ABSTRACT
The toxidrome associated with death from the synthetic cathinones includes hyperthermia as part of the sympathomimetic syndrome. Here, we examine the gender differences in the development of tolerance to the hyperthermia mediated by the synthetic cathinone methylone. In addition to temperature changes, expression differences in genes encoding the uncoupling proteins (UCP) 1 & 3, and TGR5 in skeletal muscle (SKM) and brown adipose tissue (BAT) were examined. Male and female rats were treated weekly with methylone (10 mg/kg). The females developed a tolerance to the methylone-induced hyperthermia by week two of drug exposure. By the third week, females displayed a hypothermic response to methylone. Conversely, males continued to display a hyperthermic response up to and including week four. At week four, the males demonstrated a significantly lower hyperthermia and a complete tolerance seen at week five with no significant hyperthermia. Tissue samples collected after treatment on the sixth week indicate that chronic exposure to methylone reduced UCP1 expression in SKM and BAT of the female rats. Only the females displayed increased TGR5 expression in BAT. UCP3 expression increased in both the SKM and BAT of the males and females. The differences between responses in male and female subjects further demonstrate the need for gender studies in the toxicology associated with drugs with abuse potential.
Graphical abstract
Introduction
The hyperthermia induced by synthetic cathinones (“bath salts”) has been linked to acute kidney injury, rhabdomyolysis, and ultimately death [Citation1,Citation2]. Methylone, the β-keto analog of 3,4-methylenedioxymethamphetamine (MDMA), continues to be commonly seen in forensic laboratories [Citation3]. We have previously demonstrated that methylone is the most potent thermogen of the synthetic cathinones [Citation4]. Both males and females demonstrate an increase in plasma norepinephrine levels following MDMA treatment; however, males display a significantly greater acute increase in norepinephrine levels relative to female animals [Citation5]. Additionally, skeletal muscle uncoupling protein 3 (UCP3) expression is 80% less in females than in males. This depressed expression level is dependent upon estrogen levels and correlates with a reduced thermogenic response in the female rats following acute MDMA treatment [Citation5]. UCP1 and UCP3 play complementary roles in the onset (UCP1) and maintenance (UCP3) of sympathomimetic-induced hyperthermia [Citation6].
Most preclinical studies have focused on the acute pharmacological effects of synthetic cathinone analogs, despite prevailing epidemiological evidence that these drugs are abused repeatedly [Citation7]. Clemens et al. [Citation8] demonstrated that female rats given MDMA at a dose of 8 mg/kg (ip) at an ambient temperature of 28°C developed tolerance to the hyperthermic response after 8 wk of treatment. Those authors did not directly compare females to males. In the present study, we examine the gender differences in the development of tolerance to the hyperthermia mediated by methylone.
Materials and methods
Animals
Adult, male (n = 12, 275–300 g) and female (n = 12, 225–250 g) Sprague-Dawley (Rattus norvegicus domesticus) rats (N = 24) were obtained from Envigo (Indianapolis, IN). Animals were housed one per cage (cage size: 21.0 × 41.9 × 20.3 cm) and maintained on a 12:12 h light/dark schedule. To maximize the thermogenic response, animals were maintained at an ambient temperature of 25°C to 27°C and fed a minimum 10% fat diet [Citation9,Citation10]. Animal maintenance and research were conducted in accordance with the eighth edition of the Guide for the Care and Use of Laboratory Animals; as adopted and promulgated by the National Institutes of Health, with protocols approved by the Bowling Green State University Animal Care and Use Committee.
Drug and chemicals
Methylone was obtained from Cayman Chemicals (Ann Arbor, MI) as a hydrochloride salt. On the day of the study, methylone solutions were made fresh at a concentration of 10 mg/mL in 0.9% normal saline. All other chemicals and reagents were obtained from Sigma Chemical (St. Louis, MO).
Study design
Male and female rat cohorts were randomly assigned into two groups of six (6) each, the first group being the treatment group and the second serving as the saline controls. On testing day, all subjects were weighed prior to drug challenge, and a core temperature reading was taken with a rectal thermometer at time zero. On treatment days, the ambient temperature averaged 27.1 ± 0.4°C. Following the first temperature measurement, male and female treatment groups received a 10 mg/kg subcutaneous (sc) dose of methylone, and control groups received an equal volume of saline solution (sc). The total volume injected was adjusted weekly based on changes in body weight. On the first day of treatment, the males weighed 340.7 ± 5.1 g and the females weighed 252.2 ± 2.1 g. This difference in weight between the males and females was observed throughout the study period. Following drug challenge, core temperature readings were recorded every 30 min for a total of 90 min. This treatment schedule was maintained once a week for a total of 5 wk. On the sixth testing session, the above protocol was performed up to the 90-min time point, upon which rats were euthanized with CO2. Brown adipose tissue (BAT) and skeletal muscle (SKM), namely the gastrocnemius, were removed and flash frozen with liquid nitrogen, then stored at −80°C.
RNA isolation and qRT-PCR
To isolate total RNA, samples from SKM and BAT were homogenized, then extracted using PureZOL™ RNA Isolation reagent (BioRad, CA). The concentration and quality of the RNA were determined using a NanoDrop Spectrophotometer (Thermo, MI) and by 1% agarose gel electrophoresis, respectively. cDNA was synthesized from 200 ng of total RNA using the iScript™ Select cDNA Synthesis Kit (Biorad, CA). Real-time quantitative PCR (qRT-PCR) was carried out in the CFX Connect Real-Time PCR Detection System (Biorad, CA) using iTaq™ universal SYBR® Green supermix (Biorad, CA). The PCR parameters were as follows: 3 min at 95°C; 40 cycles of 95°C for 10 s, 52–58°C for 30s, 68°C, 10 s; followed by melt curve analysis (65°C −95°C). Quantification cycle (Cq) values for all genes were compared and analyzed by using the ∆∆C(t) method [Citation11]. All primer pairs used for the analysis of UCP1, UCP3, TGR5, and actin controls were as described [Citation12].
Statistical analysis
GraphPad InStat v.6.0 software was used to complete all statistical analyses of data. The results are presented as the mean ± SEM of the rectal core body temperatures of the treatment/control groups. Between-group differences were compared with a one-way ANOVA followed by Student–Newman–Keul’s multiple comparison test. Dunnet’s post-hoc tests were performed to analyze the significance of within-group changes over the six-week time course. When only two groups were compared, a two-tailed t-test was performed. Significance was established at p < 0.05 a priori.
Results
Gender differences in the tolerance to methylone-mediated hyperthermia
The first week of treatment with methylone (10 mg/kg, sc) resulted in a significant hyperthermic response in both the male (p < 0.01; ) and female (p < 0.01; ) rats in measures of ∆°C from baseline temperature. The male rats continued to express a hyperthermic response (p < 0.001) to methylone at weeks two and three. By the fourth week of methylone treatment, the male response, while still hyperthermic (p < 0.05), yielded a significantly lower ∆°C from baseline temperature than week three (p < 0.001), suggesting a developing tolerance response. A significant difference in maximal temperature change from baseline in males between weeks three and four (p < 0.001) further illustrates a tolerance effect (). There was no hyperthermic effect in males () at either week five or six, suggesting a sustained tolerance effect (). Conversely, week one was the only time point where hyperthermia occurred in female rats treated with methylone (). By week two, female rats not only displayed no hyperthermic response to methylone but also demonstrated a tolerance to the hyperthermia, with a significantly lower ∆°C from baseline temperature compared to week one (p < 0.01; ). Furthermore, a hypothermic effect was observed after methylone treatments in female rats at weeks three, four, five and six, shown by significant ∆°C from baseline temperature readings that were below zero (p < 0.01). Weeks four and five in measures of maximal temperature change from baseline were significantly lower when compared to week one (p < 0.01), in addition to being maximally below zero, further illustrating the hypothermic effect of methylone in female treatment group (). Significant differences between males and females ∆°C from baseline temperature were observed at the 30-min time point only on week one (p < 0.001; ). Nevertheless, there was no significant difference in maximal temperature change (p = 0.1169; ). Weeks two and three resulted in significant differences in ∆°C from baseline temperature following methylone exposure between male and female’s treatment groups at 30, 60, and 90-min time points (p < 0.001). At week four, there was a significant difference ∆°C from baseline temperature at both the 30 and 60-min time points (p < 0.05). Significant differences in maximal temperature change between males and females were seen at weeks two, three, four, and five (p < 0.002).
Figure 1. (a) Weekly comparison of ∆°C from baseline temperature in male treatment group at 30 (●), 60 (■), and 90-min (▲) post treatment vs male saline controls at 30 (○), 60 (□), and 90-min (△) time points. Significance of hyperthermic response is denoted by asterisks; * = p < 0.05, ** = p < 0.01, *** = p < 0.001, while significant tolerance effects are denoted by †. (b) Weekly comparison of ∆°C from baseline temperature in female treatment group at 30 (●), 60 (■), and 90-min (▲) post treatment vs female saline controls at 30 (○), 60 (□), and 90-min (△) time points. Significance of hyperthermic response is denoted by asterisks; * = p < 0.05, ** = p < 0.01, *** = p < 0.001, while significant tolerance effects are denoted by †. Significant hypothermic effect is denoted by cent sign. (c) Weekly comparison of ∆°C from baseline temperature in male treatment group at 30 (●), 60 (■), and 90-min (▲) post treatment vs female treatment group at 30 (○), 60 (□), and 90-min (△) time points. Significance of hyperthermic response is denoted by asterisks; * = p < 0.05, ** = p < 0.01 =, *** = p < 0.001.
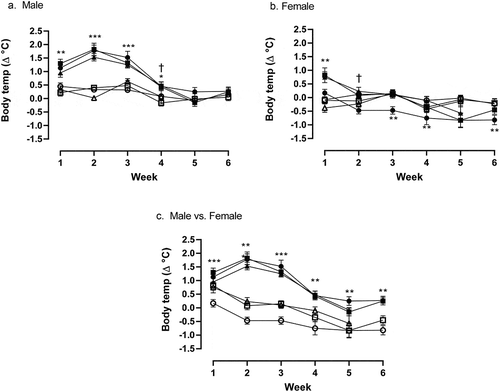
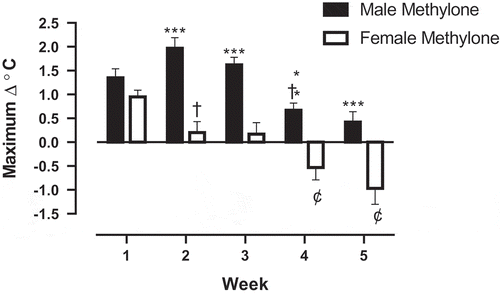
Figure 2. Weekly maximal temperature change (°C) from baseline in male and female rats following weekly treatment with methylone (10 mg/kg, sc) for 5 wk. Each value is the mean ± SEM; n = 6. Significance of between group differences are denoted by asterisks; * = p < .05, ** = p < .01, *** = p < .001, while significant (p < 0.002) tolerance effects are denoted by †. Significant hypothermic effect is denoted by ¢.
Expression of genes associated with hyperthermia in response to chronic methylone treatment
Gene expression analysis by qRT-PCR in SKM indicated down-regulation of TGR5 expression in both male and female methylone-tolerant rats. The down-regulation of TGR5 expression was significantly larger in methylone-tolerant males than in methylone-tolerant females (p <0.0001). The expression of UCP1 was almost 100-fold increased in methylone-tolerant males whereas a down-regulation of UCP1 was observed in methylone-tolerant females (p < 0.0001). For UCP3, the comparable significant fold changes were observed in both males and females methylone-tolerant rats compared to controls, with UCP3 expression in females being slightly higher than that observed in males (p = 0.0004; ).
Figure 3. qPCR gene expression analysis (Fold Change) of TGR5, UCP1, and UCP3 in (a) skeletal muscle and (b) brown adipose tissue following 6 wk of chronic methylone (10 mg/kg, sc) treatment of male (■) and female (□) rats. ** = male and female fold changes for that specific gene are significantly different from each other (p < 0.0004). ** = male and female fold changes for that specific gene are significantly different from each other (p < 0.0001). Each value is the mean ± SEM (n = 6).
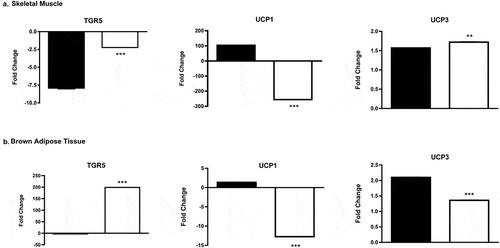
In brown adipose tissue, there was significant up-regulation in TGR5 expression in methylone-tolerant females relative to methylone-tolerant males (p < 0.0001). The expression of UCP1; however, showed a significant down-regulation in methylone-tolerant females compared a small positive up-regulation in methylone-tolerant males (p < 0.0001). For UCP3, expression was increased in both methylone-tolerant males and females, with significantly increased expression in males relative to females (p < 0.0001; ).
Discussion
Here, we demonstrate for the first time that chronic treatment with the synthetic cathinone methylone results in tolerance to the overall hyperthermic effects of the drug in both male and female rats. However, significant differences were seen between males and females. The females rapidly developed a tolerance effect evident after the first week of exposure. By the fourth week of methylone exposure, the response of female rats demonstrated a marked hypothermic response to the drug. Conversely, the males continued to display an acute hyperthermic response for the first 3 wk of treatment. By the fourth week of treatment, the male hyperthermic response was blunted, and by the fifth week, the hyperthermic response was dissipated altogether. Clemens et al. [Citation8] treated female rats with MDMA (8 mg/kg), methamphetamine (8 mg/kg) or the combination weekly for 16 weeks. Those authors measured changes in body temperature following drug exposure on weeks one, eight and sixteen. They found that the hyperthermic effects of MDMA or MDMA/methamphetamine were lost by week eight. These findings are similar to our present finding, where the hyperthermic effects in our female animals were lost after 1 wk of treatment and hypothermia was seen by 3 wk of methylone treatment. Piper et al. [Citation13] utilized adolescent female rats also demonstrated a hypothermic response after MDMA (10 mg/kg) every 5 d from postnatal day 35 to 60.
Peripherally, sympathomimetic- induced hyperthermia is mediated by an inability to dissipate heat through norepinephrine-mediated vasoconstriction [Citation14] and an increase in heat generation through the activation of mitochondrial uncoupling [Citation15]. Previously, we had demonstrated that the acute differences in thermogenic responses to MDMA in males and females rats could be attributed to four sex-specific mechanisms: (1) female subjects have reduced sympathetic activation; (2) female vasculature is less sensitive to α1-adrenergic stimulation; (3) female vasculature has an increased sensitivity to nitric oxide and (4) female expression of UCP3 in skeletal muscle is less than that seen in males [Citation5]. Sympathomimetic-induced hyperthermia and activation of UCP are in part dependent upon free fatty acids liberated from white adipose tissue [Citation16,Citation17]. Given that the male rats weighed more than the female rats throughout the current study, weight may have been a contributing factor to the temperature differences observed.
Alteration of gene expression can also be mediated by epigenetic regulation via DNA methylation and chromatin/histone modifications. Exposure to drugs of abuse can lead to the alteration of gene expression via epigenetic mechanism. Sprague-Dawley rats given cocaine demonstrated alteration in the expression of genes that correspond with drug-seeking and addiction behaviors via histone modifications [Citation18]. Sex-dependent DNA methylation has been observed in the cortex of male and female rats during development and in adult rat [Citation19]. Hypermethylation of the estrogen receptor promoter was reported in the rat following neonatal bisphenol A exposure [Citation20]. The difference in the expression of genes in our experiment between males and females following chronic methylone could be due, in part, to epigenetic regulation, which warrants further investigation.
Roles of TGR5, UCP1, and UCP3 in sympathomimetic-induced hyperthermia
UCP3 knockout mice exhibit a blunting of both sympathomimetic and norepinephrine-induced thermogenesis compared to their wild type littermates [Citation15]. Subsequently, UCP3 was demonstrated to be an important thermogenic target of thyroid hormone-induced body temperature regulation in skeletal muscle [Citation21,Citation22]. Recently, UCP1 and UCP3 were found to play complementary roles in the onset (UCP1) and maintenance (UCP3) of sympathomimetic-induced hyperthermia [Citation6]. Bile acids increase energy expenditure in a UCP-dependent fashion in BAT and SKM [Citation23]. Through the activation of the G-protein coupled bile acid receptor TGR5 (aka. M-BAR, GPBAR1), bile acids increase uncoupling to generate heat (for a review see [Citation24],). Antagonism of the TGR5 receptor with triamterene has also been demonstrated to attenuate the hyperthermic effects of MDMA [Citation12]. In the present study, chronic weekly exposure to methylone resulted in a significant UCP1 reduction in SKM and BAT of the female rats. Although the males and females both demonstrated a reduction in TGR5 in SKM, in BAT the male displayed no change in TGR5 and the females an increase in TGR5. Finally, UCP3 expression increased in both the SKM and BAT of the males and females. The changes in gene expression seen here may not be related to the temperature differences and further research is needed to determine if these gene changes are related to the important acclimation differences seen between genders.
Clinical implications
In a recent review of synthetic cathinone-related fatalities, Zaami et al. [Citation25] found that death was attributed to hyperthermia, hypertension and serotonin syndrome. Further, a majority of the victims were white males, with a previous history of drug abuse. In the present study, we found that weekly exposure to methylone resulted in a hyperthermic response in males over a longer period and that the females became tolerant within a week of first exposure. Pharmacologic targeting of the peripheral mediators of MDMA-induced or methylone-induced hyperthermia with carvedilol providing both β1-3AR antagonisms with α1AR antagonism has also been shown to effectively reverse this hyperthermia in animal [Citation26–Citation28] and human subjects [Citation29]. Additionally, targeting the central triggers of the peripheral response with the atypical antipsychotic agent clozapine has also been effective in reducing MDMA-mediated thermogenesis [Citation28]. The gender differences in tolerance to the hyperthermia mediated by methylone were also associated with differences in the expression of genes associated with heat generation in the target tissue.
Conclusions
Male and female rats treated weekly to methylone develop a tolerance to the drug’s ability to induce hyperthermia. Female rats develop this tolerance much more rapidly and subsequently develop a hypothermic response to repeated dosing of methylone. The differences between male and female subjects further demonstrate the need for gender studies when examining the toxicologic effects of drugs with abuse potential.
Abbreviations
| (MDMA) | = | 3,4-methylenedioxymethamphetamine |
| (UCP3) | = | uncoupling protein 3 |
| (UCP1) | = | uncoupling protein 1 |
| (sc) | = | subcutaneous |
| (ip) | = | intraperitoneal |
| (BAT) | = | brown adipose tissue |
| (SKM) | = | skeletal muscle |
| (qRT-PCR) | = | quantitative PCR |
| (Cq) | = | quantification cycle |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emer Med. 2012;60:103–105.
- O’Connor AD, Padilla-Jones A, Gerkin RD, et al. Prevalence of rhabdomyolysis in sympathomimetic toxicity: a comparison of stimulants. J Med Toxicol. 2015;11:195–200.
- U.S. Drug Enforcement Administration, Diversion Control Division. Synthetic cannabinoids and synthetic cathinones reported in NFLIS, 2013–2015. Springfield (VA): U.S. Drug Enforcement Administration; 2016.
- Grecco GG, Sprague JE. Impact of functional group modifications on designer phenethylamine induced hyperthermia. Chem Res Toxicol. 2016;29(5):871–878.
- Wyeth RP, Mills EM, Ullman A, et al. The hyperthermia mediated by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is sensitive to sex differences. Toxicol Appl Pharmacol. 2009;235(1):33–38.
- Riley CL, Dao C, Kenaston MA, et al. The complementary and divergent roles of uncoupling proteins 1 and 3 in thermoregulation. J Physiol. 2016;594(24):7455–7464.
- Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs. 2014;46(5):369–378.
- Clemens KJ, Cornish JL, Hunt GE, et al. Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioral and neurochemical effects in rats. Drug Alcohol Dep. 2007;86:183–190.
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) in rats. Psychopharmacology. 1994;114:505–508.
- Mills EM, Weaver KL, Abramson PM, et al. Influences of dietary fats on ecstasy-induced hyperthermia. Br J Pharmacol. 2007;151:1103–1108.
- Kiraly DD, Walker DM, Calipari ES, et al. Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep. 2016;6:35455.
- Ridge EA, Pachhain S, Choudhury SR, et al. The influence of the host microbiome on 3,4-ethylenedioxymethamphetamine (MDMA)-induced hyperthermia and vice versa. Sci Rep. 2019;9(1):4313.
- Piper BJ, Henderson CS, Meyer JS. Adolescent MDMA exposure diminishes the physiological and neurotoxic consequences of an MDMA binge in female rats. Dev Psychobiol. 2014;56:924–934.
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654.
- Mills EM, Banks ML, Sprague JE, et al. Pharmacology: uncoupling the agony from ecstasy. Nature. 2003;426:403–404.
- Dao CK, Nowinski SM, Mills EM. The heat is on: mechanisms of drug-induced hyperthermia. Temperature. 2014;1(3):183–191. doi: 10.4161/23328940.2014.985953.
- Hrometz SL, Ebert JA, Grice KE, et al. Potentiation of Ecstasy-induced hyperthermia and FAT/CD36 expression in chronically exercised animals. Temperature. 2016;3(4):557–566. doi: 10.1080/23328940.2016.1166310.
- Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314.
- Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Hormones Behav. 2011;59(3):353–357.
- Doshi T, Mehta SS, Dighe V, et al. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289(2–3):74–82.
- Sprague JE, Yang X, Sommers J, et al. Roles of norepinephrine, free Fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J Pharmacol Exp Ther. 2007;320:274–280.
- Flandin P, Lehr L, Asensio C, et al. Uncoupling protein-3 as a molecular determinant of the action of 3,5,3ʹ-triiodothyronine on energy metabolism. Endocrine. 2009;36(2):246–254.
- Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489.
- Fiorucci S, Mencarelli A, Palladino G, et al. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–580.
- Zaami S, Giorgetti R, Pichini S, et al. Synthetic cathinones related fatalities: an update. Eur Rev Med Pharmacol Sci. 2018;22:268–274.
- Sprague JE, Moze P, Caden D, et al. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) in an animal model. Crit Care Med. 2005;33(6):1311–1316.
- Zona LC, Grecco GG, Sprague JE. Cooling down the bath salts: carvedilol attenuation of methylone and mephedrone mediated hyperthermia. Toxicol Let. 2016;263:11–15.
- Kiyatkin EA, Ren S, Wakabayashi KT, et al. Clinically relevant pharmacological strategies that reverse MDMA-induced brain hyperthermia potentiated by social interaction. Neuropsychopharmacol. 2016;41:549–559.
- Hysek C, Schmid Y, Rickli A, et al. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol. 2012;166(8):2277–2288.
