ABSTRACT
In studies of human thermoregulation, ingestible temperature pills are being increasingly used as a convenient alternative to more clinically relevant indices of deep-body (core) temperature (e.g., rectal temperature). It remains unclear whether the time between pill ingestion and the measurement period influences the validity of telemetry pills as a surrogate index of core temperature. We therefore assessed the influence of pill ingestion timing on the agreement between rectal temperature (criterion method) and ingestible pill temperature during exercise-heat stress. To achieve this, nine young men (21–31 years) completed two trials involving 15-min rest, 90-min exercise at an average metabolic heat production of 200 W/m2 (~40% peak oxygen consumption), and 45-min recovery. Core temperature was measured throughout using rectal temperature and four telemetric temperature pills (VitalSense®) ingested 12, 6, 3 and 1 h(s) prior to the start of each trial. Data from the two trials were combined and averaged over the final 10-min of rest, exercise, and recovery for analysis. Our primary finding was that the mean squared difference between rectal temperature and each pill did not differ significantly across ingestion times during rest, exercise or recovery (p = 0.056), with those errors ranging from 0.1–0.2°C, 0.2–0.2°C, 0.1–0.2°C, and 0.1–0.2°C for the pills ingested 12, 6, 3, and 1 h(s) before data collection, respectively. While there is a need for larger confirmatory studies, our findings indicate that pill ingestion timing does not significantly influence the validity of telemetry pill temperature as an index of core temperature.
Introduction
Obtaining valid measures of deep-body (core) temperature is fundamental for studying human thermoregulation. Unfortunately, the most clinically relevant measures of core temperature (e.g., rectal, esophageal, and pulmonary artery) necessitate the use of wired recording devices, which are often impractical. For that reason, temperature measurements from an ingestible, telemetric pill (Tpill) and wireless recording device, are being increasingly used as a more convenient alternative for both field- and laboratory-based experiments. This relatively new technology is generally considered to provide a valid index of core temperature that shares good agreement with rectal temperature (Tre) during exercise-heat stress [Citation1]. However, unlike Tre, which is measured from a fixed anatomical location, Tpill is measured as it passes through the gastrointestinal tract. This presents potential issues that may impact validity including food and fluid consumption, and temperature differences along the gastrointestinal tract.
While the effects of food and fluid consumption on Tpill are relatively well studied [Citation2,Citation3], we know considerably less regarding the effects of pill positioning within the gastrointestinal tract on Tpill validity. It has been suggested that pills ingested earlier prior to data collection, which have presumably progressed further toward the lower gastrointestinal tract, may be more stable and share better agreement with Tre [Citation4–6]. However, since previous studies assessing Tpill ingestion timing are sparse and have not simultaneously measured Tre [Citation7,Citation8], direct evidence supporting this suggestion is unavailable. Perhaps for this reason, there is extensive variation in pill ingestion timing in the literature (0.5–12 hours) [Citation1], with no apparent consensus on optimal ingestion time.
For reasons beyond our control, a field-based experiment planned by our group to assess occupational heat strain using telemetric temperature pills was postponed, leaving a large quantity of pre-purchased temperature pills due to expire in the coming months. We were therefore presented with a unique opportunity to conduct an exploratory study on the effect of pill ingestion timing on the validity of Tpill as an index of core temperature. To achieve this, we incorporated the measurement of Tpill within an ongoing study directed at evaluating the effects of the partitioning of work intensity on heat exchange and Tre under hot, dry conditions [Citation9]. In this study, young men performed two trials involving 15-min rest, 90-min exercise eliciting an average metabolic heat production of 200 W/m2 (~40% peak oxygen consumption), and 45-min recovery. At 12, 6, 3 and 1 hour(s) prior to the start of each trial, temperature pills were ingested and compared with Tre (criterion). Based on previous suggestions [Citation4–6], we anticipated that agreement between Tre and Tpill would improve with increases in the ingestion time prior to data collection.
Methods
Ethical approval
The experimental protocol was approved by the University of Ottawa Health Sciences and Science Research Ethics Board (file: H04-17-05) and conformed to the latest version of the Declaration of Helsinki, except for registration in a database. Written and informed consent was obtained from all volunteers prior to their participation.
Participants
Nine men participated (mean [SD]; age: 26 [Citation4] years; height: 176 [Citation8] cm; mass: 78 [Citation7] kg; body surface area: 1.9 [0.1] m2; body fat: 11% [Citation3]; peak oxygen consumption: 46 [Citation5] mL/kg/min). All participants were healthy and physically active, performing ≥150 min/week of moderate-to-vigorous physical activity. Participants were not taking any medication, were nonsmokers and did not report a history of cardiovascular, respiratory, or metabolic disease. As noted above, this study was part of a larger investigation [Citation9], with data from some participants (n = 8) being reproduced here.
Experimental design
Participants completed one screening session and two randomly ordered experimental trials, wearing minimal clothing (sports shoes and shorts). Experimental trials were performed at the same time of day (separated by ~1 week). Prior to each session, participants were asked to refrain from exercise, alcohol, caffeine and non-steroidal anti-inflammatory drugs for >24 h, and to ensure they were adequately hydrated before arriving.
Screening session
During the screening visit, standing height, body mass, body surface area, body density, and peak oxygen consumption were determined. Body surface area was derived from measures of standing height (model 2391, Detecto, Webb City, MO, USA) and body mass (IND560, Mettler Toledo Inc., Mississauga, ON, Canada) [Citation10]. Body density was measured using the hydrostatic weighing technique and used to estimate body fat percentage [Citation11]. Indirect calorimetry was used to quantify peak oxygen consumption (MCD Medgraphics Ultima Series, MGC Diagnostics, MN, USA) during an incremental exercise to volitional fatigue on a semi-recumbent cycle ergometer (Corival, Lode B.V., Groningen, Netherlands).
Experimental trials
On the day prior to each trial, participants were provided with four ingestible temperature pills (see details in “Measurements”), which had been activated according to manufacturer instructions. Participants were instructed to consume each pill with warm water (to better reflect internal temperature) at 12, 6, 3 and 1 hour(s) prior to the start of each trial, with ingestion being confirmed via phone by one of the authors (SRN). Since all experimental trials commenced at ~12:00 pm, participants consumed each pill at 12:00 am (midnight), 6:00 am, 9:00 am, and 11:00 am ()). Following ingestion of the last pill (11:00 am), no food or fluid consumption was permitted. These ingestion times will be referred to hereafter as Tpill−12 h, Tpill−6 h, Tpill−3 h, and Tpill−1 h (respectively), and were selected to span the range of ingestion times reported in the literature [Citation1]. We acknowledge that pill placement within the gastrointestinal tract may be influenced by meal consumption; however, we did not document the timing or nutritional content of meals consumed.
Figure 1. Panel A: A schematic of the time points at which temperature pills were ingested: 12, 6, 3 and 1 hour(s) prior to data collection within each experimental trial (Exp. trial). Since trials commenced at ~12:00 pm, this meant that participants consumed each pill at 12:00 am (midnight) (Tpill−12 h), 6:00 am (Tpill−6 h), 9:00 am (Tpill−3 h), and 11:00 am (Tpill−1 h), before inserting a rectal temperature probe (Tre) prior to the trial. Panel B: An example of time-dependent changes in all temperature indices (1-min averages) from one subject during one of the two experimental trials. Each trial involved 15-min rest, 90-min of cycling eliciting a metabolic heat production of ~200 W/m2 in dry heat (40°C, ~20% relative humidity), and 45-min recovery. Each trial differed only in the pattern of external work performed, with one involving a constant external work rate of 40 W/m2 and the other involving alternating cycles of 5 min at 15 W/m2 then 5 min at 60 W/m2. The gray zones denote the times at which data were extracted for statistical comparisons. Panel C: An example of time-dependent changes in all temperature indices (1-min averages) for the same subject in Panel B, but this time presented as a difference from rectal temperature. The gray zone shows the error threshold (±0.3°C) we considered to be acceptable, with data points falling within this area being used to assess agreement between each pill and rectal temperature (see “Data and statistical analysis” for details).
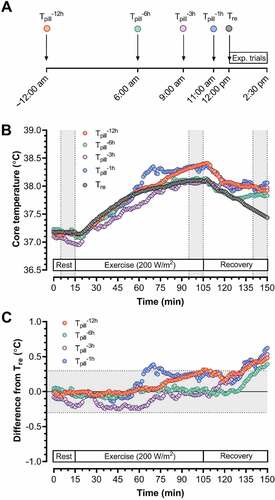
Upon arrival to the laboratory for each trial, participants provided a urine sample to confirm euhydration (urine specific gravity: ≤1.025) [Citation12], before being instrumented in a temperate room (~25°C). Participants then sat in the semi-recumbent position in dry-heat (40°C, ~20% relative humidity; air flow ~0.3 m/s) and completed 15-min rest, 90-min of cycling eliciting an average metabolic heat production of ~200 W/m2 (~40% of peak oxygen consumption), and 45-min recovery. Each trial differed only in the pattern of external work performed to elicit that heat production, with one involving a constant external work rate of 40 W/m2 (constant-intensity exercise) and the other involving cycles of 5 min at 15 W/m2 then 5 min at 60 W/m2 (variable-intensity exercise). Since our previous work demonstrated similar Tre responses across constant- and variable-intensity exercise [Citation9], and given our preliminary analysis revealed comparable agreement between Tpill and Tre in both conditions [Citation13], we performed all statistical analysis on data combined across conditions to maximize our sample size (n = 18) and simplify our analysis.
Measurements
Metabolic heat production
During each trial, oxygen consumption, carbon dioxide production and minute ventilation were derived from measures of expired gases and air flows (Moxus modular metabolic system, AEI Technologies, Bastrop, TX, USA), and used to approximate metabolic rate [Citation14]. External work was controlled using a modified, constant load cycle ergometer. Metabolic heat production was calculated as the difference between metabolic rate and external work.
Thermometry
Core (rectal) temperature was measured using a thermocouple probe (Mon-a-therm General Purpose Temperature Probe, Mallinckrodt Medical, St. Louis, MO, USA) inserted 12 cm past the anal sphincter. Skin temperature was measured at four sites (bicep, chest, thigh, and calf) using T-type thermocouples (Concept Engineering, Old Saybrook, CT, USA) attached with surgical tape, and used to approximate mean skin temperature [Citation15]. Rectal and skin temperature sensors were calibrated using a mercury-in-glass thermometer, yielding an error of <0.1°C. Data were sampled at 15-s intervals using a HP Agilent data-acquisition module (model 3497A), and recorded with LabVIEW software (version 7.0, National Instruments).
Core temperature was also monitored during each trial using four ingestible temperature pills (VitalSense® ingestible capsule thermometer; Mini Mitter, Bend, OR, USA). Data were recorded at 15-s intervals using a single hip-worn, recording device (VitalSense® Monitor, Mini Mitter Company, Bend, OR, USA) set to Medic Mode™. In this mode of recording, the monitor detects and records signals from all VitalSense® pills within range. Since recent evidence indicated that VitalSense® capsules demonstrate low systematic bias (<0.1°C) when assessed against a reference compared to other systems [Citation16], we opted not to calibrate each pill. Data were extracted to spreadsheet format using manufacturer software. Unfortunately, one subject expelled a pill (Tpill−12 h) prior to arriving at the laboratory for one experimental trial. We therefore report a reduced sample size for comparisons across pills (n = 17).
Heart rate and urine specific gravity
Heart rate was recorded at 1-s intervals using a Polar M400 monitor (Polar Electro Oy, Finland). Pre-trial urine specific gravity was assessed using a refractometer (Reichert TS 400, Reichert, Depew, NY, USA).
Data and statistical analysis
Metabolic heat production, Tre, each Tpill, mean skin temperature, and heart rate were expressed as 1-min averages, with a mean of the final 10-min of pre-exercise rest, exercise and recovery (gray zones in )) being used for statistical analysis unless stated otherwise. These mean data were used to calculate mean squared errors for each Tpill against Tre (expressed as absolute and percent differences). Mean core temperatures from all indices, as well as the mean squared errors for each Tpill, were compared across experimental phases (rest, exercise, recovery) using a repeated measures ANOVA. If a significant interaction or main effect occurred, pairwise comparisons were conducted using Bonferroni-adjusted, two-tailed t-tests.
To further assess agreement between Tre and each Tpill, we calculated the mean bias and limits of agreement (mean bias ±1.96 SD) during each experimental phase according to the methods provided by Bland and Altman for repeated, non-constant measurements [Citation17]. To identify systematic bias, the mean bias for each Tpill was compared to zero at each time point using one-tailed t-tests, with the p-value being adjusted using the Bonferroni procedure to control for type one error due to multiple comparisons. The limits of agreement were compared to an acceptance threshold defined a priori (±0.3°C), with limits falling on or outside this threshold indicating that Tpill does not offer the level agreement required to be used interchangeably with Tre. This threshold has been used previously in comparative studies of Tpill and Tre [Citation8], as it represents the typical day-to-day variation in resting Tre in young men [Citation18], and a difference that may be considered physiologically meaningful.
The analysis above ignores data collected outside of the final 10-min of rest, exercise, and recovery. We therefore calculated the percentage of Tpill measurements (1-min intervals) falling within the acceptance threshold noted above (±0.3°C) across the entire 150-min protocol (gray zone in )). These percentages were compared across ingestion times using a Friedman test, with Dunn’s multiple comparison test being used in the event of a significant main effect. All statistical analyses were performed using Prism 8 (GraphPad, CA, USA). Alpha was set at 0.05 for all analyses, with data being reported as means (SD) unless stated otherwise. All raw data are freely available in an online repository (https://osf.io/c3r4x/).
Results
Metabolic heat production, heart rate and mean skin temperature averaged 54 (7) W/m2, 75 (9) beats/min and 35.1°C (0.3) at baseline, 208 (5) W/m2, 126 (13) beats/min and 35.9°C (0.4) during exercise, and 51 (5) W/m2, 89 (11) beats/min and 35.5°C (0.3) during recovery. When the mean temperatures from all indices were compared across experimental phases (), we observed a temperature index-by-phase interaction (p = 0.016). All indices were elevated from rest during exercise and recovery (all p < 0.001). Further, while Tpill−1 h was lower than Tpill−12 h, Tpill−6 h, and Tpill−3 h at rest (all p < 0.028), responses did not differ between indices during exercise and recovery (all p ≥ 0.135). Likewise, the mean squared errors for each Tpill did not differ between ingestion times or experimental phases (p ≥ 0.056) ().
Table 1. Statistical comparisons of the mean and mean squared errors for rectal temperature and each ingestible pill.
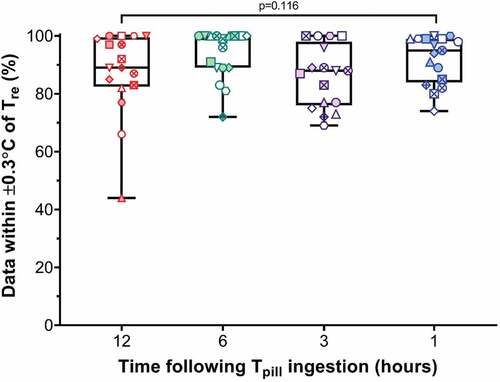
Agreement between each Tpill and Tre as assessed using Bland–Altman plots is illustrated in . No significant systematic bias was detected across all temperature pills (all p ≥ 0.090), with mean bias ranging from −0.1°C (0.1) to 0.1°C (0.1). However, all temperature pills displayed limits of agreement (range: ±0.3°C to ±0.5°C) falling outside our acceptance threshold (±0.3°C). We also calculated the percentage of Tpill measurements (1-min intervals) falling within this acceptance threshold across the entire 150-min protocol (). Those median percentages (range: 44–100%), however, did not differ across ingestion times (p = 0.116).
Figure 2. Bland-Altman plots showing agreement between rectal temperature (Tre) and gastrointestinal temperature pills ingested 12 (Tpill−12; A), 6 (Tpill−6 h; B), 3 (Tpill−3 h; C) and 1 hour(s) (Tpill−1 h; D) prior to data collection. Data were obtained from nine participants who completed two trials involving 15-min rest, 90-min cycling eliciting an average metabolic heat production of ~200 W/m2, followed by 45-min recovery in dry heat (40°C, ~20% relative humidity), with data presented as averaged of the final 10-min of rest, exercise and recovery. Each trial differed only in the pattern of external work performed, with one involving a constant external work rate of 40 W/m2 and the other involving 10-min cycles of 5 min at 15 W/m2 then 5 min at 60 W/m2 (variable-intensity exercise). Each subject has a unique symbol, with the open and shaded symbols representing the constant- and variable-intensity exercise trials, respectively. The dotted gray lines show zero bias, the solid black lines show the mean bias, and the dashed black lines show the limits of agreement (mean bias ±1.96 SD). All limits of agreement fell on or outside of the acceptance threshold (±0.3°C) defined a priori (gray shaded zone), indicating that none of the temperature pills could be used interchangeably with rectal temperature to assess core temperature.
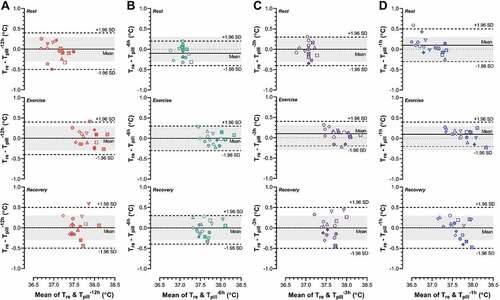
Figure 3. Box-plots showing the median, interquartile range and min/max for the percentage of measurements (1-min intervals) from each pill falling within our pre-established acceptance threshold (±0.3°C from rectal temperature) across the entire 150 min protocol (gray zone in )). These percentages were compared across ingestion times using a Friedman test, with Dunn’s multiple comparison test being used in the event of significant main effect. between rectal temperature (Tre) and temperature pills ingested 12 (Tpill−12; A), 6 (Tpill−6 h; B), 3 (Tpill−3 h; C) and 1 hour(s) (Tpill−1 h; D) prior to data collection. Data were obtained from nine participants who ingested temperature pills 12, 6, 3 and 1 hour(s) prior to completing two trials involving 15-min rest, 90-min cycling eliciting an average metabolic heat production of ~200 W/m2, followed by 45-min recovery in dry heat (40°C, ~20% relative humidity). Each trial differed only in the pattern of external work performed, with one involving a constant external work rate of 40 W/m2 and the other involving 10-min cycles of 5 min at 15 W/m2 then 5 min at 60 W/m2 (variable-intensity exercise). Each subject has a unique symbol, with the open and shaded symbols representing the constant- and variable-intensity exercise trials, respectively. No statistically significant differences were observed across ingestion times (p = 0.116).
Discussion
In the present study, we evaluated the agreement between rectal temperature (criterion) and ingestible telemetric temperature pills consumed 12, 6, 3 and 1 hour(s) prior to data collection, during exercise-heat stress in young men. We hypothesized that agreement between rectal and pill temperature would improve with increases in time following ingestion. However, neither the mean temperature nor mean squared error were significantly influenced by ingestion timing. Further, the limits of agreement for each pill fell on or exceeded our acceptance criteria (±0.3°C). Thus, while ingestible temperature pills did not offer the precision required to be used interchangeably with rectal temperature, pill ingestion timing appears not to influence the validity of telemetry pill temperature as an index of core temperature.
Several authors have documented increases in gastrointestinal temperature as an ingestible pill transitions through the gastrointestinal tract [Citation4,Citation7,Citation8], with some suggesting that gastrointestinal temperature may become more stable and reflective of rectal temperature as a pill moves along the gastrointestinal tract [Citation5,Citation6,Citation19]. We therefore anticipated that agreement between rectal and pill temperature may improve with time following pill ingestion. In contrast, the mean squared error recorded from pills ingested 12, 6, 3 and 1 hour(s) prior to data collection did not differ significantly during baseline, exercise or recovery (). This was paralleled by a similar percentage of data points (range: 44–100%) falling within our acceptance threshold (±0.3°C from rectal temperature) across the four ingestion times, indicating that pill ingestion timing does not significantly influence agreement between rectal and ingestible pill temperature.
Since we were unable to image the gastrointestinal tract during the experiment, it is difficult to explain the unexpected similarities in the agreement between rectal and pill temperature across ingestion times. It is possible, however, that any purported temperature differences across the gastrointestinal tract [Citation4,Citation7,Citation8], may be of insufficient magnitude to influence agreement with rectal temperature. Another possibility, is that such temperature discrepancies may simply represent that pill equilibrating with the surrounding, warmer tissues if cool fluid was used to consume a pill prior to use [Citation3], which we attempted to minimize by instructing participants to consume pills with warm water. Identifying strategies to mitigate temperature artifacts during experiments where cool food or fluid consumption cannot be avoided (e.g., field-based studies) may therefore represent an important area of future research.
Despite the observed similarities in gastrointestinal temperature across ingestible pills (), none demonstrated acceptable agreement with rectal temperature (). Indeed, although all pills demonstrated a low systematic bias (range: −0.1 to 0.1°C), the limits of agreement (range: ±0.3 to ±0.5°C) fell on or outside our acceptance threshold (±0.3°C). Therefore, while ingestible temperature pills provide a suitable means to approximate core temperature, they did not provide the agreement required to be used interchangeably with rectal temperature to assess core temperature. This outcome is consistent with previous comparisons of rectal temperature and one ingestible pill [Citation4,Citation19–22], although we extend those observations by demonstrating such agreement is not appreciably modified by pill ingestion timing.
Perspectives
Investigators utilizing ingestible pills to assess core temperature have employed a broad range of pill ingestion times prior to use (0.5–12 h), with no apparent consensus on appropriate timing [Citation1]. For instance, two groups suggested 6 h before data collection would be optimal [Citation1,Citation19], while another proposed 12 h [Citation6]. Based on our findings, ingesting a temperature pill between 12 and 1 hour(s) prior to use would seemingly offer similar utility. However, it is important to note that we prevented food and fluid consumption during data collection to avoid the associated artifacts, which can persist for ~8 h [Citation3]. Further, one subject arrived at the laboratory having already expelled a pill ingested ~11 h prior. Taking these considerations into account, we therefore propose the following: (i) for experiments where food and fluid consumption can be avoided or consumed at body temperature during data collection, pills should be ingested 1–6 h prior to data collection, whereas (ii) for instances where food and fluid consumption cannot be avoided, pills should be ingested between 8–10 hours prior to data collection. Nonetheless, we acknowledge there may be instances where the latter is impossible (e.g., field-based research). The only suitable approach we are aware of for such scenarios is to use an ingestible pill as a suppository [Citation23]. While there is a need to verify these suggestions in differing exercise and environmental conditions as well as in different populations, we hope they may assist in the design of studies utilizing ingestible temperature pills to assess core temperature.
Considerations
As noted earlier, this study was conducted as we were provided with a unique opportunity to utilize pre-purchased ingestible temperature pills, which were due to expire in the coming months. We therefore incorporated the measurement of gastrointestinal temperature within an ongoing study directed at evaluating the effects of the partitioning of work intensity on heat exchange and rectal temperature in young men in dry-heat [Citation24], as opposed to designing a study specifically for this purpose. As such, there are several design limitations worth noting. First, we observed core temperatures spanning a relatively narrow range (~37.0–37.9°C). We therefore cannot be certain whether our findings hold for more extreme environmental or exercise conditions associated with hypothermia or more profound hyperthermia. Second, given that gastrointestinal motility can be modified by various individual factors [Citation25,Citation26], it is possible that pill position within the gastrointestinal tract and its subsequent validity may differ in other populations (e.g., women, older adults). Finally, since the measurement characteristics of the ingestible temperature systems can differ based on the manufacturer [Citation16,Citation22], it remains unknown whether our findings hold for other systems.
Conclusion
When assessed in young men during exercise-heat stress, we demonstrated that the agreement between rectal temperature and gastrointestinal temperature, as measured from telemetry pills ingested 12, 6, 3 and 1 hour(s) prior to data collection, was not significantly modified by pill ingestion timing. While there is a need for larger, confirmatory studies, these findings indicate that pill ingestion timing appears not to influence the validity of ingestible temperature pills as an index of core temperature.
Author contributions
S.R.N., R.D.M., and G.P.K. conceptualized and designed the research; S.R.N., and R.D.M. performed experiments; S.R.N. analyzed data, prepared figures and drafted the manuscript. All authors interpreted results of experiments, edited and revised the manuscript, and approved the final version.
Grants
This research was supported by the Government of Ontario, Canada (all funds held by Dr. Glen P. Kenny). G.P. Kenny is supported by a University of Ottawa Research Chair. S.R. Notley is supported by a Postdoctoral Fellowship from the Human and Environmental Physiology Research Unit. R.D. Meade was supported by an Ontario Graduate Scholarship.
Acknowledgments
We thank all the participants who volunteered for the present study, and Mr. Andrew W. D’Souza and Ms. Maura M. Rutherford, who assisted with data collection.
Disclosure statement
No conflict of interest, financial or otherwise, are declared by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41(3):126–133.
- Savoie FA, Dion T, Asselin A, et al. Intestinal temperature does not reflect rectal temperature during prolonged, intense running with cold fluid ingestion. Physiol Meas. 2015;36(2):259.
- Wilkinson DM, Carter JM, Richmond VL, et al. The effect of cool water ingestion on gastrointestinal pill temperature. Med Sci Sports Exercise. 2008;40(3):523–528.
- Kolka MA, Levine L, Stephenson LA. Use of an ingestible telemetry sensor to measure core temperature under chemical protective clothing. J Therm Biol. 1997;22(4–5):343–349.
- Livingstone S, Grayson J, Frim J, et al. Effect of cold exposure on various sites of core temperature measurements. J Appl Physiol. 1983;54(4):1025–1031.
- O’Brien C, Hoyt RW, Buller MJ, et al. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc. 1998;30(3):468–472.
- Domitrovich JW, Cuddy JS, Ruby BC. Core-temperature sensor ingestion timing and measurement variability. J Athl Train. 2010;45(6):594–600.
- Goodman D, Kenefick R, Cadarette B, et al. Influence of sensor ingestion timing on consistency of temperature measures. Med Sci Sports Exerc. 2009;41(3):597–602.
- Notley SR, Meade RD, D’Souza AW, et al. Heat exchange in young and older men during constant- and variable-intensity work. Med Sci Sports Exerc. 2020; Published ahead of print. doi:10.1249/MSS.0000000000002410.
- Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Internal Med. 1916;17(6_2):863–871.
- Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–280.
- Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70(Suppl 2):S137–142.
- D’Souza AW, Notley SR, Meade RD, et al. The influence of ingestion time on the validity of gastrointestinal pill temperature as an index of body core temperature during work in the heat. FASEB J. 2019;33. ( 842.847-842.847).
- Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol. 2013;3:1689–1719.
- Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19(3):531–533.
- Bongers CC, Daanen HA, Bogerd CP, et al. Validity, reliability, and inertia of four different temperature capsule systems. Med Sci Sports Exercise. 2018;50(1):169–175.
- Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–582.
- Consolazio C, Johnson R, Pecora L. Physiological variability in young men. In: Physiological measurements of metabolic functions in man. 1963. p. 453–480. McGraw Hill Publishing Co. Ltd: London, U.K.
- Lee S, Williams W, Fortney SS. Core temperature measurement during supine exercise: esophageal, rectal, and intestinal temperatures. Aviat Space Environ Med. 2000;71(9):939–945.
- Casa DJ, Becker SM, Ganio MS, et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42:333.
- Ganio MS, Brown CM, Casa DJ, et al. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J Athl Train. 2009;44(2):124–135.
- Travers GJ, Nichols DS, Farooq A, et al. Validation of an ingestible temperature data logging and telemetry system during exercise in the heat. Temperature. 2016;3(2):208–219. doi: 10.1080/23328940.2016.1171281.
- Kenefick RW, Sollanek KJ, Charkoudian N, et al. Impact of skin temperature and hydration on plasma volume responses during exercise. J Appl Physiol (1985). 2014;117(4):413–420.
- Notley SR, Meade RD, D’Souza AW, Rutherford MM, Kim JH, Kenny GP. Heat Exchange in Young and Older Men during Constant- and Variable-Intensity Work [published online ahead of print, 2020 May 18]. Med Sci Sports Exerc. 2020.
- Degen L, Phillips S. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39(2):299–305.
- Graff J, Brinch K, Madsen JL. Gastrointestinal mean transit times in young and middle-aged healthy subjects. Clin Physiol. 2001;21(2):253–259.
