747; doi:10.1016/S0166-2236(02)02143-4.
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–9. PMID:19666694; doi:10.1113/expphysiol.2009.048512.
- Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451(2):152–5. PMID:19133315; doi:10.1016/j.neulet.2008.12.043.
- Nakagawa T, Ono-Kishino M, Sugaru E, Yamanaka M, Taiji M, Noguchi H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab Res Rev. 2002;18(3):185–91. PMID:12112936; doi:10.1002/dmrr.290.
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27(52):14265–74. PMID:18160634; doi:10.1523/JNEUROSCI.3308-07.2007.
- Hargreaves M, Angus D, Howlett K, Conus NM, Febbraio M. Effect of heat stress on glucose kinetics during exercise. J Appl Physiol. (1985). 1996;81(4):1594–7. PMID:8904574.
- Jeukendrup AE. Modulation of carbohydrate and fat utilization by diet, exercise and environment. Biochem Soc Trans. 2003;31(Pt 6):1270–3. PMID:14641041; doi:10.1042/.
- Slivka DR, Dumke CL, Tucker TJ, Cuddy JS, Ruby B. Human mRNA response to exercise and temperature. Int J Sports Med. 2012;33(2):94–100. PMID:22113536; doi:10.1055/s-0031-1287799.
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18(5):649–59. PMID:24120943; doi:10.1016/j.cmet.2013.09.008.
- Goekint M, Roelands B, Heyman E, Njemini R, Meeusen R. Influence of citalopram and environmental temperature on exercise-induced changes in BDNF. Neurosci Lett. 2011;494(2):150–4. PMID:21385602; doi:10.1016/j.neulet.2011.03.001.
- Gaoua N, Racinais S, Grantham J, El Massioui F. Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia. 2011;27(1):1–9. PMID:21070137; doi:10.3109/02656736.2010.516305.
- Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol (Lond). 2008;586(19):4751–62. PMID:18703579; doi:10.1113/jphysiol.2008.157420.
- Watkins SL, Castle P, Mauger AR, Sculthorpe N, Fitch N, Aldous J, Brewer J, Midgley AW, Taylor L. The effect of different environmental conditions on the decision-making performance of soccer goal line officials. Res Sports Med. 2014;22(4):425–37. PMID:25295479; doi:10.1080/15438627.2014.948624.
- Sun G, Qian S, Jiang Q, Liu K, Li B, Li M, Zhao L, Zhou Z, von Deneen KM, Liu Y. Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One. 2013;8(4):e61157. PMID:23593416; doi:10.1371/journal.pone.0061157.
- Thomas TR, Etheridge GL. Hydrostatic weighing at residual volume and functional residual capacity. J Appl Physiol Respir Environ Exerc Physiol. 1980;49(1):157–9. PMID:7399988.
- Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9(5):480–91. discussion 480, 492. PMID:8286893.
- Laursen TL, Zak RB, Shute RJ, Heesch MW, Dinan NE, Bubak MP, La Salle DT, Slivka DR. Leptin, adiponectin, and ghrelin responses to endurance exercise in different ambient conditions. Temperature. 2017;4(2):166–175. doi:10.1080/23328940.2017.1294235.
- Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med. 2005;26(Suppl 1):S28–37. PMID:15702454; doi:10.1055/s-2004-830512
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–38. PMID:18627534; doi:10.1111/j.1469-8986.2008.00676.x.
- Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. 2008;36(3):280–91. PMID:18825471; doi:10.1007/s12160-008-9064-5.
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–22. PMID:21282661; doi:10.1073/pnas.1015950108.
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–7. PMID:19828787; doi:10.1523/JNEUROSCI.3566-09.2009.
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–53. PMID:20604989; doi:10.1017/S1461145710000738.
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. PMID:25566019; doi:10.3389/fnhum.2014.00985.
- Williams JS, Ferris LT. Effects of endurance exercise training on BrainDerived neurotrophic factor. J Exercise Physiologyonline. 2012;15(4):11. PMID:17414812; doi:10.1249/mss.0b013e31802f04c7.
- Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104(5):934–41. PMID:21722657; doi:10.1016/j.physbeh.2011.06.005.
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. PMID:25566019; doi: 10.3389/fnhum.2014.00985.
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–34. PMID:12008958.
- Fujinami A, Ohta K, Obayashi H, Fukui M, Hasegawa G, Nakamura N, Kozai H, Imai S, Ohta M. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008;41(10–11):812–7. PMID:18402781; doi:10.1016/j.clinbiochem.2008.03.003.
- Dumke CL, Slivka DR, Cuddy JS, Hailes WS, Rose SM, Ruby BC. The effect of environmental temperature on glucose and insulin after an oral glucose tolerance test in healthy young men. Wilderness Environ Med. 2015;26(3):335–42. PMID:25937547; doi:10.1016/j.wem.2015.03.002.
- Mazloumi A, Golbabaei F, Mahmood Khani S, Kazemi Z, Hosseini M, Abbasinia M, Farhang Dehghan S. Evaluating effects of heat stress on cognitive function among workers in a hot industry. Health Promot Perspect. 2014;4(2):240–6. PMID:25649311; doi:10.5681/hpp.2014.031.
- Gaoua N. Cognitive function in hot environments: a question of methodology. Scand J Med Sci Sports. 2010;20(s3):60–70. PMID:21029192; doi:10.1111/j.1600-0838.2010.01210.x.
- Nilsson A, Radeborg K, Bjorck I. Effects on cognitive performance of modulating the postprandial blood glucose profile at breakfast. Eur J Clin Nutr. 2012;66(9):1039–43. PMID:22781020; doi:10.1038/ejcn.2012.80.