 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
As endotherms, humans exposed to a compensable cold environment rely on an increase in thermogenic rate to counteract heat lost to the environment, thereby maintaining a stable core temperature. This review focuses primarily on the most important contributor of heat production in cold-exposed adult humans, shivering skeletal muscles. Specifically, it presents current understanding on (1) the origins of shivering, (2) the contribution of shivering to total heat production and (3) the metabolic requirements of shivering. Although shivering had commonly been measured as a metabolic outcome measure, considerable research is still needed to clearly identify the neuroanatomical structures and circuits that initiate and modulate shivering and drives the shivering patterns (continuous and burst shivering). One thing is clear, the thermogenic rate in humans can be maintained despite significant inter-individual differences in the thermogenic contribution of shivering, the muscles recruited in shivering, the burst shivering rate and the metabolic substrates used to support shivering. It has also become evident that the variability in burst shivering rate between individuals, despite not influencing heat production, does play a key role in orchestrating metabolic fuel selection in the cold. In addition, advances in our understanding of the thermogenic role of brown adipose tissue have been able to explain, at least in part, the large inter-individual differences in the contribution of shivering to total heat production. Whether these differences in the thermogenic role of shivering have any bearing on cold endurance and survival remains to be established. Despite the available research describing the relative thermogenic importance of shivering skeletal muscles in humans, the advancement in our understanding of how shivering is initiated and modulated is needed. Such research is critical to consider strategies to either reduce its role to improve occupational performance or exploit its metabolic potential for clinical purposes.
Endotherms face the vital challenge of maintaining an elevated and constant core temperature (Tcore at 36–38°C) independently of climatic fluctuations. In addition to developing adequate behaviors to help maintain Tcore, evolution has resulted in key biochemical, physiologic and morphological adaptations to modulate rates of heat loss (Hloss) and heat production (Hprod) in response to various levels of thermal stress. On both extremes of the spectrum, these adaptations have allowed some endotherms to optimize life in the hottest climates on Earth (e.g., high sweat rate, high surface-to-volume ratio), while others have evolved to thrive the most extreme cold conditions (i.e., high body insulation, low surface-to-volume ratio, highly thermogenic tissues). Within this thermoregulatory continuum, humans are generally well adapted for dissipating heat in warm climates but are particularly maladapted at conserving it in the cold. In fact, without proper shelter and/or clothing, cold exposure can rapidly result in temporary loss of function, permanent cell damage or even death. When ambient temperature decreases, the increase in Hloss experienced by individuals is (1) attenuated by a peripheral vasoconstriction and (2) compensated by an increase in the rate of heat production (Hprod) from the activation of nonshivering (NST) and shivering (ST) thermogenic processes. In this review, we focus mainly on the cold-induced activation of involuntary muscle contractions or ST, the main contributor of heat in cold-exposed adult humans. Building on previous reviews on this topic, we present recent findings on the: (1) origins of ST, (2) contribution of ST to total Hprod and (3) metabolic requirements of ST. We also bring forth new findings on the role played by NST mainly through the activation of brown adipose tissue (BAT). Novel research has shown that the activation of highly thermogenic BAT may explain in part the large inter-individual variability in ST response observed in cold exposed humans.
Origins of shivering
Establishing the origins of involuntary muscle contractions during ST in endotherms has been the subject of numerous studies in this field of physiology. Despite the breadth of research available, little is still known regarding the processes that regulate the initiation and control of ST. However, over the last decades, significant progress has been made in characterizing the central thermoregulatory circuits as a whole, using a variety of in situ and in vivo techniques. These include trans-synaptic retrograde and anterograde tracing techniques, neural substrate metabolism, electrophysiology, direct electrical brain stimulation, pharmacological stimulation and inhibition of neural pathways in various animal models (see ref. Citation1 for review) and using imagery techniques, including functional magnetic resonance imaging (fMRI)Citation2–Citation5 and positron emission tomography/computerized tomography (PET/CT)Citation6–Citation8 in humans. From these studies, it has been possible to derive the principal anatomic structures and neurophysiological substrates that make up the central thermoregulatory circuits. provides a general overview of the core pathways providing thermoregulatory control, including the sensory afferent axis (green boxes), the thermoregulatory control center and the efferent pathway (red box). This schematic reflects one proposed model, which posits that deep body temperature is perpetually controlled via a negative feedback loop, whereby deep (core) temperature serves as both the control variable (Tcore) and the predominant feedback signal (i.e., from temperature-sensitive neurons in the brain or transient receptor potential (TRP) cation channels in the brain, spinal cord and viscera), while skin temperature provides a rapidly-responding auxiliary feedback signal (from TRP found in epidermis), providing negative or positive control.Citation9,Citation10 Others suggest that thermoregulatory responses are under feedforward control driven by changes in skin temperature, thereby activating cold-defense effectors before any detectable change in Tcore can occur.Citation11,Citation12
Figure 1. Simplified schematic representation of cold-induced thermogenesis. Afferent input represented by green boxes, efferent output represented by red box, thermoregulatory control center (hypothalamus) and integrated response (core temperature) represented by black boxes. Model adapted from refs. Citation9,Citation10,Citation12.
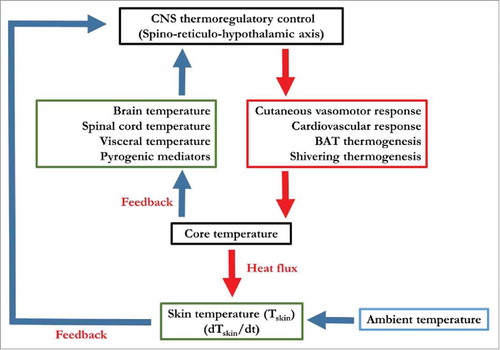
Regardless of the preferred model used to illustrate this thermoregulatory control, it has become evident that each cold-defense effector response (i.e., vasomotor tone, BAT, ST) is independently controlled, with each effector being driven by different combinations of Tcore and Tskin inputs. Further, recent evidence in mice suggests that under cooling conditions, it is the change in Tskin rather than the absolute Tskin that leads to the stimulation of temperature-sensing spinal dorsal root ganglia (DRG) neurons.Citation13 How this ultimately influences the recruitment of each cold-defense thermoeffector or whether these cold-responding neurons also detect changes rather than absolute temperature in humans still remains to be determined. There are some indications in humans and monkeys that both the peripheral and central cold-sensitive receptors exhibit a vigorous increase in nerve impulse activity upon a decrease in Tskin followed by a steady-state continuous discharge when the temperature is held constant,Citation14,Citation15 demonstrating an acute habituation effect. Both steady-state and nonsteady-state firing patterns can also be seen in the activation of the effector responses to an innocuous cold stimulus. Upon cooling in humans, ST electromyography (EMG) increases dramatically, before stabilizing to a lower amplitude.Citation16 Unfortunately, in humans, brain mapping and functional imaging of the central control of thermoregulation has remained limited and somewhat controversial. Nevertheless, EMG measurements have been able to characterize distinct and important muscle recruitment patterns indicative of large inter-individual differences in human ST response.
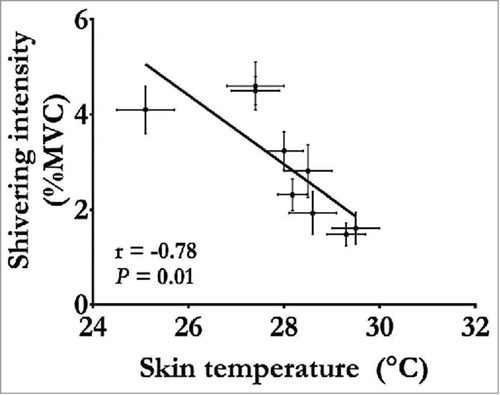
Traditional methods for assessing changes in muscle recruitment during ST have included indwelling and surface EMG. From these electrophysiological signals, ST intensity and muscle recruitment pattern can be quantified and compared at different levels of cold exposure as well as between treatments and individuals (for example, ref. Citation17). At the whole-body level, as average Tskin and Tcore decrease, ST intensity increases mainly in proximal trunk and leg muscles, whereas arm and lower leg muscles contribute little to Hprod.Citation18,Citation19 When this increase in ST intensity and associated increase in Hprod is sufficient to compensate fully for the increase in Hloss, Tcore remains fully defended as long as this compensation is maintained.Citation20–Citation22 Changes in Hprod and Tcore as a function of alterations in mean Tskin under such compensable cold conditions are presented in . It is important to note that these changes in ST and Hprod can occur quickly when Tskin is modified.Citation16 During uncompensable cold exposure, where Hloss surpasses the increase in ST intensity and associated increase in Hprod, both changes in Tskin and Tcore become the ultimate driving force for the thermogenic response.Citation23 As Tcore decreases to ∼35°C, increases in Hprod through ST can reach maximal values equivalent to ∼5 times resting metabolic rate (xRMR) or ∼40% of maximal oxygen consumption (VO2max).Citation23,Citation24 For example, when individuals were rewarmed following a 45 min cold water immersion at 7°C where average Tskin and Tcore were reduced to ∼10°C and ∼34.5°C, respectively, Hprod reached maximal ST intensity.Citation24 Most importantly, if the decrease in Tcore continues without compensation, ST stimulation is reduced or halted once a threshold Tcore is reached (∼31°C;Citation25). Evidently, this reduction in ST and associated decrease in Hprod results in an even quicker reduction in Tcore. Under such advanced stage of hypothermia, rewarming is not possible without an external source of heat. Exact physiologic reasons why the ST response is inhibited at a specific Tcore and why maximal thermogenesis is three to four times lower during shivering compared with the metabolic rate attainable from muscle contractions through exercise are far from being well understood. However, maximal ST intensity reached by humans is consistent with values observed in avian species and large mammalian species, which rely almost entirely on this mode of Hprod during cold exposure.Citation26,Citation27 Since high ST intensities interfere with voluntary movements,Citation28,Citation29 it could be speculated that lower ST intensities produced sufficient heat to increase odds of survival without compromising locomotion and/or cold survival.
Figure 2. Changes in shivering intensity as a function of changes in mean skin temperature measured under compensable conditions (no change in Tcore). Data adapted from refs. Citation20–Citation22, Citation42, Citation43, Citation52, Citation56, Citation68.
On the methodological front, indwelling and surface EMG only provide superficial information on the recruitment of muscles and thus, cannot inform researchers on the role played by deeper parts of the muscles being monitored or by deep muscles. To assess the potential contribution of deep muscles to whole-body ST, Blondin et al.Citation19 combined PET with 18fluro-dexoxyglucose (18FDG PET) and surface EMG. Using this approach, it was shown that, in superficial muscles, ST intensity measured by EMG covaried closely with increases in 18FDG uptake quantified by 18FDG PET. Examining deep muscles, this approach provided the first estimates of the activation of ST from changes in 18FDG uptake. Results showed that 18FDG uptake increased in deep muscles especially in the neck area (i.e., longus colli) and are therefore more active than many other surface muscles during ST. While it is well established that distal muscles contribute less to total ST, this new evidence indicates that both surface and deep proximal trunk muscles are involved in sustaining total Hprod in the cold.
Despite a general pattern emerging using 18FDG PET, muscle recruitment patterns are highly variable, even among morphologically similar men and women.Citation18,Citation30–Citation34 Indeed, cold exposure studies have shown that for the same given Hprod, some individuals rely almost entirely on upper body muscles, while others depend more on upper leg muscles.Citation17,Citation18,Citation30,Citation31 Also, important inter-individual differences in motor unit recruitment have also been reported within the same muscles. Quantification of shivering EMG has revealed the presence of two distinct muscle recruitment patterns based on differences in intensity (2–5 versus 7–15% of maximal voluntary contractions (%MVC)) and rate of occurrence (8–10 versus 0.1–0.2 Hz).Citation31,Citation35 Continuous, low-intensity ST is associated with the recruitment of type I fibers, whereas high-intensity bursts are linked with the recruitment of type II fibers.Citation28 The proportion of burst shivering rate to total ST activity is highly variable between muscles and between individuals for reasons that remain unknown. However, recently, Blondin et al.Citation36 have indicated that burst shivering rates are related in part to differences in muscle fiber composition between individuals. This observation is consistent with what is found in birds, where burst activity has also been linked to the fiber composition of a specific muscle.Citation37,Citation38 Whether these differences in the proportion of bursts shivering to continuous shivering provide survival advantage remains unclear. In humans, ST research has consistently shown that thermogenic response remains unchanged even when different muscles are recruited and/or when high proportion of burst shivering activity is observed.Citation17,Citation19,Citation21,Citation36 However, differences in burst shivering activity have important effects on whole body fuel selection and potentially on the depletion of carbohydrate (CHO) reserves (see Metabolic requirements of ST).
Contribution of ST to total heat production
Heat is a by-product of all exothermic biochemical reactions, which includes the combustion of metabolic substrates. As mentioned above, during cold exposure, metabolic processes are activated to stimulate Hprod in an attempt to prevent substantial decreases in Tcore. Of all cold-activated mechanisms, the highest amount of heat is obtained by muscle contractions during voluntary movements or exercise. From 4 to 6 kJ min−1 at RMR, humans can reach rates of thermogenesis 15 to 20-fold higher during maximal exercise. However, when exercise is not possible or advisable (e.g., overexertion, limited food supply, injury, risk of becoming disoriented), ST become the most important line of defense for compensating for increases in Hloss.
To assess differences in cold stress between various studies, ST responses may be characterized based on differences in thermogenesis from RMR to cold-induced MR. As such, changes in cold exposure can be reported as mild (1.0–2.0 xRMR; e.g., Citation19, Citation20, Citation22, Citation36, Citation39, Citation40–Citation51), moderate (2.0–3.0; e.g., Citation34, Citation52, Citation53–Citation61) and high (3.0–maximum; e.g., Citation21, Citation24, Citation62–Citation64) (). Of great importance, cold exposure intensities below moderate intensity for 2–3 h tend to be compensable, whereas high-intensity cold exposure may lead to a rapid decrease in Tcore. In theory, under compensable conditions, Tcore can be maintained as long as cold-induced Hprod is sustained. For example, during mild cold exposure in a thermal chamber at 7.5°C, we showed that Tcore, Hprod and ST intensity at ∼1.8 xRMR can be sustained in men for up to 24 h independently of a negative energy balance (∼44% less than 24 h energy demand) and independently of a substantial change in metabolic fuel utilizationCitation65 (see also Metabolic requirements of ST). However, it remains that even under this compensable condition, only four of the eight recruited subjects were willing to remain in the cold for the full 24 h. This exemplifies that the challenges for human survival even under mild, compensable conditions extend far beyond sustaining adequate thermogenic and physiologic responses.
Figure 3. Changes in thermogenic rate (times resting metabolic rate (xRMR)) as a function of changes in average skin temperature found in various cold exposure studies in lean healthy men. Thermogenic rates are characterized as mild (1–2 xRMR), moderate (2–3 xRMR) and high (>3 xRMR). Points represent data from end of cold exposure for following studies.Citation19–Citation22,Citation24,Citation34,Citation36,Citation39–Citation64
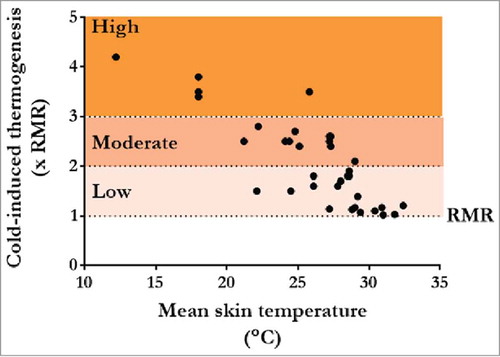
Above in Origins of ST, we reported that substantial inter-individual variability in ST muscle recruitment patterns (i.e., group of muscles recruited, rate of burst shivering activity) is found in humans. Adding to the complexity of these individual differences, recent work has also shown that the contribution of ST to total Hprod varies greatly between individual and is modulated by the presence of other thermogenic sources, such as BAT.Citation20 This tissue is essential to sustain endothermia, especially in newborn and small mammals. However, in humans, highly thermogenic BAT was thought to disappear after the first years of life. Recent studies have shown that not only is BAT present, but it is also metabolically active in some individuals.Citation20 Most importantly, Ouellet et al.Citation20 demonstrated that the presence of BAT was inversely correlated with whole-body ST activity in men. While it is clear that BAT contributes to NST in adult humans, its contribution to Hprod is unclear. Further work is also needed to determine the effects of cold acclimation or the administration of specific food (e.g., green tea, capsinoids) or pharmaceutical (e.g., nicotinic acid, β blockers) compounds in modulating the contribution of ST to total Hprod.
Metabolic requirements of ST
Combined CHO, lipids and proteins oxidation provide the necessary substrates to sustain ST (see Citation32, Citation66 for review). Metabolic research in the cold has clearly shown that the respective contribution of each fuel to ST can be highly variable and will depend on differences in the status of CHO reserves,Citation17 in burst shivering rateCitation31 and in the availability of exogenous substrates.Citation53–Citation55 Over the last decades, metabolic research in the cold has focused on understanding whether, like exercise, the depletion of CHO reserves could limit ST endurance.Citation67 However, to date, mounting evidence indicates that adult humans are able to sustain ST and whole-body thermogenesis using a wide variety of metabolic fuels. When one fuel source is depleted or reduced, others compensate to maintain ATP production, ST intensity and whole body Hprod. The following sections overview the respective contribution of plasma glucose, muscle glycogen, lipids and proteins at various shivering intensities for different shivering patterns and with different levels of CHO availability.
Shivering intensity. As mentioned above, ST intensity is modulated by reducing mean Tskin and/or Tcore. As ST intensifies, muscles rely progressively more on CHO, whereas the relative contribution of both lipids and proteins is reduced. The relative contribution of CHO to total thermogenic rate varies between ∼20% and 80% Hprod (see Citation32 for review) with rates of oxidation ranging from ∼130 to 500 mg kg−1 h−1.Citation21,Citation34 The highest rates of whole-body CHO oxidation were found during passive rewarming, where men recovered passively from a Tcore of ∼34.5–36.5°C following a 7°C water immersion.Citation24 Under these extreme conditions, CHO oxidation rates reached as much as ∼1.5 g min−1 and accounted for three quarters of all the heat produced. It is also important to note that under all reported cold conditions, muscle glycogen reserves provide as much as ∼75–80% of the total glucose needed to sustain ST while the contribution of plasma glucose (hepatic) remains limited to ∼20–25% of total CHO oxidation.Citation21,Citation52,Citation68 Therefore, similar to exercise, muscle glycogen is always the greatest source of glucose for sustaining whole-body CHO oxidation and energy demands in the cold.
Lipids also play a key role to sustain Hprod in the cold. As previously stated, the relative contribution of lipids dominates at all intensities below 50% of maximal shivering intensity (∼20% VO2max). However, for reasons that remain unknown, the oxidation rate of lipids rises to ∼140 mg kg−1 h−1 and remains constant as shivering intensifies. This maximal lipid oxidation rate in the cold is ∼3 times lower than what is found during exercise at a similar metabolic rate.Citation69 In women, lipid oxidation rates were reported to be higher, ranging between ∼190 and 200 mg kg−1 h−1 in the luteal and follicular phases of the menstrual cycle.Citation34 Consequently, in women, the relative contributions of lipids to total heat production were higher at both menstrual phases (∼75% Hprod) compared with men (∼50% Hprod).
Fatty acids are obtained from the lipolysis of triacylglycerol (TAG) stores located in adipose tissue, in the liver or in muscles. In the cold, the relative contribution of each compartment is still unknown. Using stable isotope tracer methods, Vallerand et al.Citation59 and Ouellet et al.Citation20 showed that the turnover rate of circulating fatty acids and rates of lipolysis increase proportionally to the increase in metabolic rate found during mild cold exposure. This indicates that circulating fatty acids play an important role to sustain ATP production in the cold. However, two earlier studies by Martineau and JacobsCitation70,Citation71 identified the effects of a reduction in plasma fatty acids availability in men with normal or reduced CHO reserves immersed at 18°C for 90 min. In both studies, the reduction in circulating fatty acids was induced by oral nicotinic acid administration (3.2 mg kg−1 as niacin), an inhibitor of intracellular TG lipolysis, given before and during cold exposure. When fatty acid supply was suppressed in men with normal glycogen reserves, results showed that heat production remained unchanged presumably by increasing the use of intra-muscular lipids and glycogen reserves.Citation70 Similarly, in the second study, where both plasma fatty acid availability and glycogen reserves were reduced, changes in Hprod, immersion times and rectal temperature were not different from results found in CHO loaded and normal plasma fatty acid treatments.Citation71 More recently, we showed that suppressing intracellular lipolysis by ingesting nicotinic acid, suppresses BAT oxidative metabolism, increases ST and alters fuel selection by increasing CHO utilization.Citation36 Independently of these important modifications in NST/ST responses and fuel selection, Hprod remained constant and unaffected by nicotinic acid. This further highlights the great versatility for sustaining thermogenic rate in cold exposed humans.
Finally, for proteins, it was customary, as for exercise, to assume that involuntary muscle contractions during ST were exclusively sustained by the oxidation of CHO and lipids. The contribution of protein oxidation was thought negligible at best. Proteins generally contribute as much as plasma glucose at low ST intensities (∼10% HprodCitation52) and their role decreases progressively as ST intensifies.Citation21 The initial contribution of this fuel to total ST is directly related to the individual's average dietary protein consumption. High protein intake will result in a higher initial contribution of proteins to total energy budget. In addition, as described later, any reductions in CHO availability have been shown to substantially increase the role played by proteins in the cold.Citation68
Shivering pattern. Previous work has shown that ST pattern and burst shivering activity does not modify Hprod but they can play a key role in orchestrating fuel selection.Citation31 At a shivering intensity of ∼3.5 xRMR, Haman et al.Citation31 reported that fuel selection in men ranged from 33% to 78% for CHO and from 14% to 60%
for lipids. Detailed EMG analysis in eight large muscles revealed that burst shivering activity also exhibited large variability among individuals ranging from ∼2 to 8 bursts per minutes. Because burst activity was previously associated with the recruitment of glycolytic type II muscle fibers,Citation28,Citation29 it was assumed that whole body CHO oxidation rate would be correlated with burst shivering activity. Results showed that both muscle glycogen and whole-body CHO oxidation co-vary closely with individual variations in burst activity, whereas the use of plasma glucose does not. This demonstrates that individuals with higher burst rates not only oxidize more glucose at the whole body level but would deplete muscle glycogen faster than the ones that display low bursting activity at the same given shivering intensity. However, it remains unclear whether CHO are essential for sustaining shivering for prolonged exposures.
CHO availability. Of all metabolic fuel stores, CHO account for only ∼1% of total reserves but still represent a large fraction of all the heat produced in the cold. Three independent studies modified CHO availability artificially by dietary and exercise manipulations to determine whether endogenous CHO reserves were essential to sustain ST. Following this decrease and increase in CHO stores, men were exposed to either moderate 2.5 xRMRCitation31 or to high cold exposure 3.5 xRMR.Citation64,Citation72 While in all studies glycogen depletion and loading caused a respective large shift in fuel use from lipid dominance (up to ∼80% ) to CHO dominance (up to ∼80%
), changes in Tcore and Tskin remained unaffected by the modification in CHO availability. This indicates that decreases and increases in CHO availability result in a respective up- or downregulation in the use of lipids and proteins. This metabolic flexibility may not be surprising considering the relatively low metabolic rates achieved in the cold. Adding to this conclusion, Haman et al.Citation17,Citation68 showed that, during mild shivering, humans are able to sustain a constant thermogenic rate by oxidizing these widely different fuel mixtures without modifying EMG ST pattern or muscle fiber recruitment (i.e., intensity, burst versus continuous ST). Therefore, the drastic switch in fuel metabolism found as a result of glycogen depletion and loading (CHO, ∼28 versus 65%; lipids, ∼53 versus 23%; proteins, ∼19 versus 12%
, respectively) can be maintained within the same muscle fibers. These results emphasize the importance of proteins and lipids in compensating for large decreases in CHO availability and indicate that CHO might not be essential to sustain ATP production during ST.
It is important to note that before obtaining detailed quantifications of the partitioning of CHO reserves for Hprod in the cold, WisslerCitation67 had proposed an elaborate model to predict shivering endurance based on empirical observations from Beckman, Reeves and Goldman.Citation73 In his model, shivering fatigue was determined from the onset of muscle cramping observed in a group of men exposed to 24°C water. Under conditions previously describe in glycogen depleted and glycogen loaded individuals,Citation68 the model of Wissler predicts that shivering at 200 W could be sustained for 33 to 42 h. In addition, Tikuisis et al.Citation74 later suggested that the Wissler model may underestimate true values. In a more recent paper, Haman et al.Citation65 estimated a 20 h time to glycogen depletion based on whole-body oxidation of glycogen; a value clearly shorter than that predicted by Wissler et al.Citation67 or Tikuisis et al..Citation74 Again, calculations of ST endurance based on time to glycogen depletion have two possible shortcomings: (1) glycogen may not be essential, and low-intensity shivering is sustainable solely on lipids and proteins and/or, (2) a significant shift in fuel selection to spare glycogen takes place after 2 h of shivering (in fact, a progressive increase in fat oxidation was observed by Tikuisis et al.Citation74 during prolonged shivering lasting for up to 4 h). No detailed information is currently available on fuel selection for shivering in excess of 4 h in fasted individual, and it is still unclear whether glycogen depletion coincides with muscle fatigue. Future studies should address this interesting problem. At the moment, however, research has demonstrated that lipid and protein oxidation can compensate for any reductions in CHO availability over a period of 90–120 min at shivering intensities ranging between ∼2.5 and 3.5 xRMR. Of course, longer exposure times and different shivering intensities are needed to validate this assumption.
Conclusion
This review focused on ST and presented the origins, contribution to total Hprod and metabolic requirements of this key thermogenic process for human survival in the cold. While much work remains to clearly understand the origins of the dual ST pattern (continuous and burst ST), research has shown that differences in burst shivering rate between individuals does not seem to influence Hprod but does play a key role in orchestrating metabolic fuel selection in the cold. In addition, advances in our understanding of the thermogenic role of BAT have been able to explain at least in part the large inter-individual differences in the contribution of ST to total Hprod. Whether these differences in the thermogenic role of ST have any bearing on cold endurance and survival remains to be established. However, it seems that under compensable conditions, fasted humans can sustain ST and Hprod using a variety of metabolic fuels and changes in endogenous CHO stores does not alter Hprod at low to moderate intensity for up to 4h. Clearly, improving the fundamental knowledge of the role played by muscles, BAT and other metabolic processes in sustaining thermogenic rate in cold humans is extremely important to provide additional breakthroughs in this field of research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Morrison SF. 2010 Carl ludwig distinguished lectureship of the APS neural control and autonomic regulation section: central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137-49. PMID:21270352; doi:10.1152/japplphysiol.01227.2010.
- McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF. Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc Natl Acad Sci USA. 2006;103:809-13. PMID:16407125; doi:10.1073/pnas.0509862103.
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533-46. PMID:9744957.
- Kanosue K, Sadato N, Okada T, Yoda T, Nakai S, Yoshida K, Hosono T, Nagashima K, Yagishita T, Inoue O, et al. Brain activation during whole body cooling in humans studied with functional magnetic resonance imaging. Neurosci Lett. 2002;329:157-60. PMID:12165401; doi:10.1016/S0304-3940(02)00621-3.
- Muzik O, Diwadkar VA. In vivo correlates of thermoregulatory defense in humans: temporal course of sub-cortical and cortical responses assessed with fMRI. Hum Brain Mapp. 2016;37:3188-202. PMID:27220041; doi:10.1002/hbm.23233.
- Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley MJ, Lancaster J, Denton D, Fox PT. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci USA. 2005;102:5262-7. PMID:15793009; doi:10.1073/pnas.0409753102.
- Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76:571-81. PMID:8836245.
- Muzik O, Mangner TJ, Leonard WR, Kumar A, Granneman JG. Sympathetic innervation of cold-activated brown and white fat in lean young adults. J Nucl Med. 2017;58:799-806. PMID:27789721; doi:10.2967/jnumed.116.180992.
- Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210:498-507. PMID:24716231; doi:10.1111/apha.12231.
- Werner J. System properties, feedback control and effector coordination of human temperature regulation. Eur J Appl Physiol. 2010;109:13-25. PMID:19787369; doi:10.1007/s00421-009-1216-1.
- Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci. 2016;196:14-24. PMID:26924538; doi:10.1016/j.autneu.2016.02.010.
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207-28. PMID:21900642; doi:10.1152/ajpregu.00109.2011.
- Ran C, Hoon MA, Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci. 2016;19:1201-9. PMID:27455110; doi:10.1038/nn.4350.
- Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855-65. PMID:11559780; doi:10.1111/j.1469-7793.2001.t01-1-00855.x.
- Darian-Smith I, Johnson KO, Dykes R. "Cold" fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol. 1973;36:325-46. PMID:4196271.
- Imbeault M-A, Mantha OL, Haman F. Shivering modulation in humans: effects of rapid changes in environmental temperature. J Ther Biol. 2013;38:582-7. doi:10.1016/j.jtherbio.2013.10.002.
- Haman F, Legault SR, Rakobowchuk M, Ducharme MB, Weber J-M. Effects of carbohydrate availability on sustained shivering II: relating muscle recruitment to fuel selection. J Appl Physiol. 2004;96:41-9. PMID:12949017; doi:10.1152/japplphysiol.00428.2003.
- Bell DG, Tikuisis P, Jacobs I. Relative intensity of muscular contraction during shivering. J Appl Physiol. 1992;72:2336-42. PMID:1629089.
- Blondin DP, Labbé SM, Phoenix S, Guérin B, ÉE T, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701-14. PMID:25384777; doi:10.1113/jphysiol.2014.283598.
- Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545-52. PMID:22269323; doi:10.1172/JCI60433.
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Weber JM. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol. 2005;566:247-56. PMID:15831534; doi:10.1113/jphysiol.2005.086272.
- Gosselin C, Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr. 2013;110:282-8. PMID:23237788; doi:10.1017/S0007114512005089.
- Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100-6. PMID:11394237; doi:10.1007/s004210000329.
- Haman F, Scott CG, Kenny GP. Fueling shivering thermogenesis during passive hypothermic recovery. J Appl Physiol. 2007;103:1346-51. PMID:17641212; doi:10.1152/japplphysiol.00931.2006.
- Parsons K. Human thermal environments: the effects of hot, moderate, and cold environments on human health, comfort, and performance. New York, (NY): Taylor & Francis Inc; 2003.
- Bishop CM. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc Biol Sci. 1999;266:2275-81. PMID:10629977; doi:10.1098/rspb.1999.0919.
- Rezende EL, Swanson DL, Novoa FF, Bozinovic F. Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J Exp Biol. 2002;205:101-7. PMID:11818416.
- Meigal A. Gross and fine neuromuscular performance at cold shivering. Int J Circumpolar Health. 2002;61:163-72. PMID:12078964; doi:10.3402/ijch.v61i2.17449.
- Meigal A, Lupandin V, Kuzmina GI. Electromyographic patterns of thermoregulatory activity of motor units in the course of body cooling. Fiziol Cheloveka. 1993;19:106-14 (in Russian;). PMID:8354435.
- Blondin DP, Tingelstad HC, Mantha OL, Gosselin C, Haman F. Maintaining thermogenesis in cold exposed humans: relying on multiple metabolic pathways. Compr Physiol. 2014;4:1383-402. PMID:25428848; doi:10.1002/cphy.c130043.
- Haman F, Legault SR, Weber JM. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol. 2004;556:305-13. PMID:14742724; doi:10.1113/jphysiol.2003.055152.
- Haman F. Shivering in the cold: From mechanisms of fuel selection to survival. J Appl Physiol 2006;100:1702-8; PMID:16614367; doi:10.1152/japplphysiol.01088.2005.
- Haman F, Blondin DP, Imbeault MA, Maneshi A. Metabolic requirements of shivering humans. Front Biosci. 2010;2:1155-68. PMID:20515847; doi:10.2741/s124.
- Blondin DP, Maneshi A, Imbeault MA, Haman F. Effects of the menstrual cycle on muscle recruitment and oxidative fuel selection during cold exposure. J Appl Physiol. 2011;111:1014-20. PMID:21737827; doi:10.1152/japplphysiol.00293.2011.
- Israel DJ, Pozos RS. Synchronized slow-amplitude modulations in the electromyograms of shivering muscles. J Appl Physiol. 1989;66:2358-63. PMID:2745301.
- Blondin DP, Frisch F, Phoenix S, Guérin B, Turcotte ÉE, Haman F, Richard D, Carpentier AC. Inhibition of intracellular triglyceride lipolysis suppresses cold-induced brown adipose tissue metabolism and increases shivering in humans. Cell Metab. 2017;25(2):438-47. PMID:28089568; doi:10.1016/j.cmet.2016.12.005.
- Olson JM. The ontogeny of shivering thermogenesis in the red-winged blackbird (agelaius phoeniceus). J Exp Biol. 1994;191:59-88. PMID:9317339.
- Hohtola E, Henderson RP, Rashotte ME. Shivering thermogenesis in the pigeon: the effects of activity, diurnal factors, and feeding state. Am J Physiol. 1998;275:R1553-62. PMID:9791073.
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395-403. PMID:23867626; doi:10.1172/JCI68993
- Din M, et al. Human brown adipose tissue [O]O PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016; 43:1878-1886.
- Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089-99. PMID:25056438; doi:10.2337/db14-0746.
- Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC, Richard D, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab. 2014;99:E438-46. PMID:24423363; doi:10.1210/jc.2013-3901.
- Blondin DP, Tingelstad HC, Noll C, Frisch F, Phoenix S, Guérin B, ÉE Turcotte, Richard D, Haman F, Carpentier AC, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun. 2017;8:14146. PMID:28134339; doi:10.1038/ncomms14146.
- Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299-305. PMID:17785356; doi:10.1210/jc.2007-1065.
- Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96:E598-605. PMID:21270329; doi:10.1210/jc.2010-1957.
- Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106-13. PMID:22872233; doi:10.2337/db12-0288.
- Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes. 2015;39:1696-702. PMID:26189600; doi:10.1038/ijo.2015.130.
- van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Cold-induced heat production preceding shivering. Br J Nutr. 2005;93:387-91. PMID:15877879; doi:10.1079/BJN20041362.
- Glickman-Weiss EL, Nelson AG, Hearon CM, Windhauser M, Heltz D. The thermogenic effect of a carbohydrate feeding during exposure to 8, 12 and 27 °C. Eur J Appl Physiol Occup Physiol. 1994;68:291-7. PMID:8055885; doi:10.1007/BF00571445.
- Vallerand AL, Jacobs I. Rates of energy substrates utilization during human cold exposure. Eur J Appl Physiol. 1989; 58:873-8. PMID:2767069; doi:10.1007/BF02332221.
- Glickman-Weiss EL, Nelson AG, Hearon CM, Vasanthakumar SR, Stringer BT, Shulman SS. Does feeding regime affect physiologic and thermal responses during exposure to 8, 20, and 27°C? Eur J Appl Physiol Occup Physiol. 1993;67:30-4. PMID:8375361; doi:10.1007/BF00377700.
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber JM. Effect of cold exposure on fuel utilization in humans: Plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77-84. PMID:12070189; doi:10.1152/japplphysiol.00773.2001.
- Blondin DP, Dépault I, Imbeault P, Péronnet F, Imbeault MA, Haman F. Effects of two glucose ingestion rates on substrate utilization during moderate-intensity shivering. Eur J Appl Physiol. 2010;108:289-300. PMID:19779734; doi:10.1007/s00421-009-1210-7.
- Blondin DP, Peronnet F, Haman F. Effects of ingesting [13C]glucose early or late into cold exposure on substrate utilization. J Appl Physiol. 2010;109:654-62. PMID:20651221; doi:10.1152/japplphysiol.00440.2010.
- Blondin DP, Peronnet F, Haman F. Coingesting glucose and fructose in the cold potentiates exogenous CHO oxidation. Med Sci Sports Exer. 2012;44:1706-14. PMID:22453246; doi:10.1249/MSS.0b013e318254e952.
- Blondin DP, et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol. 2017; 595:2099-2113.
- Vallerand AL, Zamecnik J, Jacobs I. Plasma glucose turnover during cold stress in humans. J Appl Physiol. 1995;78:1296-302. PMID:7615436.
- Vallerand AL, Jacobs I, Kavanagh MF. Mechanism of enhanced cold tolerance by an ephedrine-caffeine mixture in humans. J Appl Physiol. 1989;67:438-44. PMID:2759973.
- Vallerand AL, Zamecnik J, Jones PJ, Jacobs I. Cold stress increases lipolysis, FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med. 1999;70:42-50. PMID:9895020.
- Vallerand AL, Jacobs I. Influence of cold exposure on plasma triglyceride clearance in humans. Metabolism. 1990;39:1211-8. PMID:2233284; doi:10.1016/0026-0495(90)90097-V.
- Vallerand AL, Tikuisis P, Ducharme MB, Jacobs I. Is energy substrate mobilization a limiting factor for cold thermogenesis. Eur J Appl Physiol. 1993;67:239-44. PMID:8223537; doi:10.1007/BF00864222.
- Tikuisis P, Jacobs I, Moroz D, Vallerand AL, Martineau L. Comparison of thermoregulatory responses between men and women immersed in cold water. J Appl Physiol. 2000;89:1403-11. PMID:11007575.
- Martineau L, Jacobs I. Muscle glycogen utilization during shivering thermogenesis in humans. J Appl Physiol. 1988;65:2046-50. PMID:3209549.
- Martineau L, Jacobs I. Muscle glycogen availability and temperature regulation in humans. J Appl Physiol. 1989;66:72-8. PMID:2917958.
- Haman F, Mantha OL, Cheung SS, DuCharme MB, Taber M, Blondin DP, McGarr GW, Hartley GL, Hynes Z, Basset FA. Oxidative fuel selection and shivering thermogenesis during a 12- and 24-h cold-survival simulation. J Appl Physiol. 2016;120:640-8. PMID:26718783; doi:10.1152/japplphysiol.00540.2015.
- Weber JM, Haman F. Fuel selection in shivering humans. Acta Physiol Scand. 2005;184:319-29. PMID:16026423; doi:10.1111/j.1365-201X.2005.01465.x.
- Wissler EH. Mathematical simulation of human thermal behavior using whole-body models. In: Shitzer A, Eberhart RC, editors. Heat transfer in medicine and biology, Vol. 1. New York: Plenum Press; 1985. p. 347-55.
- Haman F, Peronnet F, Kenny GP, Doucet E, Massicotte D, Lavoie C, Weber JM. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol. 2004;96:32-40. PMID:12949018; doi:10.1152/japplphysiol.00427.2003.
- Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exer. 2002;34:92-7. PMID:11782653; doi:10.1097/00005768-200201000-00015.
- Martineau L, Jacobs I. Free fatty acid availability and temperature regulation in cold water. J Appl Physiol. 1989;67:2466-72. PMID:2606855.
- Martineau L, Jacobs I. Effects of muscle glycogen and plasma FFA availability on human metabolism responses in cold water. J Appl Physiol. 1991;71:1331-9. PMID:1757356.
- Young AJ, Sawka MN, Neufer PD, Muza SR, Askew EW, Pandolf KB. Thermoregulation during cold water immersion is impaired by low glycogen levels. J Appl Physiol. 1989;66:1806-16. PMID:2732173.
- Beckman EL, Reeves E, Goldman RF. Current concepts and practices applicable to the control of body heat loss in aircrew subjected to water immersion. Aerosp Med. 1966;37:348-57. PMID:5333592.
- Tikuisis P, Eyolfson DA, Xu X, Giesbrecht GG. Shivering endurance and fatigue during cold water immersion in humans. Eur J Appl Physiol. 2002;87:50-8. PMID:12012076; doi:10.1007/s00421-002-0589-1.
