ABSTRACT
As most fish are ectotherms, their physiology is strongly affected by temperature. Temperature affects their metabolic rate and thus their energy balance and behavior, including locomotor and feeding behavior. Temperature influences the ability/desire of the fish to obtain food, and how they process food through digestion, absorb nutrients within the gastrointestinal tract, and store excess energy. As fish display a large variability in habitats, feeding habits, and anatomical and physiological features, the effects of temperature are complex and species-specific. The effects of temperature depend on the timing, intensity, and duration of exposure as well as the speed at which temperature changes occur. Whereas acute short-term variations of temperature might have drastic, often detrimental, effects on fish physiology, long-term gradual variations might lead to acclimation, e.g. variations in metabolic and digestive enzyme profiles. The goal of this review is to summarize our current knowledge on the effects of temperature on energy homeostasis, with specific focus on metabolism, feeding, digestion, and how fish are often able to “adapt” to changing environments through phenotypic and physiological changes.
Overall effects of temperature in fish
Most fish are classified as ectotherms, i.e. their metabolic heat production, and retaining mechanisms are insufficient to provide body warming [Citation1]. They are therefore strict temperature conformers and obligate poikilotherms i.e. the ambient environmental temperature determines their body temperatures. As a consequence, in fish, temperature sets the rates of virtually all biochemical reactions and thus the pace of physiological processes [Citation2,Citation3]. Since temperature has such a large impact on fish, it is labeled the abiotic ecological master factor [Citation4].
When uncompensated, metabolic processes increase 2- to 3-fold with a 10°C increase in environmental temperature, the influence of temperature on physiological processes being described by the Q10 temperature coefficient (i.e. the rate at which a physiological response changes with a 10°C increase in temperature) [Citation5]. However, the magnitude of these changes varies with the temperatures considered, as Q10 values are not constant for different 10°C increments and usually decrease at higher temperatures [Citation5,Citation6]. Consequently, enzymatic reactions, cellular respiration, oxygen consumption, and thus metabolic rates vary with temperature [Citation7,Citation8].
Since biochemical reaction rates increase with temperature, the standard metabolic rate (SMR, the metabolic rate required to maintain life and routine activity) in the ectothermic fish also increases with temperature ()). The maximum metabolic rate (MMR or metabolic rate at maximum sustained exercise) on the other hand, usually has a dome-shaped response to temperature, where it increases, and subsequently plateaus or decreases [Citation9]. The metabolic (or aerobic) scope, calculated as the difference between MMR and SMR (plotted in )), is the surplus energy left after the basal maintenance costs are met and is available for functions such as digestion, locomotion, growth, and reproduction [Citation9,Citation10]. Aerobic scope is used as a proxy for performance ()).
Figure 1. (a) Changes in standard metabolic rate (SMR) and maximum metabolic rate (MMR) and aerobic scope (AS) as a function of temperature in fish; (b) Performance curve as a function of temperature. Performance is maximal at an optimal temperature (Topt). Performance can no longer be sustained at temperatures below the minimal critical temperature (CTmin) or beyond the maximal critical temperature (CTmax) [based on [Citation18]]
![Figure 1. (a) Changes in standard metabolic rate (SMR) and maximum metabolic rate (MMR) and aerobic scope (AS) as a function of temperature in fish; (b) Performance curve as a function of temperature. Performance is maximal at an optimal temperature (Topt). Performance can no longer be sustained at temperatures below the minimal critical temperature (CTmin) or beyond the maximal critical temperature (CTmax) [based on [Citation18]]](/cms/asset/3a669816-4ab2-465a-b324-b41cb2760f56/ktmp_a_1765950_f0001_oc.jpg)
In all ectotherms, including fish, thermal performance curves, i.e. performance (related to traits such as growth, reproduction, and locomotion) as a function of temperature is bell-shaped curves [Citation11,Citation12]. Performance is maximal within a range of optimal temperatures, declines when outside that optimal range, to become zero at the upper and lower critical temperatures ()), which define the organism’s thermal tolerance range [Citation13].
Fish live in species-specific temperature ranges where they can optimize physiological performance, so that performance curves vary among species [Citation14,Citation15]. Fish living at low temperatures have a left-shifted performance curve compared to high-temperature adapted fish [Citation6]. For example, the maximum thermal tolerance varies from −1/−2°C in Antarctic fish (Notothenioidei) [Citation16] to 44.6°C for desert pupfish (Cyprinodon macularius, C. salinus) [Citation17]. In general, temperate ectothermic species, inhabiting mid-latitudes where seasonal differences in temperatures are largest, have wider thermal performance ranges, lower optimal temperatures, and are more cold-tolerant and less heat-tolerant compared to tropical species [Citation18,Citation19].
Temperature preference also varies with other intrinsic factors such as size (small animals have a higher metabolic rate per unit weight than do large animals) age, and developmental and reproductive stages [Citation9,Citation20].
Individual fish exposed to moderate temperature changes are usually able to maintain close to optimal performance by altering either their behavior (preference/avoidance) or their physiology by acclimatization/acclimation (i.e. reversible physiological plasticity, responses without changes in genotype) [Citation1]. Acclimatory responses act to keep biological processes functioning at an acceptable rate [Citation5] and include changes in the synthesis of isozymes and modification of cell membrane structure. Longer exposures over several generations result in evolutionary changes and genetic adaptation [Citation6]. Metabolic acclimation requires time and is valuable as an adaptation to seasonal or slow changes in temperature, while it fails to enable the fish to respond to rapid episodic changes such as those due to heat waves in the summer. In addition, concurrent factors such as heat waves and bloom of toxic algae may interact with detrimental synergistic effects on survival: for example, in larval cobia, survival is reduced by 16% in fish exposed to either heat or algae toxin, but drops by 60% when both stressors are present [Citation21].
Temperature tolerance and performance curves shift with acclimation and lethal thermal limits are affected by the temperatures that fish are placed in prior to exposure [Citation22]. With this as a starting point, two types of lethal thermal limits can be defined: incipient (or chronic) and ultimate (acute) lethal temperatures [Citation5]. Incipient lethal temperature is the temperature at which significant mortality (50% of the group is often the standard, TL50) occurs, following continuous exposure of fish to this temperature for a long time period. Exposure time is critical, and fish will survive exposure to higher/lower temperatures if that exposure is shorter. The acute lethal temperature is the temperature at which death occurs when water temperature is raised rapidly. At the ultimate (acute) lethal temperature mortality is almost instantaneous (<10 min) [Citation5].
The thermal limits for fish acclimated to specific temperatures can be summarized graphically as a temperature tolerance polygon [Citation23]. Pioneering studies by Fry and coworkers used these thermal tolerance polygons to compare eight salmonid species [see refs in [Citation24]]. The incipient lethal levels were defined within a tolerance zone where salmon survived over 7 days. The data for Atlantic salmon (Salmo salar), the most robust of these species [Citation24], are shown in . Resistance to thermal stress outside the tolerance zone was a function of time: the ultimate lethal level (defined as survival for 10 min) increased with acclimation temperature to a maximum of 33°C, whilst the minimum value remained close to 0°C.
Figure 2. Thermal tolerance polygon for Atlantic salmon. The purple area outlines the tolerance zone while the blue area is the limit for food intake. The incipient lethal temperature (incipient Ct) is the temperature where 50% of individuals acclimated to a certain temperature can survive for a long time, in this study defined as 7 days. The ultimate lethal temperature (Ultimate Ct) is the highest temperature to which fish can acclimate [Citation123]. Note that both lower and upper limits for food intake, incipient, and ultimate lethal temperatures are positively correlated with acclimation temperature [modified from [Citation24]]
![Figure 2. Thermal tolerance polygon for Atlantic salmon. The purple area outlines the tolerance zone while the blue area is the limit for food intake. The incipient lethal temperature (incipient Ct) is the temperature where 50% of individuals acclimated to a certain temperature can survive for a long time, in this study defined as 7 days. The ultimate lethal temperature (Ultimate Ct) is the highest temperature to which fish can acclimate [Citation123]. Note that both lower and upper limits for food intake, incipient, and ultimate lethal temperatures are positively correlated with acclimation temperature [modified from [Citation24]]](/cms/asset/cb8be480-5395-4515-ac72-75afcf4947bb/ktmp_a_1765950_f0002_oc.jpg)
Fish with poor swimming ability are sometimes forced to adapt to habitats where temperature fluctuations are large. For example, fish faced with harsh winter conditions become dormant and down-regulate metabolic enzyme activity [e.g. cunner Tautogolabrus adspersus [Citation25,Citation26]] or are freeze tolerant [e.g. winter flounder Pseudopleuronectes americanus, sculpins [Citation27]]. Interestingly, the temperature sensors in fish remain to be described. Fish do not seem to have the TRPM8 gene, which encodes for the principal molecular receptor of cold temperatures in mammals. However, there is evidence in zebrafish that they possess TRPV1, a cation-selective ion channel activated by hot temperatures [Citation28] (and also by noxious chemicals like capsaicin, although this may not be relevant in fish). However, there is ample evidence that fish sense and react to temperature, as they are capable of behavioral thermoregulation [Citation4,Citation29]. High temperatures induce frantic activity which assists fish in nature to flee [Citation30] and can cause apparent panic and intense swimming activity, as seen in Atlantic salmon treated against salmon louse during short-term exposure, i.e. 30 s in water at 34–36°C [Citation31]. Low temperatures most likely are also sensed, but cold induces lethargy which might prevent fish from escaping very low temperatures [Citation30].
The close relationships between water temperature and the rates of physiological processes also affect the food intake required to meet the demands for chemical energy and substrates necessary for survival. Ingested food, following processing by the gastrointestinal tract (GIT), provides the chemical energy used for basal maintenance and the excess is allocated to activity/locomotion, somatic growth or reproduction and storage/reserves [Citation32]. Some of the ingested energy is also lost during processing of nutrients via specific dynamic action (SDA), the increase in metabolic rate in response to feeding resulting from ingestion, digestion, absorption, and assimilation of a meal ()). Temperature, through its effects on metabolism, affects this energy budget by influencing nutrient digestion and assimilation and the investment of surplus energy into reproduction and growth, and the intake of energy via feeding [Citation9]. However, although this relationship has often been described using an energetics approach, the mechanisms underlying some of the observed changes are poorly understood. It has been speculated that food intake ultimately is a function of energy requirements, including the energy required to grow. However, this assumption lacks empirical support not only in fish but also in mammals, and, to date, there is no convincing demonstration that energy expenditure influences within-day appetite control [Citation33]. Several models for physiological control of appetite mainly developed for mammals do not involve energy expenditure, but rather describe food intake as a function of signals arising from adipose tissue and the GIT [Citation34,Citation35]. The experimental evidence for making such thorough assessment does not exist in fish, due to the current lack of available methods for assessing the parameters required. However, several of the key factors involved in signaling pathways in the regulation, particularly at the gene expression levels have been explored in several studies over the last few years (see sections below).
Figure 3. (a) Dynamic energy budget in a fish. Energy is provided by ingestion of food, which is processed in the gastrointestinal tract (GIT). Food undergoes digestion and portion of the digesta is absorbed, and some is not absorbed and is lost via the feces (egestion). Absorbed nutrients provide energy that is used for basal maintenance (SMR). Energy in excess is allocated to activity/locomotion, storage/reserves, somatic growth, or reproduction. Energy is also lost during processing of nutrients via SDA and some due to excretion of N-containing compounds, i.e. NH3 [based on [Citation124] and [Citation125]]. (b) General effects of temperature on performance (conversion efficiency) and rates of food intake, metabolic rate, and growth in fish. Numbers (1–3) indicate maxima (optima) in relation to temperature [modified from [Citation126]]
![Figure 3. (a) Dynamic energy budget in a fish. Energy is provided by ingestion of food, which is processed in the gastrointestinal tract (GIT). Food undergoes digestion and portion of the digesta is absorbed, and some is not absorbed and is lost via the feces (egestion). Absorbed nutrients provide energy that is used for basal maintenance (SMR). Energy in excess is allocated to activity/locomotion, storage/reserves, somatic growth, or reproduction. Energy is also lost during processing of nutrients via SDA and some due to excretion of N-containing compounds, i.e. NH3 [based on [Citation124] and [Citation125]]. (b) General effects of temperature on performance (conversion efficiency) and rates of food intake, metabolic rate, and growth in fish. Numbers (1–3) indicate maxima (optima) in relation to temperature [modified from [Citation126]]](/cms/asset/82166141-2527-4fa7-9910-10b40efab0a5/ktmp_a_1765950_f0003_oc.jpg)
Effects of temperature on feeding
Effects of temperature on feeding behavior and food intake
The impacts of temperature on feeding vary depending on species, but usually, voluntary food intake increases with moderate temperature increases, and decreases when temperatures are outside the fish optimal temperature range ()); [e.g. Sockeye salmon Oncorhynchus nerka [Citation4]; channel catfish Ictalurus punctatus [Citation36]; walking catfish Clarias batrachus [Citation37]; cobia Rachycentron canadum [Citation38]; Indian major carp Catla catla [Citation39]; Atlantic salmon [Citation40,Citation41]; goldfish Carassius auratus [Citation42]; perch fry Perca fluviatilis [Citation43]].
The available data show that fish will lose appetite, cease, and finally stop ingesting food at temperatures well before the ultimate maximal critical temperature for the species [Citation44]. This can also be seen in thermal tolerance polygons in Atlantic salmon [Citation45], for which the temperature limits for food intake increase slightly with acclimation temperature to upper and lower mean values of 22.5°C and 7.0°C, but these values are well below the incipient lethal temperature ().
Successful food consumption depends on the availability of appropriate food items, adequate sensory perception, and the capacity for locomotion. Food intake is closely linked to feeding behavior, which includes a series of steps including food detection, capture, and ingestion and ultimately swallowing [Citation46,Citation47]. Temperature might independently affect and modulate several of these processes and factors.
Food is detected via a wide range of chemical (olfaction and taste), visual (eyes), and mechanical (lateral line) stimuli. In most species, olfaction detects the most distant stimuli while touch and gustation detect the closest ones and vision plays the most prominent role in prey/food detection [Citation48]. However, there is variation among fish species. For example, plaice Pleuronectes platessa is mostly dependent on vision for feeding but sole Solea solea relies principally on chemoreception and mechanoreception [Citation49]. In Chinese perch Siniperca chuatsi, blocking of olfaction, but not vision or lateral line, decreases feeding behavior [Citation50]. Similarly, in goldfish, destruction of the eyes [Citation51] or reduced visibility (increased water turbidity) [Citation42] does not affect food intake, although it increases locomotion and the time taken to reach food, whereas impairment of olfaction decreases feeding behavior [Citation52]. In red drum Sciaenops ocellatus, blocking vision alone or olfaction alone does affect predation, whereas fish with the lateral line system blocked exhibited low predation rate [Citation53]. Some fish species are also reliant on hearing for detection of predators and prey, particularly in muddy or dark habitats when vision is limited [Citation54].
Temperature has been shown to influence the sensitivity of sensory systems, including vision, hearing, and olfaction/taste, likely affecting feeding behavior. For example, in rockfish (Sebastes sp.), the low-light sensitivity of the retina decreases tenfold with a 10°C increase in temperature [Citation55]. In channel catfish Ictalurus punctatus, hearing sensitivity is lowest at 10°C and increases between 10°C and 26°C [Citation54], and the average life span of taste bud cells on the barbels is on the order of 40, 30, 15, and 12 days at 14°C, 18°C, 22°C, and 30°C, respectively [Citation56]. These changes in perception might explain why temperature can affect taste preferences in some fish. For example, in stellate sturgeon Acipenser stellatus, palatability of some amino acids (L‐glutamic acid, L‐alanine, L‐tryptophan, L‐valine, and L‐leucine) changes with temperature [Citation57,Citation58].
Temperature can also affect fish cognitive abilities, perhaps also disturbing their ability to forage for food. In wild-type zebrafish, extreme temperatures (18°C and 34°C, 26°C being control) have been shown to not only affect energy metabolism but also to down-regulate the expression of proteins associated to synapses and neurotransmitter release, resulting in reduced interest for the novel environment and an impairment of cognitive abilities during behavioral tests [Citation59].
Furthermore, temperature can affect locomotor performance of fish and their ability to capture food (and also to escape from predators), with an increase up to an optimal temperature and a rapid decrease as temperatures increase above that optimal value. For example, the best swimming performances for goldfish are attained at temperatures from 20°C to 30°C, with lower values at 5°C, 10°C, 15°C, 35°C, and 38°C [Citation60]. Similar curves are seen in other fish such as Atlantic cod Gadus morhua [Citation61], channel catfish [Citation36], five-banded damsel-fish A. vaigiensis and A. whitleyi [Citation62], yellow catfish Pelteobagrus fulvidraco [Citation63]. Similarly, decreases in locomotor performance negatively affect the ability of prey to survive an attack from a predator [Citation64].
It is not clear to what extent these temperature-dependent changes in swimming performances are due to physiological changes in fish muscle and neurons or to variations in the physical properties of water. Fish propulsion is driven by muscular contractions controlled by the nervous system. Temperature strongly affects muscle physiology [for example, muscle takes twice as long to contract and relax when temperature drops by 10°C and have faster contractile properties in warmer waters [Citation65]] and nerve conduction is reduced by temperature [Citation66]. Changes in temperature also cause variations in the physical properties of water, which may affect fish movements, as a decrease in temperature induces increases in viscosity and density, and thus drag. For example, in Atlantic herring Clupea harengus, swimming speed increases by 60% in fish as temperature is increased from 6°C to 13°C and reduced viscosity accounts for 54% of this increase [Citation67].
Effects of temperature on appetite-regulating endocrine factors
In fish, as in mammals, the control of food intake is affected by a number of endocrine factors that are produced by the brain or peripheral organs (such as the intestine, pancreas, liver, and possibly adipose tissue and muscle) (), which influence feeding centers in the brain and thus inhibit (anorexigenic factors) or stimulate (orexigenic factors) feeding [Citation68,Citation69]. Peripheral endocrine factors are released in the blood and cross the blood-brain barrier and have a direct action on receptors on complex neural networks that act as feeding centers. Sensory information (chemical, sensed by chemoreceptors, and mechanical, such as distension, sensed by mechanoreceptors) from the GIT is also carried by the vagus nerve and will also affect feeding centers, via innervation from the brainstem [Citation69,Citation70]. Recent evidence suggests that plasma concentrations of glucose can be directly sensed by the brain and appetite-controlling centers, and the existence of comparable mechanisms for other nutrients is currently being explored [Citation71]. The major endocrine brain factors include neuropeptide Y (NPY), orexin, cocaine- and amphetamine-regulated transcript (CART), Agouti-related protein peptide, melanocyte-stimulating hormone [post-translatory cleavage product from the proopiomelanocortin] and corticotropin-releasing factor (CRF), while major peripheral endocrine factors include cholecystokinin (CCK), peptide YY and glucagon-like peptide (intestine), ghrelin (intestine/stomach), and leptin (liver) [Citation68,Citation69,Citation72].
Figure 4. General overview of the endocrine regulation of feeding in fish. Information on the presence and composition of food in the gastrointestinal tract (GIT) is transmitted to the brainstem and subsequently the brain feeding centers via receptors [GIT hormone receptors as well as mechanoreceptors (stretch) and chemoreceptors] located on vagal afferents. Some GIT hormones also act via the circulation and cross the blood-brain barrier and affect the secretion of brain appetite hormones, which in turn affect feeding centers. AgRP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; CCK, cholecystokinin; CRF, corticotropin-releasing factor; GHRL, ghrelin; GLP, glucagon-like peptide; MCH, melanocyte-concentrating hormone; NPY, neuropeptide Y; OX, orexin; POMC, proopiomelanocortin; PYY, peptide YY. Hormones are characterized as orexigenic (green) or anorexigenic (red) if they stimulate or inhibit feeding, respectively
![Figure 4. General overview of the endocrine regulation of feeding in fish. Information on the presence and composition of food in the gastrointestinal tract (GIT) is transmitted to the brainstem and subsequently the brain feeding centers via receptors [GIT hormone receptors as well as mechanoreceptors (stretch) and chemoreceptors] located on vagal afferents. Some GIT hormones also act via the circulation and cross the blood-brain barrier and affect the secretion of brain appetite hormones, which in turn affect feeding centers. AgRP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; CCK, cholecystokinin; CRF, corticotropin-releasing factor; GHRL, ghrelin; GLP, glucagon-like peptide; MCH, melanocyte-concentrating hormone; NPY, neuropeptide Y; OX, orexin; POMC, proopiomelanocortin; PYY, peptide YY. Hormones are characterized as orexigenic (green) or anorexigenic (red) if they stimulate or inhibit feeding, respectively](/cms/asset/2f8630cc-b700-4b2d-9086-517a35839754/ktmp_a_1765950_f0004_oc.jpg)
Similar to mammals, food consumption in fish may be viewed in terms of short- and long-term signals. However, the large variability in feeding habits, food availability, ecology, and anatomical features, such as the lack of stomach in some fish, adds much complexity to this view. In the short term, initiation of food consumption during a meal is believed to be regulated by sensory stimuli, gut mechanical and chemical sensors, nutrient concentrations in the plasma, and levels of gut hormones secreted by specialized enteroendocrine cells [Citation34]. Dynamic changes in these stimuli eventually induce “satiation,” leading to a decrease and termination of food intake while food is present in the anterior part of the GIT. In many species, there will be a period of “satiety” that is characterized by an inhibition of further ingestion and minimal hunger. For example, in rainbow trout Oncorhynchus mykiss, appetite stops shortly after feeding and returns when 70–80% of the previous meal has been evacuated from the stomach [Citation73]. The long-term regulation of feeding in fish is more controversial. Fish can survive for long periods of time without food, and fasting periods are part of the natural life cycle of many fish species, as it is the case during winter months and spawning migrations. In mammals, leptin, secreted by adipose tissue, acts as a lipostatic signal which relays information regarding energy status to the brain and allows animals to maintain a relatively constant body weight [Citation74]. It is still unclear if and how any adiposity signal (i.e. leptin in mammals), modulates hunger, satiation, and feeding behavior in fish. While there are indications that leptin may act as a “the lipostatic signal” in fish [Citation74], thus providing a potential negative feedback for the regulation of adipose tissue, it has also been suggested that leptin is mainly involved in glucose homeostasis and/or maintenance of adequate energy stores for survival during periods of energy deficit [Citation74,Citation75].
In fish, the argument for a long-term homeostatic control of energy, in particular in the context of body weight stability, is not convincing, since many fish continue to grow their whole life. Overfeeding does not lead to any significant down-regulation of energy intake as would be predicted in a homeostatic system, but rather the fish grow faster and continue to allocate more of the ingested energy into fat deposits in body. Similar to mammals, there is no complete generic or species-specific model that describes the signaling pathways and factors involved in the control of appetite and growth in fish. Again, similar to mammals [Citation34] the current conceptualization in fish should not be regarded as permanent but rather as an evolving representation based on the current state of understanding.
Very few studies have examined the effects of temperature on the endocrine factors controlling feeding in fish mentioned above.
In goldfish, higher temperatures increase orexin mRNA levels and decrease CART mRNA levels in the hypothalamus and decrease peptide YY and CCK mRNA levels in the intestine [Citation42]. In Atlantic cod, low temperatures inhibit food intake, and this inhibition is in part mediated by increases in CART transcript expression [Citation76]. In Atlantic salmon, temperature-induced variations in feeding have been correlated to changes in plasma concentrations of ghrelin [Citation41,Citation77] and leptin [Citation78]. In cunner, orexin, NPY, CART, and CCK mRNA levels decrease during natural torpor (when fish are exposed to low temperatures and do not feed) as compared to fed summer fish, suggesting these hormones mediate different physiological responses to torpor-induced long-term fasting [Citation79].
The hypothalamic–pituitary–interrenal axis has also been shown to be involved in temperature-mediated changes in feeding. Stressful environmental conditions such an increase in temperature or extremely high and low water temperatures induce the release of CRF and cortisol, which both inhibit feeding in fish [e.g. rainbow trout [Citation80], grouper Epinephelus akaara [Citation81]; Atlantic salmon [Citation40]; carp [Citation82]].
Effects of temperature on digestive processes
Digestion consists of a series of complex series of processes with the overall aim to maximize absorption of dietary nutrients (). After ingestion, food is mainly degraded by digestive enzymes and to some extent mechanically by muscular movements of the GIT. Among fish species, different feeding habits (e.g. herbivore, omnivore, carnivore) result in different GIT morphologies. Carnivores usually have short and straight intestines, most often with the presence of a true stomach and pyloric ceca (finger-like appendages in the proximal intestine, which increase the overall intestinal absorptive surface area) whereas herbivores tend to have longer intestines without ceca and sometimes no true stomach [Citation83]. Agastric fish may possess an intestinal bulb or an enlargement in the anterior intestine that might somewhat increase retention time [Citation84]. A number of gastrointestinal factors (hormones, neurotransmitters) act locally to regulate digestive processes [Citation85]. Temperature affects the secretory activity of digestive juices (by its effect on food ingestion), GIT motility, the activity of digestive enzymes, and digestion and absorption rates [Citation86].
Figure 5. Processes involved in digestion. After food is ingested (1), digestive juices are secreted (2) and allow enzymes to digest food to very simple molecules (3) that are transported across the intestinal epithelium into the blood (4). The food is actively transported through the gastro-intestinal tract (GIT) by smooth muscular movements (5). The remaining indigestible food is eventually evacuated (6). The gut transit time is the time from ingestion (1) to elimination (6). Digestion consists of closely orchestrated processes (7) that are integrated in ways that is believed to optimize efficiency and maximize absorption. *The GIT also has a very important function as a barrier to prevent entry of vectors for disease. Structural and enzymatic barriers are characteristic of the entire GIT. Microbiota and symbiotic digestion may occur not only in posterior section, but also in the anterior sections. Interactions between the digesta, microbiota, and gut tissue likely also occur
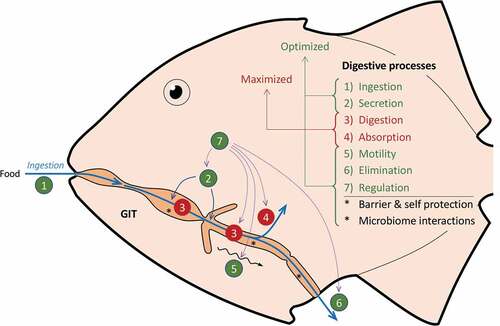
Temperature influences gut transit time and digestion/absorption rates
Cooler water temperatures may reduce nutrient digestibility, by reducing digestion rates, increasing gut transit time (time for all digesta to be voided from the fish), and lowering gastrointestinal evacuation rates [Citation87]. For example, in yellowtail kingfish (Seriola lalandi), gut transit time is 12–16 h in the summer compared to 36–48 h at winter temperatures [Citation87]. Similarly, gastric evacuation rates are higher at ≥20°C than under ≤15°C in the delicate loach Niwaella delicata [Citation88], at 20°C than at 10°C in horse mackerel [Citation89], at 30°C than 22°C in mahseer Tor tambroides [Citation90], 30°C than 22°C in the hybrid grouper Epinephelus fuscoguttatus ×E. lanceolatus [Citation91] and blood snapper Lutjanus malabaricus [Citation92], 34°C than at 30°C in cobia Rachycentron canadum [Citation93].
Temperature might also change environmental conditions in the GIT. For example, in cobia, increasing temperature from 30°C to 34°C increases the acidity levels in the intestine [Citation93], and in several seasonal species, intestinal pH decreases in summer and increases during the cold season [Citation94]. This suggests that temperature- and species-dependent pH changes in the intestine may be necessary to optimize the activity of digestive enzymes. The fatty acid composition of lipids in the intestinal mucosa of goldfish has also been shown to be temperature-dependent [Citation95], which might modify transport processes (e.g. lipid solubility of amino acids and the rate at which they cross the intestinal membranes). Temperature also affects the composition of the intestinal microbiota [Citation96,Citation97], which in turn might affect digestive processes [Citation98].
SDA (the increase in metabolic rate in response to feeding resulting from ingestion, digestion, absorption, and assimilation of a meal) is also affected by temperature. Higher temperatures usually induce higher increases in metabolic rates and decreased durations of SDA, as seen in lionfish Pterois ssp [Citation99]., Southern catfish Silurus meridionalis [Citation100], Atlantic cod [Citation101], Caribbean neon goby Elacatinus lobeli [Citation102], goldfish Carassius auratus [Citation103] and yellowfin tuna Thunnus albacares [Citation104].
Temperature affects digestive enzymes
Digestive processes and nutrient/energy digestibility usually decrease at temperatures outside the optimal range [e.g. brook trout Salvelinus fontinalis [Citation105]]. These changes are due in part to the effects of temperature on digestive enzyme activities. Efficient degradation of nutrients in the digestive tract of fish largely depends on the availability and activity of digestive enzymes, although a longer gut transit time will increase the time during which enzymes can work.
Most fish possess the similar main digestive enzymes, i.e. proteolytic enzymes (i.e. trypsin, carboxypeptidases), carbohydrate enzymes (i.e. maltase, amylase), lipolytic enzymes (i.e. lipase), and phosphatases (i.e. alkaline phosphatase) [Citation106,Citation107]. Some enzyme may have several forms within one species, forms that differ in specificity and activity [e.g. trypsin/trypsinogen in Atlantic salmon [Citation108], Atlantic cod [Citation109], albacore tuna Thunnus alalunga [Citation110], anchovy Engraulis japonicus [Citation111]]. Digestive enzyme profiles and activities vary between species, depending on food preferences. Carnivorous species have higher levels of proteolytic enzyme activity whereas carbohydrases are predominant in herbivorous and omnivorous fish [Citation112,Citation113].
The nature of digestive enzymes also varies depending on the habitat/climate fish live in. Temperature adaptation of enzymes is genetically determined and can involve phenotypic changes. These include differences in structure, affinity to substrates and activation energy, as well as changes in rate of secretion and production of isozymes – which catalyze the same reaction but with optimal efficiencies at different temperatures [Citation114]. For example, digestive enzymes from fish adapted to cold environments, such as the Atlantic cod [Citation115] and the Antarctic icefish (Chionodraco hamatus) [Citation116], have higher catalytic efficiencies at low and moderate temperatures, compared to mammals or fish living in warmer temperatures.
The effects of water temperature on fish digestive enzyme activities seem species-specific, as the optimal temperature for enzyme activity usually falls within the temperature range corresponding to the fish habitats, as seen for example, in some subtropical/tropical species: in seabass (Dicentrarchus labrax, carnivorous, range 8–24°C), α-amylase and lipase activities peak at 23°C and trypsin at 17°C [Citation117], in threespine stickleback (Gasterosteus aculeatus, carnivorous, 4–20°C), highest levels of amylase and trypsin activity occur at 18°C [Citation118], in walking catfish (Clarias batrachus, omnivore, 10–30°C) protease activities are higher at 25°C and lipase activity is higher at 30°C compared to 10°C, 15°C, 20°C, 25°C, 30°C, and 35°C, with lowest enzyme activities were recorded at 10°C [Citation37] and in Catla catla (omnivore, 18–28°C) fish exposed at 10°C show reduced digestive enzyme (amylase, protease, lipase, trypsin) activities compared to fish held at 25°C [Citation39].
Species-specific seasonal variations in enzymatic activities are also seen, likely related to not only temperature effects on enzymes but also changes in diet. In Atlantic salmon, trypsin activity levels are lower during the winter months compared to spring months [Citation119]. In yellowtail kingfish (Seriola lalandi), intestinal protease and lipase activity levels are higher in winter, possibly as a compensation to slower gut motility at colder water temperatures [Citation87]. In cunner, low temperatures induce a reduction in feeding and in the activities of intestinal trypsin, alkaline phosphatase, and lipase [Citation26]. In the Japanese grenadier anchovy Coilia nasus (planktivore), water temperature affects pepsin activity during the spawning migration [Citation120]. One seasonal species can also produce different isozymes depending on the season, each form being adapted for specific temperature ranges [Citation114].
As water temperature affects the activity of all enzymes, it directly affects the digestibility and metabolism of nutrients such as proteins and lipids [Citation121]. For example, in spotted seabass (Lateolabrax maculatus), the optimal dietary protein level is higher at 27°C than at 33°C, and higher water temperature lead to higher serum triglyceride concentration, the differences being related to differentially expressed hepatic genes involved in the metabolism of amino acids, fatty acids, and glucose [Citation122].
Conclusion
Temperature is likely the major physical environmental factors affecting the physiology of fish through effects on overall metabolism and energy balance Temperature determines how much energy fish obtains (through regulation of feeding behavior and food intake), how much of that energy is acquired (through digestion and absorption) and how much of it can be allocated to key processes such as activity, growth (including development in larvae and juveniles), and reproduction. Each species usually has a range of temperatures for which physiological processes are optimized, and any deviation from these optimal temperatures might have dramatic effects on the overall health of fish and their survival. To date, our knowledge on the effects of temperature on fish energy homeostasis is still very limited, in particular given the high diversity of fish with regards to feeding habits and habitats and capacity to “adapt” to changing temperatures. A better understanding of temperature-dependent is crucial for fish conservation and aquaculture, in particular in view of future global environmental changes.
List of abbreviations
Acknowledgments
HV acknowledges funds from the Natural Sciences and Engineering Research Council (NSERC, grant # 261414-03). IR acknowledges funds from the Regional Research Fund West (grant # 259183 Greenbag), Research Council of Norway, RCN (grants # 267626 LeuSense; # 261753 ExcelAQUA; # 311627 Gut2Brain2020), Norwegian Programme for Capacity Development in Higher Education and Research for Development (grant # QZA-0485 SRV-13/0010), and Mobility grants from Meltzer Foundation and University of Bergen.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Prosser CL, Nelson DO. The role of nervous systems in temperature adaptation of poikilotherms. Annu Rev Physiol. 1981;43(1):281–300.
- van de Pol I, Flik G, Gorissen M. Comparative physiology of energy metabolism: fishing for endocrine signals in the early vertebrate pool. Front Endocrinol. 2017;8:36.
- Kovacevic A, Latombe G, Chown SL. Rate dynamics of ectotherm responses to thermal stress. Proc R Soc B. 2019;286(1902):20190174.
- Brett JR. Energetic responses of salmon to temperature. a study of some thermal relations in the physiology and freshwater ecology of Sockeye Salmon (Oncorhynchus nerka). Am Zool. 1971;11(1):99–113.
- Willmer P, Stone J, Johnston I. Environmental physiology of animals. Malden, MA, USA: Wiley-Blackwell; 2009.
- Tattersall GJ, Sinclair BJ, Withers PC, et al. Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol. 2012;2(3):2151–2202.
- Kamunde C, Sappal R, Melegy TM. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. Plos One. 2019;14(7):e0219792.
- Liu Y, Liu J, Ye S, et al. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comp Biochem Physiol D. 2019;29:308–319.
- Neubauer P, Andersen KH. Thermal performance of fish is explained by an interplay between physiology, behaviour and ecology. Conserv Physiol. 2019;7(1):coz025–coz025.
- Rosewarne PJ, Wilson JM, Svendsen JC. Measuring maximum and standard metabolic rates using intermittent-flow respirometry: a student laboratory investigation of aerobic metabolic scope and environmental hypoxia in aquatic breathers. J Fish Biol. 2016;88(1):265–283.
- Huey RB, Stevenson RD. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool. 1979;19(1):357–366.
- Nati JJH, Lindström J, Halsey LG, et al. Is there a trade-off between peak performance and performance breadth across temperatures for aerobic scope in teleost fishes? Biol Letters. 2016;12(9):20160191.
- Miller NA, Stillman JH. Physiological optima and critical limits. Nat Educ Knowledge. 2012;3(10):1.
- Whitney JE, Al-Chokhachy R, Bunnell DB, et al. Physiological basis of climate change impacts on North American inland fishes. Fisheries. 2016;41(7):332–345.
- Pörtner H-O, Bock C, Mark FC. Oxygen- and capacity-limited thermal tolerance. Bridging Ecol Physiol. 2017;3(4):5.
- Sandersfeld T, Mark FC, Knust R. Temperature-dependent metabolism in Antarctic fish: do habitat temperature conditions affect thermal tolerance ranges? Polar Biol. 2017;40(1):141–149.
- Lowe CH, Heath WG. Behavioral and physiological responses to temperature in the desert Pupfish Cyprinodon macularius. Physiol Zool. 1969;42(1):53–59.
- Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol. 2010;77(8):1745–1779.
- Spicer JI, Morley SA, Bozinovic F. Physiological diversity, biodiversity patterns and global climate change: testing key hypotheses involving temperature and oxygen. Philos Trans Roy Soc B. 2019;374(1778):20190032.
- Rezende EL, Bozinovic F. Thermal performance across levels of biological organization. Philos Trans Roy Soc B. 2019;374(1778):20180549.
- Le M-H, Dinh K, Nguyen M, et al. Combined effects of a simulated marine heatwave and an algal toxin on a tropical marine aquaculture fish cobia (Rachycentron canadum). Aquac Res. 2020.
- Beitinger TJ, Lutterschmidt WI. Measures of thermal tolerance. In: Farrell AP, Stevens ED, Cech JJeditors. Encyclopedia of fish physiology—from genome to environment. San Diego, CA: Academic Press; 2011. p. 1695–1702.
- Fry FEJ. The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ, editors. Fish physiology. Vol. 6. Cambridge, Massachusetts: Academic Press; 1971. p. 1–98.
- Elliott JM. Tolerance and resistance to thermal stress in juvenile Atlantic salmon, Salmo salar. Freshw Biol. 1991;25(1):61–70.
- Speers-Roesch B, Norin T, Driedzic WR. The benefit of being still: energy savings during winter dormancy in fish come from inactivity and the cold, not from metabolic rate depression. Proc R Soc B. 2018;285(1886):20181593.
- Hayes J, Volkoff H. Characterization of the endocrine, digestive and morphological adjustments of the intestine in response to food deprivation and torpor in cunner, Tautogolabrus adspersus. Comp Biochem Physiol D. 2014;170:46–59.
- Soyano K, Mushirobira Y. The mechanism of low-temperature tolerance in fish. In: Iwaya-Inoue M, Sakurai M, Uemura M, editors. Survival strategies in extreme cold and desiccation: adaptation mechanisms and their applications. Singapore: Springer Singapore; 2018. p. 149–164.
- Gracheva EO, Bagriantsev SN. Evolutionary adaptation to thermosensation. Curr Opin Neurobiol. 2015;34:67–73.
- Schurmann H, Christiansen JS. Behavioral thermoregulation and swimming activity of two arctic teleosts (subfamily gadinae)—the polar cod (Boreogadus saida) and the navaga (Eleginus navaga). J Therm Biol. 1994;19(3):207–212.
- Beitinger TL, Bennett WA, McCauley RW. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish. 2000;58(3):237–275.
- Nilsson J, Moltumyr L, Madaro A, et al. Sudden exposure to warm water causes instant behavioural responses indicative of nociception or pain in Atlantic salmon. Vet An Sci. 2019;8:100076.
- Weidner J, Jensen CH, Giske J, et al. Hormones as adaptive control systems in juvenile fish. Biol Open. 2020;9(2):bio046144.
- Hopkins M, Blundell JE. Energy metabolism and appetite control: separate roles for fat-free mass and fat mass in the control of food intake in humans. In: Harris RBS, editor. Appetite and food intake: central control. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. p. 259–276.
- Blundell JE, Finlayson G, Gibbons C, et al. The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav. 2015;152:473–478.
- Speakman JR. If body fatness is under physiological regulation, then how come we have an obesity epidemic? Physiol. 2014;29(2):88–98.
- Buentello JA, Gatlin DM, Neill WH. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture. 2000;182(3–4):339–352.
- Ahmad T, Singh SP, Khangembam BK, et al. Food consumption and digestive enzyme activity of Clarias batrachus exposed to various temperatures. Aquacult Nutr. 2014;20(3):265–272.
- Nguyen MV, Espe M, Conceição LEC, et al. The role of dietary methionine concentrations on growth, metabolism and N-retention in cobia (Rachycentron canadum) at elevated water temperatures. Aquacul Nutr. 2019;25(2):495–507.
- Sharma J, Singh SP, Chakrabarti R. Effect of temperature on digestive physiology, immune-modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture. 2017;479:134–141.
- Folkedal O, Torgersen T, Olsen RE, et al. Duration of effects of acute environmental changes on food anticipatory behaviour, feed intake, oxygen consumption, and cortisol release in Atlantic salmon parr. Physiol Behav. 2012;105(2):283–291.
- Hevrøy EM, Waagbø R, Torstensen BE, et al. Ghrelin is involved in voluntary anorexia in Atlantic salmon raised at elevated sea temperatures. Gen Comp Endocrinol. 2012;175(1):118–134.
- Nadermann N, Seward RK, Volkoff H. Effects of potential climate change -induced environmental modifications on food intake and the expression of appetite regulators in goldfish. Comp Biochem Physiol D. 2019;235:138–147.
- Smirnov AK, Smirnova ES. Behavior of perch fry Perca fluviatilis (Percidae) in a heterothermal environment at different levels of food availability. Biol Bull. 2019;46(9):1065–1074.
- Shafland PL, Pestrak JM. Lower lethal temperatures for fourteen non-native fishes in Florida. Environ Biol Fish. 1982;7(2):149–156.
- Elliott JM, Elliott JA. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. J Fish Biol. 2010;77(8):1793–1817.
- Rønnestad I, Yúfera M, Ueberschär B, et al. Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquacult. 2013;5(s1):S59–S98.
- Golovanov VK, Smirnov AK, Garina DV. Thermoregulatory behavior as a form of the temperature adaptation in freshwater teleosts in a boreal climatic zone. In: Carone S, editor. Teleosts: evolutionary development, diversity and behavioral ecology. Hauppauge, NY: Nova Science Pub.; 2014. p. 153–198.
- Pavlov DS, Kasumyan AO. Sensory principles of the feeding behaviour of fishes. J Ichthyol. 1990;30(6):77–93.
- Batty RS, Hoyt RD. The role of sense organs in the feeding behaviour of juvenile sole and plaice. J Fish Biol. 1995;47(6):931–939.
- Liang XF, Kiu JK, Huang BY. The role of sense organs in the feeding behaviour of Chinese perch. J Fish Biol. 1998;52(5):1058–1067.
- Springer AD, Agranoff BW. Effect of temperature on rate of goldfish optic nerve regeneration: A radioautographic and behavioral study. Brain Res. 1977;128(3):405–415.
- Stacey NE, Kyle AL. Effects of olfactory tract lesions on sexual and feeding behavior in the goldfish. Physiol Behav. 1983;30(4):621–628.
- Liao IC, Chang EY. Role of sensory mechanisms in predatory feeding behavior of juvenile red drum Sciaenops ocellatus. Fish Sci. 2003;69(2):317–322.
- Wysocki LE, Montey K, Popper AN. The influence of ambient temperature and thermal acclimation on hearing in a eurythermal and a stenothermal otophysan fish. J Exp Biol. 2009;212(19):3091.
- Reilly CRL, Thompson SH. Temperature effects on low-light vision in juvenile rockfish (Genus Sebastes) and consequences for habitat utilization. J Comp Physiol A. 2007;193(9):943–953.
- Raderman-Little R. The effect of temperature on the turnover of taste bud cells in catfish. Cell Proliferat. 1979;12(3):269–280.
- Kasumyan AO, Sidorov SS, Pashchenko NI. Effect of water temperatures on taste sensitivity of fry of the stellate sturgeon Acipenser stellatus to free amino acids. Dokl Biol Sci. 1993;331:265–267.
- Kasumyan AO. The taste system in fishes and the effects of environmental variables. J Fish Biol. 2019;95(1):155–178.
- Toni M, Angiulli E, Miccoli G, et al. Environmental temperature variation affects brain protein expression and cognitive abilities in adult zebrafish (Danio rerio): A proteomic and behavioural study. J Proteomics. 2019;204:103396.
- Fry FEJ, Hart JS. Cruising speed of goldfish in relation to water temperature. J Fish Res Board Can. 1948;7b(4):169–175.
- Clark DS, Brown JA, Goddard SJ, et al. Activity and feeding behaviour of Atlantic cod (Gadus morhua) in sea pens. Aquaculture. 1995;131(1–2):49–57. .
- Djurichkovic LD, Donelson JM, Fowler AM, et al. The effects of water temperature on the juvenile performance of two tropical damselfishes expatriating to temperate reefs. Sci Rep. 2019;9(1):13937.
- Zhang L, Zhao Z-G, Fan Q-X. Effects of water temperature and initial weight on growth, digestion and energy budget of yellow catfish Pelteobagrus fulvidraco (Richardson, 1846). J Appl Ichthyol. 2017;33(6):1108–1117.
- Higham TE, Stewart WJ, Wainwright PC. Turbulence, temperature, and turbidity: the ecomechanics of predator-prey interactions in fishes. Integr Comp Biol. 2015;55(1):6–20.
- Coughlin DJ, Rome LC. The roles of pink and red muscle in powering steady swimming in scup, Stenotomus chrysops. Am Zool. 1996;36(6):666–677.
- Moran O, Melani R. Temperature-dependent conduction properties in Arctic fish peripheral nerves. Polar Biol. 2001;24(1):9–15.
- Fuiman L, Batty R. What a drag it is getting cold: partitioning the physical and physiological effects of temperature on fish swimming. J Exp Biol. 1997;200(12):1745.
- Rønnestad I, Gomes AS, Murashita K, et al. Appetite-controlling endocrine systems in teleosts. Front Endocrinol. 2017;8:73.
- Volkoff H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front Neurosci. 2016;10:540.
- Soengas JL, Cerdá-Reverter JM, Delgado MJ. Central regulation of food intake in fish: an evolutionary perspective. J Mol Endocrinol. 2018;60(4):R171–R199.
- Conde-Sieira M, Soengas JL. Nutrient sensing systems in fish: impact on food intake regulation and energy homeostasis. Front Neurosci. 2017;10:603.
- Volkoff H. Fish as models for understanding the vertebrate endocrine regulation of feeding and weight. Mole Cell Endocrinol. 2019;497:110437.
- Ware DM. Predation by rainbow trout (Salmo gairdneri): the influence of hunger, prey density, and prey size. J Fish Res Board Canada. 1972;29(8):1193–1201.
- Deck CA, Honeycutt JL, Cheung E, et al. Assessing the functional role of leptin in energy homeostasis and the stress response in vertebrates. Front Endocrinol. 2017;8:63.
- Michel M, Page-McCaw PS, Chen W, et al. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc Natl Acad Sci U S A. 2016;113(11):3084.
- Kehoe AS, Volkoff H. The effects of temperature on feeding and expression of two appetite-related factors, neuropeptide Y and cocaine- and amphetamine-regulated transcript, in Atlantic cod, Gadus morhua. J World Aquacult Soc. 2008;39(6):790–796.
- Vikeså V, Nankervis L, Hevrøy EM. Appetite, metabolism and growth regulation in Atlantic salmon (Salmo salar L.) exposed to hypoxia at elevated seawater temperature. Aquacult Res. 2017;48(8):4086–4101.
- Kullgren A, Jutfelt F, Fontanillas R, et al. The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic salmon (Salmo salar). Comp Biochem Physiol D. 2013;164(1):44–53.
- Babichuk NA, Volkoff H. Changes in expression of appetite-regulating hormones in the cunner (Tautogolabrus adspersus) during short-term fasting and winter torpor. Physiol Behav. 2013;120:54–63.
- Madison BN, Tavakoli S, Kramer S, et al. Chronic cortisol and the regulation of food intake and the endocrine growth axis in rainbow trout. J Endocrinol. 2015;226(2):103–119.
- Park JY, Han KH, Cho JK, et al. Survival rate and hematological responses with temperature changes of red spotted grouper, Epinephelus akaara in South Korea. Dev Reprod. 2016;20(2):103–112.
- Jaxion-Harm J, Ladich F. Effects of temperature change on cortisol release by common carp Cyprinus carpio. J Fish Biol. 2014;84(4):1221–1227.
- Olsson C. Gut anatomy. In: AP F, editor. Encyclopedia of fish physiology. San Diego: Academic Press; 2011. p. 1268–1275.
- Le HTMD, Shao X, Krogdahl Å, et al. Intestinal function of the stomachless fish, Ballan wrasse (Labrus bergylta). Front Mar Sci. 2019;6:140.
- Takei Y, Loretz CA. The gastrointestinal tract as an endocrine/neuroendocrine/paracrine organ: organization, chemical messengers and physiological targets. In: Grosell M, Farrell AP, Brauner CJ, editors. Fish physiology. Vol. 30. Cambridge, Massachusetts: Academic Press; 2010. p. 261–317.
- Kapoor BG, Smit H, Verighina IA. The alimentary canal and digestion in teleosts. In: Russell FS, Yonge M, editors. Advances in marine biology. Vol. 13. Cambridge, Massachusetts: Academic Press; 1976. p. 109–239.
- Miegel RP, Pain SJ, van Wettere WHEJ, et al. Effect of water temperature on gut transit time, digestive enzyme activity and nutrient digestibility in yellowtail kingfish (Seriola lalandi). Aquaculture. 2010;308(3–4):145–151.
- Nakagawa H. Temperature-dependent gastric evacuation rate of the Japanese delicate loach Niwaella delicata (Cobitidae). Ichthyol Res. 2018;65(1):172–174.
- Temming A, Herrmann J-P. Gastric evacuation in horse mackerel. I. The effects of meal size, temperature and predator weight. J Fish Biol. 2001;58(5):1230–1245.
- Das SK, Noor NM, Kai KS, et al. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquacult Rep. 2018;12:20–24.
- De M, Ghaffar MA, Bakar Y, et al. Effect of temperature and diet on growth and gastric emptying time of the hybrid, Epinephelus fuscoguttatus ♀×E. lanceolatus ♂. Aquacult Rep. 2016;4:118–124.
- Mazumder SK, Ghaffar MA, Das SK. Exploring the suitable temperature and diet for growth and gastric emptying time of juvenile malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Thalassas. 2019;35(1):29–41.
- Yúfera M, Nguyen MV, Navarro-Guillén C, et al. Effect of increased rearing temperature on digestive function in cobia early juvenile. Comp Biochem Physiol D. 2019;230:71–80.
- Solovyev MM, Izvekova GI. Seasonal changes in pH values in the intestine of fish from Lake Chany (West Siberia). Inland Water Biol. 2016;9(4):400–404.
- Kemp P, Smith MW. Effect of temperature acclimatization on the fatty acid composition of goldfish intestinal lipids. Biochem J. 1970;117(1):9–15.
- Vasemägi A, Visse M, Kisand V. Effect of environmental factors and an emerging parasitic disease on gut microbiome of wild salmonid fish. mSphere. 2017;2(6):e00418–17.
- Wang AR, Ran C, Ringø E, et al. Progress in fish gastrointestinal microbiota research. Rev Aquacult. 2018;10(3):626–640.
- Butt RL, Volkoff H. Gut microbiota and energy homeostasis in fish. Front Endocrinol. 2019;10:9.
- Steell SC, Van Leeuwen TE, Brownscombe JW, et al. An appetite for invasion: digestive physiology, thermal performance and food intake in lionfish (Pterois spp.). J Exp Biol. 2019;222(19):jeb209437.
- Luo Y, Xie X. Effects of temperature on the specific dynamic action of the southern catfish, Silurus meridionalis. Comp Biochem Physiol D. 2008;149(2):150–156.
- Tirsgaard B, Svendsen JC, Steffensen JF. Effects of temperature on specific dynamic action in Atlantic cod Gadus morhua. Fish Physiol Biochem. 2015;41(1):41–50.
- Di Santo V, Lobel PS. Size affects digestive responses to increasing temperature in fishes: physiological implications of being small under climate change. Mar Ecol. 2016;37(4):813–820.
- Pang X, Cao Z-D, Fu S-J. The effects of temperature on metabolic interaction between digestion and locomotion in juveniles of three cyprinid fish (Carassius auratus, Cyprinus carpio and Spinibarbus sinensis). Comp Biochem Physiol D. 2011;159(3):253–260.
- Klinger DH, Dale JJ, Gleiss AC, et al. The effect of temperature on postprandial metabolism of yellowfin tuna (Thunnus albacares). Comp Biochem Physiol D. 2016;195:32–38.
- Amin MN, Carter CG, Katersky Barnes RS, et al. Protein and energy nutrition of brook trout (Salvelinus fontinalis) at optimal and elevated temperatures [Article]. Aquacul Nutr. 2016;22(3):527–540.
- Bakke AM, Glover C, Krogdahl Å. 2 - Feeding, digestion and absorption of nutrients. In: Grosell M, Farrell AP, Brauner CJ, editors. Fish physiology. Vol. 30. Cambridge, Massachusetts: Academic Press; 2010. p. 57–110.
- Kuz’mina VV. Classical and Modern conceptions of fish digestion. In: Cyrino JEP, Bureau D, Kapoor BG, editors. Feeding and digestive functions in fishes. Enfield, NH: Science Publishers; 2008. p. 85–154.
- Male R, Lorens JB, Smalås AO, et al. Molecular cloning and characterization of anionic and cationic variants of trypsin from Atlantic salmon. Eur J Biochem. 1995;232(2):677–685.
- Gudmundsdóttir A, Gudmundsdóttir E, Óskarsson S, et al. Isolation and characterization of cDNAs from Atlantic cod encoding two different forms of trypsinogen. Eur J Biochem. 1993;217(3):1091–1097.
- Klomklao S, Benjakul S. Two trypsin isoforms from albacore tuna (Thunnus alalunga) liver: purification and physicochemical and biochemical characterization. Int J Biol Macromol. 2018;107:1864–1870.
- Ahsan MN, Funabara D, Watabe S. Molecular cloning and characterization of two isoforms of trypsinogen from anchovy pyloric ceca. Mar Biotechnol. 2001;3(1):80–90.
- Hidalgo MC, Urea E, Sanz A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquacult 1999;170(3–4):267–283.
- Gioda CR, Pretto A, Freitas CDS, et al. Different feeding habits influence the activity of digestive enzymes in freshwater fish. Ciênc Rural. 2017;47(3):e20160113.
- Gelman A, Kuz’mina V, Drabkin V, et al. Temperature adaptation of digestive enzymes in fish. In: Cyrino JEP, Bureau DP, Kapoor BGeditors. Feeding and digestive functions in fishes. Enfield, NH, USA: Science Publishers; 2008. p. 155–225.
- Stefansson B, Sandholt GB, Gudmundsdottir Á. Elucidation of different cold-adapted Atlantic cod (Gadus morhua) trypsin X isoenzymes. Biochim Biophys Acta. 2017;1865(1):11–19.
- Krogdahl Å, Sundby A, Bakke AM. Gut secretion and digestion. In: Farrell AP, editor. Encyclopedia of fish physiology. San Diego: Academic Press; 2011. p. 1301–1310.
- Pereira LF, Peixoto MJ, Carvalho P, et al. Cross-effects of dietary probiotic supplementation and rearing temperature on growth performance, digestive enzyme activities, cumulative mortality and innate immune response in seabass (Dicentrarchus labrax). Aquacult Nutr. 2018;24(1):453–460.
- Hani YMI, Marchand A, Turies C, et al. Digestive enzymes and gut morphometric parameters of threespine stickleback (Gasterosteus aculeatus): influence of body size and temperature. Plos One. 2018;13(4):e0194932.
- Einarsson S, Jönsson AC, Davies PS. Seasonal variation in trypsin activity in juvenile Atlantic salmon upper and lower modal groups. J Fish Biol. 1997;51(6):1209–1218.
- Ma F, Yang Y, Jiang M, et al. Digestive enzyme activity of the Japanese grenadier anchovy Coilia nasus during spawning migration: influence of the migration distance and the water temperature. J Fish Biol. 2019;95(5):1311–1319.
- Fang J, Tian X, Dong S. The influence of water temperature and ration on the growth, body composition and energy budget of tongue sole (Cynoglossus semilaevis). Aquaculture. 2010;299(1–4):106–114.
- Cai L-S, Wang L, Song K, et al. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture. 2020;516:734615.
- Jobling M. The influences of feeding on the metabolic rate of fishes: a short review. J Fish Biol. 1981;18(4):385–400.
- McKenzie DJ, Axelsson M, Chabot D, et al. Conservation physiology of marine fishes: state of the art and prospects for policy. Conserv Physiol. 2016;4(1):cow046–cow046.
- Treberg JR, Killen SS, MacCormack TJ, et al. Estimates of metabolic rate and major constituents of metabolic demand in fishes under field conditions: methods, proxies, and new perspectives. Comp Biochem Physiol D. 2016;202:10–22.
- Jobling M. Temperature and growth: modulation of growth rate via temperature change. In: Wood CM, McDonald DG, editors. Global warming: implications for freshwater and marine fish. Society for experimental biology seminar series. Cambridge: Cambridge University Press; 1997. p. 225–254.
